Abstract
Effective chemotherapy remains a key issue for successful cancer treatment in general and neuroblastoma in particular. Here we report a chemotherapeutic strategy based on catalytic antibody-mediated prodrug activation. To study this approach in an animal model of neuroblastoma, we have synthesized prodrugs of etoposide, a drug widely used to treat this cancer in humans. The prodrug incorporates a trigger portion designed to be released by sequential retro-aldol/retro-Michael reactions catalyzed by aldolase antibody 38C2. This unique prodrug was greater than 102-fold less toxic than etoposide itself in in vitro assays against the NXS2 neuroblastoma cell line. Drug activity was restored after activation by antibody 38C2. Proof of principle for local antibody-catalyzed prodrug activation in vivo was established in a syngeneic model of murine neuroblastoma. Mice with established 100-mm3 s.c. tumors who received one intratumoral injection of antibody 38C2 followed by systemic i.p. injections with the etoposide prodrug showed a 75% reduction in s.c. tumor growth. In contrast, injection of either antibody or prodrug alone had no antitumor effect. Systemic injections of etoposide at the maximum tolerated dose were significantly less effective than the intratumoral antibody 38C2 and systemic etoposide prodrug combination. Significantly, mice treated with the prodrug at 30-fold the maximum tolerated dose of etoposide showed no signs of prodrug toxicity, indicating that the prodrug is not activated by endogenous enzymes. These results suggest that this strategy may provide a new and potentially nonimmunogenic approach for targeted cancer chemotherapy.
The design of ever-more-effective chemotherapeutic approaches promises advances in the treatment of cancer (1–3). One promising strategy involves the use of catalytic antibodies as prodrug activators. Because antibodies can be readily humanized, novel prodrug activating enzymes can potentially be prepared that are devoid of immunogenicity and applicable to treatment regimes that require repeated administration of the enzyme. Although antibodies with a wide range of catalytic activities have been prepared (4), only recently have antibodies with the efficiency and mechanism of nature's enzymes become available. One such antibody is the aldolase antibody 38C2, prepared by reactive immunization (5, 6). We have described 38C2 catalyzed tandem retro-aldol/retro-Michael reactions and the application of this reaction cascade in the development of a broad class of drug modifying linkers (7). Although catalytic antibodies have demonstrated their potency in vitro (7–9), the in vivo efficacy of a catalytic antibody/prodrug approach has yet to be reported. To explore the general applicability of our prodrug-activating strategy and its efficiency in vivo, we have tailored this approach to an animal model of neuroblastoma and a drug that is widely used to treat this cancer in humans.
Etoposide, a semisynthetic compound, was originally discovered in 1966 when analogs of the naturally occurring anticancer drug podophyllotoxin were synthesized (10). Etoposide has become a well-established anticancer drug since its approval for clinical use by the Food and Drug Administration in the late 1970s, after it clearly demonstrated efficacy as a chemotherapeutic drug. Etoposide exerts its antitumor activity by inhibition of topoisomerase II. The use of etoposide for the treatment of pediatric neuroblastoma was established soon after its initial description as an anticancer agent. In this regard, stage 4 neuroblastoma remains one of the major challenges in pediatric oncology. The majority of patients present with nonresectable primary tumors and disseminated metastasis to distant organ sites, predominantly bone marrow. The overall survival rate of stage 4 neuroblastoma patients has not been significantly improved during the last two decades, despite novel radio and/or chemotherapy protocols, including high-dose chemotherapy followed by autologous peripheral blood stem cell rescue (11). Novel immunological approaches in adjuvant settings with murine and human/mouse chimeric antibodies directed against the ganglioside GD2 extensively expressed on neuroblastoma cells are currently under clinical evaluation with some promising initial results in patients with minimal residual disease (12–15). Therefore, optimally effective reduction in tumor load is a prerequisite for adjuvant approaches such as anti-GD2 immunotherapy to be effective in a minimal residual disease setting. Etoposide has proven to be quite efficient in this regard and is therefore an established component in first-line polychemotherapy protocols for neuroblastoma throughout the world. On the basis of the typically poor outcome obtained for stage 4 neuroblastoma patients, efforts to improve chemotherapy strategies with etoposide are needed. A promising approach to achieve this goal would be the local activation of synthetic etoposide prodrugs by catalytic means. Previous studies have shown that esterification of the phenol functionality of etoposide with phosphate significantly decreases the activity/toxicity of the drug, which can be restored through enzymatic cleavage of the phosphate group by endogenous human phosphatases (1, 16, 17). Accordingly, the phosphate ester of etoposide, designed originally as a prodrug and now used primarily because of its increased water solubility, is ≈100-fold less toxic than the free drug (16). On the basis of this structure–activity relationship for etoposide, we attempted to improve this strategy with the design of prodrugs of etoposide that are activated by a tandem retro-aldol/retro-Michael reaction not known to be catalyzed in nature (7).
