Abstract
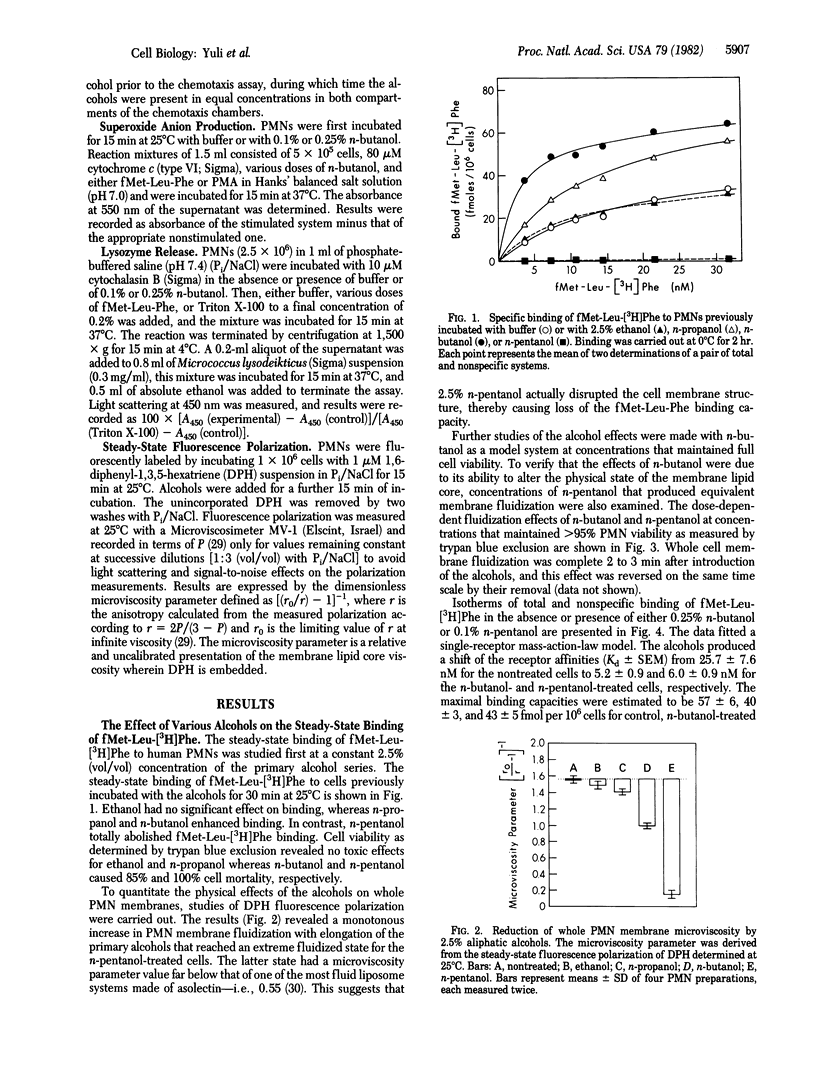
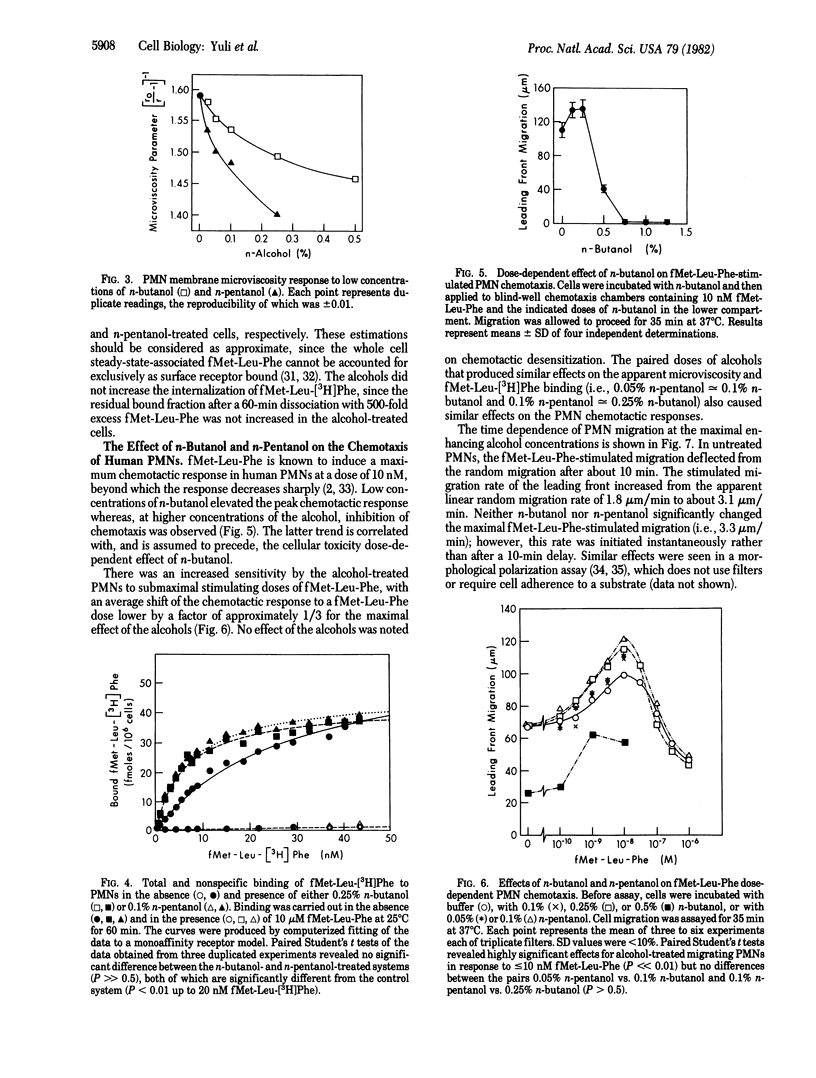
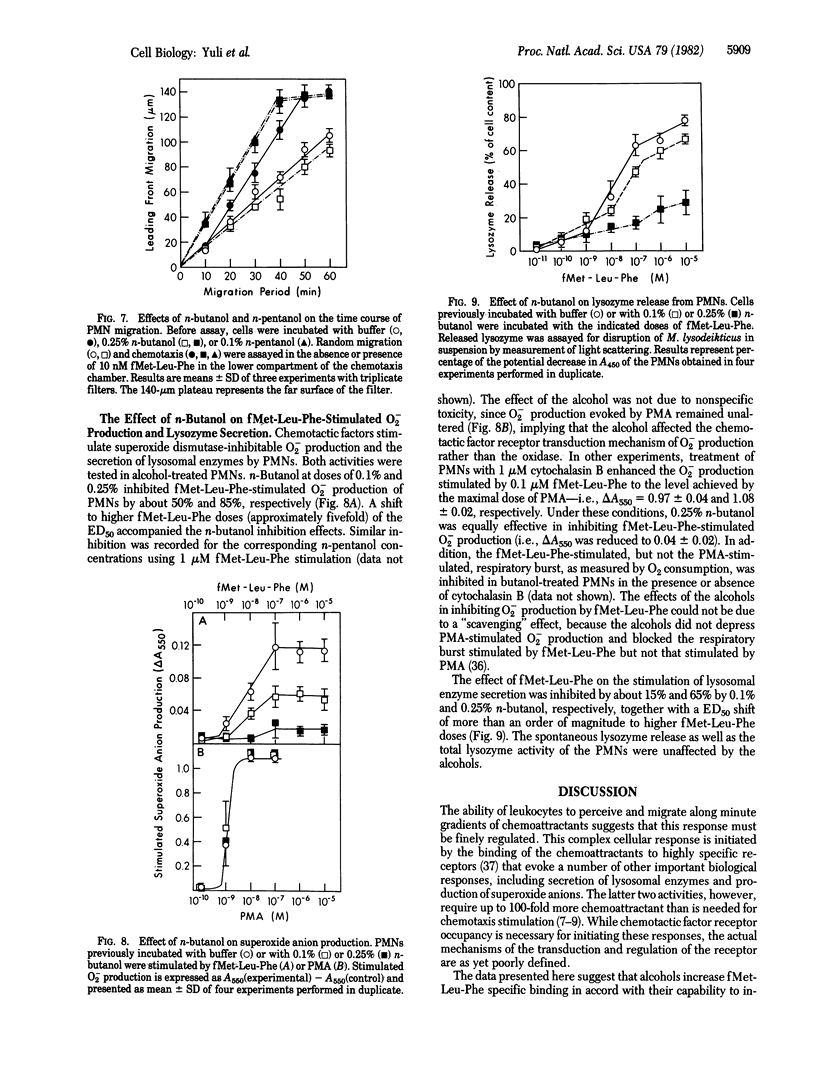
The chemotactic factor receptor on leukocytes initiates several cellular responses including chemotaxis, lysosomal enzyme secretion, and O2- production. The latter two responses require approximately 10-100 times more chemoattractant than is required for chemotaxis. We determined the effects of membrane fluidizers on the binding characteristics and the functional activities of the oligopeptide fMet-Leu-Phe chemotactic factor receptor on polymorphonuclear leukocytes. Fluidization was induced by aliphatic alcohols and monitored by diphenylhexatriene fluorescence polarization. Low doses of n-butanol (0.25%) and n-pentanol (0.1%) were nontoxic to the leukocytes yet reduced their diphenylhexatriene-induced polarization, indicating increased membrane fluidity. At these doses of alcohols, the affinity of the fMet-Leu-Phe receptor was enhanced from Kd = 25.5 +/- 7.6 nM to Kd = 5.2 +/- 0.9 nM and Kd = 6.0 +/- 0.9 nM, respectively. Chemotaxis was also increased, as indicated by the decrease, by a factor of approximately 1/3 in the ED50 for fMet-Leu-Phe, as well as by a 1.5-fold increase in the maximal distance of migration in the presence of 0.25% butanol or 0.1% pentanol. In contrast to chemotaxis, the alcohols depressed fMet-Leu-Phe stimulation of O2- production by 90% although they had no effect on phorbol 12-myristate 13-acetate-induced O2- production. Secretion of lysozyme was also inhibited. Thus, the affinity of the fMet-Leu-Phe receptor can be modulated by membrane fluidizers. The higher affinity state of the receptor induced by the alcohols is more efficient in transducing chemotactic signals but is deficient in mediating O2- production or secretion. Thus, the transduction mechanisms for the various biological activities of the chemotactic factor receptor are heterogeneous and can be differentially manipulated by membrane fluidizers.