Abstract
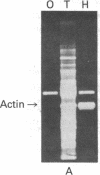
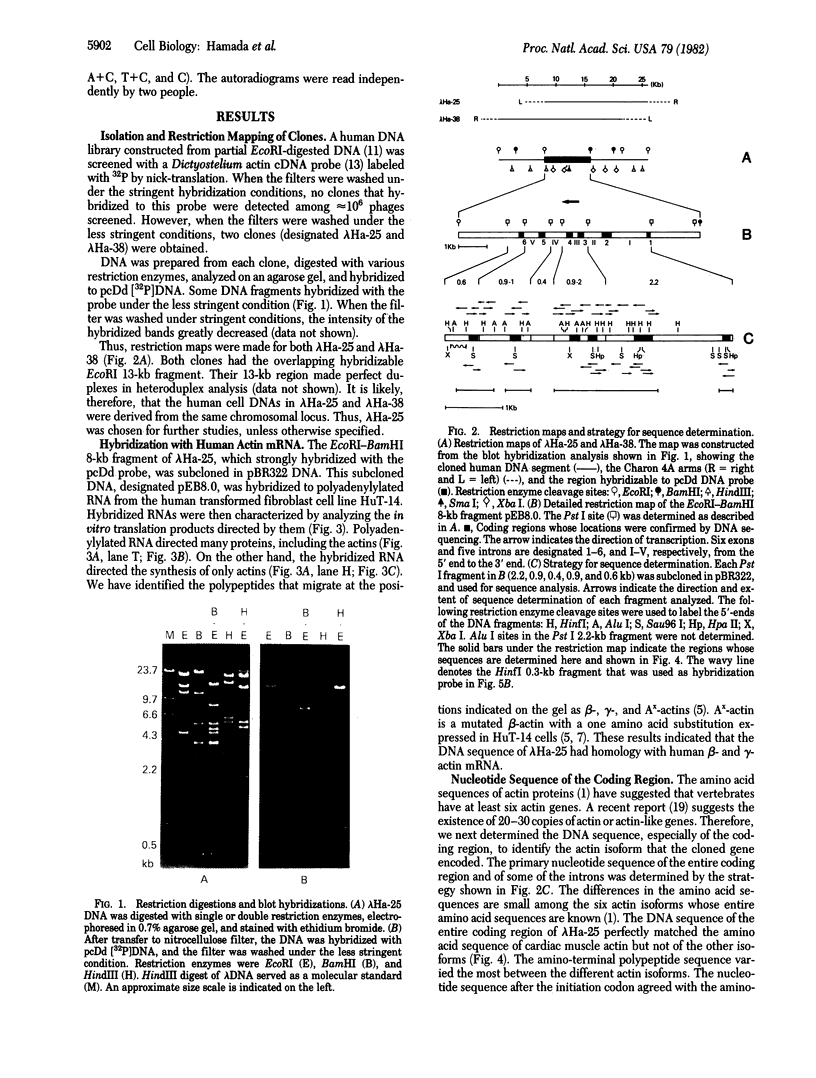
Two recombinant phages that contain cardiac muscle actin gene were isolated from a human DNA library and their structures were determined. Restriction analysis indicates that both clones carry the same EcoRI 13-kilobase fragment where the coding sequence is mapped. The cloned DNA hybridized with polyadenylylated RNA from human fibroblasts, which directs the synthesis of cytoplasmic beta- and gamma-actin in vitro. However, sequence determination of the cloned DNA showed that the entire coding sequence perfectly matched the amino acid sequence of cardiac muscle actin. The initiation codon is followed by a cysteine codon that is not found at the amino-terminal site of any actin isoform, suggesting the necessity of post-translational processing for in vivo actin synthesis. There are five introns interrupting exons at codons 41/42, 150, 204, 267, and 327/328. Surprisingly, these intron locations are exactly the same as those of the rat skeletal muscle actin gene but different from those of nonmuscle beta-actin gene. Nucleotide sequences of all exon/intron boundaries agree with the G-T/A-G rule (G-T at the 5' and A-G at the 3' termini of each intron). The 3'-untranslated sequence has no homology to that of nonmuscle beta- or gamma-actin gene, but Southern blot hybridization has shown that this region has considerable homology to that of one of the other actin genes. These results indicate that the recombinant phages, which we have isolated, contain cardiac muscle actin gene and that cardiac muscle actin gene and skeletal muscle actin genes are derived from their ancestor gene at a relatively recent time in evolutionary development.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Birdsall D. L., Leslie A. G., Ratliff R. L. Left-handed DNA helices. Nature. 1980 Feb 21;283(5749):743–745. doi: 10.1038/283743a0. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blake C. C. Exons and the structure, function and evolution of haemoglobin. Nature. 1981 Jun 25;291(5817):616–616. doi: 10.1038/291616a0. [DOI] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Nonmuscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu Rev Biochem. 1977;46:797–822. doi: 10.1146/annurev.bi.46.070177.004053. [DOI] [PubMed] [Google Scholar]
- Engel J. N., Gunning P. W., Kedes L. Isolation and characterization of human actin genes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4674–4678. doi: 10.1073/pnas.78.8.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firtel R. A., Timm R., Kimmel A. R., McKeown M. Unusual nucleotide sequences at the 5' end of actin genes in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6206–6210. doi: 10.1073/pnas.76.12.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornwald J. A., Kuncio G., Peng I., Ordahl C. P. The complete nucleotide sequence of the chick a-actin gene and its evolutionary relationship to the actin gene family. Nucleic Acids Res. 1982 Jul 10;10(13):3861–3876. doi: 10.1093/nar/10.13.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch E. F., Lawn R. M., Maniatis T. Molecular cloning and characterization of the human beta-like globin gene cluster. Cell. 1980 Apr;19(4):959–972. doi: 10.1016/0092-8674(80)90087-2. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Bond B. J., Hershey N. D., Mixter K. S., Davidson N. The actin genes of Drosophila: protein coding regions are highly conserved but intron positions are not. Cell. 1981 Apr;24(1):107–116. doi: 10.1016/0092-8674(81)90506-7. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Hamada H., Leavitt J., Kakunaga T. Mutated beta-actin gene: coexpression with an unmutated allele in a chemically transformed human fibroblast cell line. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3634–3638. doi: 10.1073/pnas.78.6.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakunaga T. Neoplastic transformation of human diploid fibroblast cells by chemical carcinogens. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1334–1338. doi: 10.1073/pnas.75.3.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Revel J. P. The molecular basis of cell movement. Sci Am. 1979 May;240(5):100–113. doi: 10.1038/scientificamerican0579-100. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Bushar G., Kakunaga T., Hamada H., Hirakawa T., Goldman D., Merril C. Variations in expression of mutant beta actin accompanying incremental increases in human fibroblast tumorigenicity. Cell. 1982 Feb;28(2):259–268. doi: 10.1016/0092-8674(82)90344-0. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Kakunaga T. Expression of a variant form of actin and additional polypeptide changes following chemical-induced in vitro neoplastic transformation of human fibroblasts. J Biol Chem. 1980 Feb 25;255(4):1650–1661. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M., Firtel R. A. Evidence for sub-families of actin genes in Dictyostelium as determined by comparisons of 3' end sequences. J Mol Biol. 1981 Oct 5;151(4):593–606. doi: 10.1016/0022-2836(81)90425-3. [DOI] [PubMed] [Google Scholar]
- Nudel U., Katcoff D., Zakut R., Shani M., Carmon Y., Finer M., Czosnek H., Ginsburg I., Yaffe D. Isolation and characterization of rat skeletal muscle and cytoplasmic actin genes. Proc Natl Acad Sci U S A. 1982 May;79(9):2763–2767. doi: 10.1073/pnas.79.9.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal A., Garapin A., Cami B., Perrin F., Mandel J. L., LeMeur M., Brégégègre F., Gannon F., LePennec J. P., Chambon P. The ovalbumin gene region: common features in the organisation of three genes expressed in chicken oviduct under hormonal control. Nature. 1979 May 10;279(5709):125–132. doi: 10.1038/279125a0. [DOI] [PubMed] [Google Scholar]
- Shani M., Nudel U., Zevin-Sonkin D., Zakut R., Givol D., Katcoff D., Carmon Y., Reiter J., Frischauf A. M., Yaffe D. Skeletal muscle actin mRNA. Characterization of the 3' untranslated region. Nucleic Acids Res. 1981 Feb 11;9(3):579–589. doi: 10.1093/nar/9.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. Speculations on RNA splicing. Cell. 1981 Mar;23(3):643–646. doi: 10.1016/0092-8674(81)90425-6. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Leavitt J., Kakunaga T., Weber K. Coexpression of a mutant beta-actin and the two normal beta- and gamma-cytoplasmic actins in a stably transformed human cell line. Cell. 1980 Dec;22(3):893–899. doi: 10.1016/0092-8674(80)90566-8. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. The complete amino acid sequence of actins from bovine aorta, bovine heart, bovine fast skeletal muscle, and rabbit slow skeletal muscle. A protein-chemical analysis of muscle actin differentiation. Differentiation. 1979;14(3):123–133. doi: 10.1111/j.1432-0436.1979.tb01021.x. [DOI] [PubMed] [Google Scholar]
- Wahli W., Dawid I. B., Wyler T., Weber R., Ryffel G. U. Comparative analysis of the structural organization of two closely related vitellogenin genes in X. laevis. Cell. 1980 May;20(1):107–117. doi: 10.1016/0092-8674(80)90239-1. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]