Here we report the synthesis and characterization of a new etoposide prodrug that is greater than 102-fold less toxic than the parental etoposide in biological assays. Furthermore, effective etoposide prodrug activation by antibody 38C2 was demonstrated with HPLC analysis and in in vitro growth inhibition assays that revealed that the antitumor activity of the etoposide prodrug was restored on activation by the antibody. Finally, antitumor activity because of localized activation of etoposide prodrug was demonstrated after intratumoral injection of antibody 38C2 in a syngeneic murine NXS2 neuroblastoma model.
Materials and Methods
General.
All reactions requiring anhydrous conditions were performed in oven-dried glassware under an Ar or N2 atmosphere. Chemicals and solvents were either puriss p.A. or purified by standard techniques. Tetrahydrofuran was distilled from sodium-benzophenone. TLC on silica gel plates. Merck 60 F254 was used, and compounds were visualized by irradiation with UV light and/or by treatment with a solution of 25 g phosphomolybdic acid/10 g Ce(SO4)2⋅H2O/60 ml concentrated H2SO4/940 ml H2O followed by heating and/or by staining with a solution of 12 g 2,4-dinitrophenylhydrazine in 60 ml of concentrated H2SO4/80 ml H2O/200 ml 95% EtOH followed by heating and/or immersing into an iodine bath (30 g I2/2 g KI, in 400 ml EtOH/H2O 1:1) and warming. Flash chromatography was done by using silica gel, Merck 60 (particle size 0.040–0.063 mm), eluent indicated in parentheses. 1H NMR: Bruker AMX 300, Bruker AMX 250. The chemical shifts are expressed in δ relative to tetramethylsilane (δ = 0 ppm) and the coupling constants J in hertz. The spectra were recorded in CDCl3 as solvent at room temperature unless stated otherwise. HR-MS: liquid secondary ionization (LSI-MS): VG ZAB-ZSE with 3-nitrobenzyl alcohol matrix. All general reagents, including salts and solvents, were purchased from Aldrich. Etoposide was obtained from Sigma. Para-nitrophenyl-carbonate (compound 1) was prepared as previously described (7).
Synthesis of the Etoposide Prodrug.
Aldol carbamate 3.
N,N-dimethyl-ethylene-diamine-mono-boc protected 2 (752 mg, 4.0 mmol) was dissolved in 10 ml of methanol. Compound 1 (1.0 g, 3.2 mmol) dissolved in 5 ml of methanol was added to the reaction mixture, which was stirred for 16 h. The solvent was removed under reduced pressure and the product purified by column chromatography over silica gel (hexane/ethyl acetate 30:70) to yield compound 3 (985 mg, 86%).