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aswanikumar S., Corcoran B., Schiffmann E., Day A. R., Freer R. J., Showell H. J., Becker E. L. Demonstration of a receptor on rabbit neutrophils for chemotactic peptides. Biochem Biophys Res Commun. 1977 Jan 24;74(2):810–817. doi: 10.1016/0006-291x(77)90375-8. [DOI] [PubMed] [Google Scholar]
- Chenoweth D. E., Hugli T. E. Demonstration of specific C5a receptor on intact human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3943–3947. doi: 10.1073/pnas.75.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciolo G. J., Snyderman R. Monocyte responsiveness to chemotactic stimuli is a property of a subpopulation of cells that can respond to multiple chemoattractants. J Clin Invest. 1981 Jan;67(1):60–68. doi: 10.1172/JCI110033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman H. A. Mathematical theory of complex ligand-binding systems of equilibrium: some methods for parameter fitting. Anal Biochem. 1972 Aug;48(2):317–338. doi: 10.1016/0003-2697(72)90084-x. [DOI] [PubMed] [Google Scholar]
- Fletcher M. P., Gallin J. I. Degranulating stimuli increase the availability of receptors on human neutrophils for the chemoattractant f-met-leu-phe. J Immunol. 1980 Apr;124(4):1585–1588. [PubMed] [Google Scholar]
- Fletcher M. P., Seligmann B. E., Gallin J. I. Correlation of human neutrophil secretion, chemoattractant receptor mobilization, and enhanced functional capacity. J Immunol. 1982 Feb;128(2):941–948. [PubMed] [Google Scholar]
- Hancock A. A., DeLean A. L., Lefkowitz R. J. Quantitative resolution of beta-adrenergic receptor subtypes by selective ligand binding: application of a computerized model fitting technique. Mol Pharmacol. 1979 Jul;16(1):1–9. [PubMed] [Google Scholar]