1H NMR (250 MHz, CDCl3) δ 4.19 (t, J = 5.4 Hz, 2H), 3.33 (m, 4H), 2.88 (m, 6H), 2.66 (d, J = 10.7 Hz, 1H), 2.61 (d, J = 10.7 Hz, 1H), 2.16 (s, 3H), 1.84 (m, 2H), 1.42 (s, 9H), 1.23 (s, 3H).
p-Nitrophenyl carbonate of etoposide (Compound 5).
p-Nitrophenyl chloroformate (201.5 mg, 1.0 mmol) was dissolved in 10 ml of tetrahydrofuran (THF) and added dropwise to a stirred solution of etoposide (588 mg, 1.0 mmol) in 20 ml of THF and 2 ml of triethylamine. The mixture was stirred for 60 min. The triethylamine hydrochloride salt was separated by filtration and the solvent removed under reduced pressure. The product was purified by column chromatography on silica gel (ethyl acetate/hexane 50:50) resulting in compound 5 (622 mg, 83%).
1H NMR (250 MHz, CDCl3) δ 8.28 (d, J = 7.0 Hz, 2H), 7.49 (d. 7.0, 2H), 6.72 (s, 1H), 6.53 (s, 1H), 6.33 (s, 2H), 6.01 (m, 2H), 4.91 (m, 1H), 4.72 (m, 1H), 4.63 (m, 2H), 4.41 (m, 1H), 4.22 (m, 2H), 3.75 (s, 6H), 3.51 (m, 6H), 2.91 (m, 1H), 1.39 (d, J = 4.1 Hz, 3H).
Etoposide Prodrug 6.
Compound 4 was prepared in situ by the following procedure. Trifluoroacetic acid (TFA) (2 ml) was added to compound 3 (360 mg, 1.0 mmol). After 2 min of stirring, excess TFA was removed under reduced pressure resulting in compound 4. The crude product was used directly for the next reaction. Thus, compound 5 (754 mg, 1.0 mmol) was added to a stirred solution of compound 4 in 5 ml of dimethylformamide (DMF), followed by the addition of 2 ml of triethylamine. The mixture was stirred for 16 h and DMF removed under reduced pressure. The remaining residue was purified by column chromatography on silica gel (ethyl acetate/hexane 70:30), yielding prodrug 6 (711 mg, 81%).
HRMS (MH+) calculated for C42H54N2O18: 874.3372; observed: 874.3363.
Drug activation assay.
The generation and purification of mouse mAb 38C2 was described previously (5). A stock solution of 15 mg/ml (100 μM) 38C2 IgG2a in PBS (pH 7.4), stored at 4°C, was used. All antibody reactions were performed in PBS (pH 7.4) at 37°C in microfuge tubes. Typically, reactions were carried out at concentrations of 20–200 μM substrate and 5 μM antibody. Antibody-catalyzed reactions were monitored at 280 nm for etoposide by RP-HPLC [Hitachi (Tokyo)] L-6200A equipped with an AS-2000 autosampler and a Supelcosil LC-18 column (25 cm × 4.6 mm, 5 m) by using various proportions of acetonitrile/water at 1 ml/min.
Cells and in vitro growth inhibition assays.
The activity of the etoposide prodrug (6) against NXS2 neuroblastoma cells was analyzed in the presence or absence of catalytic antibody 38C2. For this purpose NXS2 cells were plated in 96-well plates at 5 × 103 cells per well in 100 μl of DMEM (10% FCS/100 units penicillin/100 μg/ml streptomycin/2 mM l-Glutamin). Etoposide prodrug was added 24 h later at various concentrations diluted in 100 μl of DMEM in the presence or absence of 1 μM catalytic antibody 38C2. All plates were incubated for 72 h (37°C, 5% CO2). Cell density was determined by lactate dehydrogenase release and quantitated by a colorimetric detection kit (Promega), as previously described (7).
Mice and animal model.