- Hatch G. E., Gardner D. E., Menzel D. B. Chemiluminescence of phagocytic cells caused by N-formylmethionyl peptides. J Exp Med. 1978 Jan 1;147(1):182–195. doi: 10.1084/jem.147.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron D. S., Shinitzky M., Hershkowitz M., Samuel D. Lipid fluidity markedly modulates the binding of serotonin to mouse brain membranes. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7463–7467. doi: 10.1073/pnas.77.12.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Phospholipid methylation and biological signal transmission. Science. 1980 Sep 5;209(4461):1082–1090. doi: 10.1126/science.6157192. [DOI] [PubMed] [Google Scholar]
- Koo C., Lefkowitz R. J., Snyderman R. The oligopeptide chemotactic factor receptor on human polymorphonuclear leukocyte membranes exists in two affinity states. Biochem Biophys Res Commun. 1982 May 31;106(2):442–449. doi: 10.1016/0006-291x(82)91130-5. [DOI] [PubMed] [Google Scholar]
- Lehmeyer J. E., Snyderman R., Johnston R. B., Jr Stimulation of neutrophil oxidative metabolism by chemotactic peptides: influence of calcium ion concentration and cytochalasin B and comparison with stimulation by phorbol myristate acetate. Blood. 1979 Jul;54(1):35–45. [PubMed] [Google Scholar]
- Liao C. S., Freer R. J. Cryptic receptors for chemotactic peptides in rabbit neutrophils. Biochem Biophys Res Commun. 1980 Mar 28;93(2):566–571. doi: 10.1016/0006-291x(80)91114-6. [DOI] [PubMed] [Google Scholar]
- Muller C., Shinitzky M. Modulation of transferrin receptors in bone marrow cells by changes in lipid fluidity. Br J Haematol. 1979 Jul;42(3):355–362. doi: 10.1111/j.1365-2141.1979.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Niedel J., Wilkinson S., Cuatrecasas P. Receptor-mediated uptake and degradation of 125I-chemotactic peptide by human neutrophils. J Biol Chem. 1979 Nov 10;254(21):10700–10706. [PubMed] [Google Scholar]
- Pike M. C., Kredich N. M., Snyderman R. Phospholipid methylation in macrophages is inhibited by chemotactic factors. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2922–2926. doi: 10.1073/pnas.76.6.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike M. C., Snyderman R. Alterations of new methylated phospholipid synthesis in the plasma membranes of macrophages exposed to chemoattractants. J Cell Biol. 1981 Oct;91(1):221–226. doi: 10.1083/jcb.91.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike M. C., Snyderman R. Transmethylation reactions regulate affinity and functional activity of chemotactic factor receptors on macrophages. Cell. 1982 Jan;28(1):107–114. doi: 10.1016/0092-8674(82)90380-4. [DOI] [PubMed] [Google Scholar]
- Schiffmann E., Corcoran B. A., Wahl S. M. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta. 1978 Dec 15;515(4):367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C., Patrick R. A., Hassett C. Motility and adhesiveness in human neutrophils. Effects of chemotactic factors. J Clin Invest. 1979 Feb;63(2):221–229. doi: 10.1172/JCI109293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Goetzl E. J. Molecular and cellular mechanisms of leukocyte chemotaxis. Science. 1981 Aug 21;213(4510):830–837. doi: 10.1126/science.6266014. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Pike M. C. N-Formylmethionyl peptide receptors on equine leukocytes initiate secretion but not chemotaxis. Science. 1980 Jul 25;209(4455):493–495. doi: 10.1126/science.6248959. [DOI] [PubMed] [Google Scholar]
- Spilberg I., Mehta J. Demonstration of a specific neutrophil receptor for a cell-derived chemotactic factor. J Clin Invest. 1979 Jan;63(1):85–88. doi: 10.1172/JCI109282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S. J., Zigmond S. H. Chemotactic peptide receptor modulation in polymorphonuclear leukocytes. J Cell Biol. 1980 Jun;85(3):703–711. doi: 10.1083/jcb.85.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Pike M. C., Lefkowitz R. J. Specific receptor sites for chemotactic peptides on human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1204–1208. doi: 10.1073/pnas.74.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Sullivan S. J., Lauffenburger D. A. Kinetic analysis of chemotactic peptide receptor modulation. J Cell Biol. 1982 Jan;92(1):34–43. doi: 10.1083/jcb.92.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]