Syngeneic female A/J mice were obtained at 8 weeks of age from The Jackson Laboratory. They were housed in the pathogen-free mouse colony at our institution in groups of four mice each. Mice were fed ad libitum on standard mouse laboratory chow. Animal experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The syngeneic neuroblastoma model was essentially used as previously described (18, 19). Briefly, primary neuroblastoma tumors were induced by s.c. injection of 2 × 106 NXS2 cells into the left lateral flank. Primary tumor growth was determined by microcaliper measurements and the tumor volume calculated according to volume = (width × length × width/2). Treatment was initiated 11 days after tumor cell inoculation with an average s.c. tumor size of 100 mm3. For this purpose, a total of eight mice per group were injected with 0.5 mg of 38C2 into s.c. tumors in a total volume of 30 μl of PBS followed by i.p. injection of a total of 1,250 mg/kg of etoposide prodrug 6 subdivided into three doses injected on days 11, 13, and 16 in 100 μl of solvent (25% DMSO/12.5% Chremaphore EL, Sigma; 12.5% ethanol/50% PBS). Control groups received each agent as a monotherapy, etoposide itself 40 mg/kg total, or PBS injections, respectively. Toxicity in each experimental group was monitored by determining the body weight of mice once per week.
Statistics.
The statistical significance of differential findings between experimental groups of animals was determined by two-tailed Student's t test. Findings were regarded as significant if two-tailed P values were <0.05.
Results
Design and Synthesis of Etoposide Prodrugs.
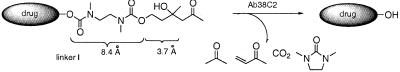
We sought to design a drug-masking linker wherein the identity of the drug itself would have a minimal impact on the recognition and activation of the linker by the catalytic antibody. To achieve this, the aldol functionality of the linker recognized by the catalytic antibody should be appended to a sterically nondemanding spacer element. On the basis of considerations of the 10-Å depth of the ɛ-amino group of the catalytic lysine residue in the antibody active site (6), a spacer was designed to be long enough (8.4 Å) that activation by retro-aldol and retro-Michael reactions could be achieved, with the bulk of the etoposide molecule remaining outside of the antibody active site. The carbonyl group of the trigger, which is unmasked by the initial retro-aldol reaction, is 3.7 Å removed from the start of the linker. Therefore the etoposide drug is placed at a distance of ≈12 Å for engagement with the catalytic lysine residue in the retro-Michael step. This length requirement of the spacer element demands that the use of a self-immolative spacer for the linker be completely removed after antibody-triggered activation. To accomplish this, we prepared a linker designed to allow for modification of the phenol group of etoposide with a minimal number of synthetic operations being performed on the etoposide drug. The linker incorporates the trigger that is activated by the tandem retro-aldol/retro-Michael reactions catalyzed by antibody 38C2 (Fig. 1). This sequence of reactions within the trigger portion of the molecule generates, on decarboxylation, a free amine in the spacer element that undergoes a spontaneous 1, 5 cyclization to release free drug and N,N-dimethyl urea. The N,N′-dimethyl-substituted ethylenediamine spacer was chosen because it provides for a more rapid cyclization reaction than the unsubstituted spacer (20).
Figure 1.
General description of our prodrug activation strategy by antibody-catalyzed retro-aldol/retro-Michael reaction sequence. The activation sequence catalyzed by 38C2 is depicted for the drug-masking linker. Catalytic cleavage of the trigger by retro-aldol/retro-Michael reactions of the linker is followed by spontaneous decarboxylation, cyclization, and release of the free drug.
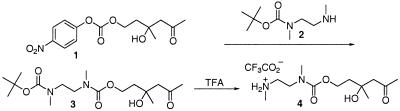
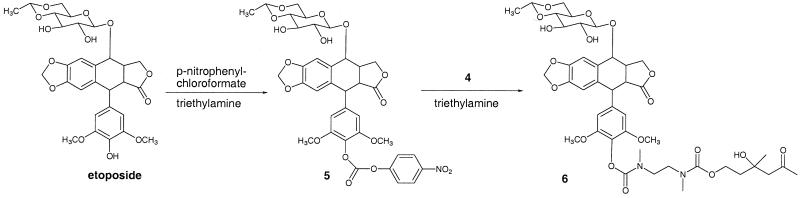
Synthesis of the linker is shown in Fig. 2. Linker 4 was prepared by reacting mono boc-protected N,N-dimethyl ethylendiamine 2 with p-nitrophenyl carbonate 1 to yield aldol-carbamate 3. The boc group was removed in the presence of TFA, yielding the trifluoroacetate salt 4. Compound 8 was reacted with the p-nitrophenylcarbonate derivative of etoposide in the presence of triethylamine to yield prodrug 6 (Fig. 3).
Figure 2.
Schematic delineating the synthesis of compound 4 that was used to mask the active phenolic oxygen functionality of etoposide.
Figure 3.
Schematic delineating the synthesis of etoposide prodrug 6 designed for activation by aldolase antibody 38C2.
Catalytic Activation of the Etoposide Prodrug.
Initial studies of the ability of antibody 38C2 to activate the prodrugs were performed by using HPLC analysis. Incubation of the catalytic antibody with the prodrug in buffered solution led to complete conversion and release of free etoposide as shown in this HPLC assay. No nonspecific activation of prodrug 6 in buffered solutions was observed during the assays. Further, no decomposition of prodrug 6 was noted after storage in buffer for several weeks.
Effect of 38C2 Antibody-Mediated Etoposide Prodrug Activation on Cell Lines.
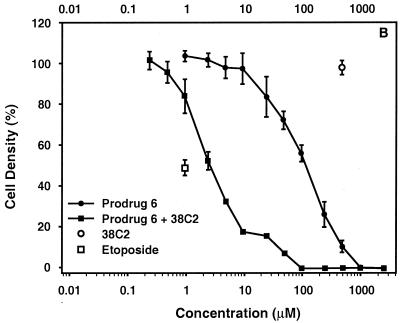
To evaluate the efficacy of 38C2-mediated etoposide prodrug activation, the activity of prodrug 6 was evaluated in vitro in the presence or absence of 1 μM antibody 38C2 with cultured murine NXS2 neuroblastoma cells. Interestingly, the prodrug revealed a clearly reduced potential for growth inhibition; >100-fold for prodrug 6 (Fig. 4). Activation of the prodrug with antibody 38C2 resulted in growth profiles similar to the unmodified etoposide control.
Figure 4.
Growth inhibition activity of etoposide prodrug 6 in the presence and absence of catalytic antibody 38C2. The growth response of NXS2 neuroblastoma cells to a 72-h incubation with increasing concentrations of etoposide prodrug 6 in the presence and absence of 1 μM 38C2 catalytic antibody was analyzed by using a standard lactate dehydrogenase release assay. A serial dilution of etoposide and wells containing 1 μM 38C2 only are shown as controls. Data points and error bars represent mean values ± standard deviation.
Catalytic Antibody-Activated Chemotherapy of Neuroblastoma with Etoposide Prodrug 6.
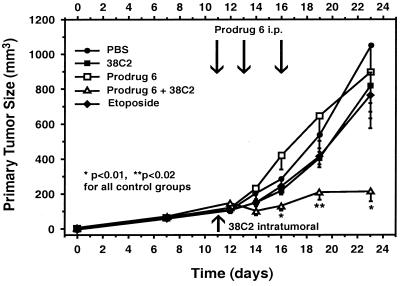
The efficacy of localized catalytic–antibody-mediated etoposide prodrug 6 activation against syngeneic murine NXS2 neuroblastoma was evaluated on established primary tumors (Fig. 5). Primary tumors were established in the lateral flank of animals, and studies were initiated when an average tumor size of 100 mm3 was obtained. Each experimental group consisted of eight animals. Catalytic antibody 38C2 was delivered locally by intratumoral injection. Prodrug 6 or etoposide was delivered systemically by i.p. injection at three time points. Two major findings were obtained in this experiment. First, an increased antitumor efficacy of locally activated etoposide prodrug 6 over systemically applied unmodified etoposide was observed at the maximum tolerated dose of etoposide. In fact, a dramatic 75% reduction in s.c. tumor growth was observed only in the group of mice receiving both intratumoral injections of catalytic antibody 38C2 and systemic treatments with etoposide prodrug 6 (Fig. 5). This is in contrast to control groups receiving each agent as monotherapy or injections with PBS. Because all animals that received both catalytic antibody 38C2 and prodrug 6 survived the 24-day experiment, they were treated at day 25 with a second cycle of catalytic antibody 38C2 and prodrug 6 therapy. After this, three of eight mice revealed the complete absence of a primary tumor, underlining the efficacy of the principle of catalytic antibody-mediated prodrug activation in vivo. Furthermore, etoposide prodrug 6 demonstrated dramatically reduced toxicity in vivo as compared with unmodified etoposide itself. In fact, no toxicity was observed at the dosage administered to the animals (1,250 mg/kg), as defined by the absence of any decrease in body weight in contrast to a 20% weight loss observed in mice treated with the maximal tolerated dose of etoposide (40 mg/kg) (21).
Figure 5.
Effect of catalytic antibody 38C2-mediated etoposide prodrug 6 activation on the growth of primary neuroblastoma tumors. Treatment of mice bearing established s.c. primary neuroblastoma tumors induced by s.c. injection with 2 × 106 NXS2 neuroblastoma cells was initiated 11 days after tumor cell inoculation. The treatment consisted of intratumoral injection of 38C2 (0.5 mg) followed by three i.p. etoposide prodrug 6 (total dose 1,250 mg/kg) injections. Arrows indicate days of treatment with each reagent, respectively. One control group received unmodified etoposide (total dose 40 mg/kg). Data points and error bars represent mean value and standard error (n = 8). The difference between the experimental group receiving etoposide prodrug 6 and 38C2 catalytic antibody and all control groups was statistically significant (*, P < 0.01, **, P < 0.02).
Discussion
Improved strategies for chemotherapy remain one of the major challenges for the effective treatment of cancer. This is particularly true for neuroblastoma, a disease in which chemotherapy has not yet lived up to its promise (11). Antibody-directed enzyme prodrug therapy (ADEPT) is a promising approach toward improving the efficacy of chemotherapy while at the same time reducing its dose-limiting peripheral toxicity (1, 2, 22). In this approach, an enzyme is targeted to the tumor site by conjugation to an antibody that binds a tumor-associated antigen or an angiogenic marker protein. After systemic delivery of such a protein, its localization at the tumor site, and clearance of unbound conjugate, a designed prodrug is delivered systemically. Ideally, because the prodrug is not activated in the periphery and is nontoxic in the prodrug form, toxicity is localized to the tumor site, thereby minimizing dose-limiting side effects. This is a three-component system consisting of a drug-activating enzyme, a prodrug(s), and a targeting antibody. With the availability of humanized or human antibodies against a range of tumor-associated antigens or angiogenic markers, the targeting component of this strategy has yet to be limiting. The enzyme and prodrug components of the strategy, however, present serious challenges to the practical application of this approach. The prodrug should be designed so that it may be catalytically converted to the active drug by the enzyme–antibody conjugate localized at the tumor site and not by endogenous enzymes. Further, because a wide range of drugs are used clinically in cancer therapy, the modification chemistry applied to inactivate the drug should be versatile, and the enzyme used to activate the prodrug should ideally be capable of processing a wide variety of drugs. To meet the specific requirement that the prodrug not be activated by enzymes endogenous to humans, bacterial enzymes have typically been used. Unfortunately, the use of nonhuman proteins in human therapy raises the issue of the immunogenicity of the drug-activating conjugate. Administration of a foreign protein typically elicits a vigorous antibody response that limits repeated administration of the protein. Indeed, immunogenicity has been problematic in clinical studies of ADEPT (23). To suppress antibody responses against the enzyme–antibody conjugate, the immunosuppressive agent cyclosporin has been used in early clinical studies of the ADEPT approach (24). Immunosuppression in cancer, however, is not desirable and offers only limited mitigation of an immune response against the conjugate. Another approach to masking the immunogenicity of a foreign protein involves the attachment of polyethylene glycol chains to the protein (25), a process known as pegylation. Although this approach has produced favorable results, not all proteins may be directly modified by using by this approach, and it would be desirable to eliminate this additional chemical conjugation step. Thus, the immunogenicity of the enzyme component of ADEPT remains a major problem seriously limiting the use of this approach.
One direct strategy proposed to overcome the immunogenicity issue of the drug-activating enzyme is to substitute a catalytic antibody as the enzyme component of the system. Because antibodies are readily humanized, the immunogenicity problems that accompany this approach might be overcome (26). Preliminary studies directed at the generation of specific drug-activating antibodies have resulted in the characterization of antibodies that activate a nitrogen mustard and 5-fluorodeoxyuridine, respectively. The in vitro activity of these antibody/prodrug systems was shown in assays that demonstrated enhanced killing of a colon cancer cell line in the case of the nitrogen mustard or Escherichia coli in the case of 5-fluorodeoxyuridine (8, 9). The two antibodies used in these studies were prepared by immunization of animals with tetrahedral phosphonate analogs, and drug activation was triggered by aromatic carbamate or ester hydrolysis. Although these antibodies were able to activate their singular prodrug substrates, the transition-state analog approach to catalytic antibodies predominately provides catalysts that operate only on a very specific substrate and not on structurally related analogs (4, 6). Therefore such antibodies would not be expected to be capable of activating drugs for which they were not specifically designed. Furthermore, each of these acyl transfer activation chemistries can potentially be accomplished by endogenous enzymes such as esterases or proteases, thereby limiting the specificity that can be achieved in the activation step.
Recently, we described an approach to catalytic antibodies that involves a process known as reactive immunization (5, 6). This approach has several advantages, especially when combined with the transition state analog approach (29). For prodrug activation, these advantages are primarily enhanced catalytic proficiency and broad substrate scope. The catalytic efficiencies of antibodies obtained by using reactive immunization for the aldol reaction match those of nature's aldolase enzymes. With respect to substrate scope or tolerance, antibody 38C2 studied here was shown to be capable of activating hundreds of substrates (5, 6). These two findings led us to perform our first studies involving prodrug activation with aldolase antibodies (7). Prodrugs of the approved anticancer drugs doxorubicin and camptothecin were prepared by design of a drug-masking linker that is activated by antibody 38C2 catalysis of sequential retro-aldol retro-Michael reactions. Unlike natural aldolase enzymes that typically operate on phosphorylated sugar derivatives, antibody aldolase 38C2 processes hydrophobic substrates and is capable of catalysis of retro-aldol reactions involving tertiary aldols (27). No natural enzymes are known to be able to catalyze this specific type of cleavage; therefore, the drug-masking linker incorporated a tertial aldol as the primary trigger. Although this study demonstrated the potential of aldolase antibody activation of two structurally different prodrugs and their in vitro activity against prostate and colon cancer cell lines, we were interested in extending this approach to other anticancer drugs and testing the efficacy of this approach in vivo.
Here we have designed, synthesized, and tested a prodrug of etoposide and established the efficacy of this approach in a mouse model of neuroblastoma. A drug-masking linker applicable to the modification of hydroxy or thiol groups of drugs was studied. This masking linker incorporates a trigger portion that is designed to be released by retroaldolization of a tertiary aldol followed by a retro-Michael reaction. Although broad substrate scope is a feature inherent to the aldolase antibodies prepared to date, we attempted to minimize the structural influence of the drug on the enzymatic release of the linker by addition of a self-immolative spacer to the masking linker. The masking linker was attached to etoposide at the phenolic oxygen, because previous studies of etoposide phosphate have indicated that modification of the drug at this position decreased the toxicity of the molecule ≈100-fold (16). The resulting prodrug 6 was shown to be a substrate for antibody 38C2-catalyzed activation. Prodrug 6 demonstrated superior chemical stability. As shown (Figs. 4B and 5), prodrug 6 demonstrated a profound ≈100-fold reduced toxicity against the NXS2 neuroblastoma cell line. This cell line was chosen because etoposide is commonly used in contemporary treatment regimens for neuroblastoma (11).
To test the principle of localized activation of the prodrug 6 in an animal model, we have used a well established syngeneic model for murine neuroblastoma that is based on the NXS2 cell line (18, 19). Intratumoral injection of the antibody catalyst into established primary tumors was applied to study the potential of local antibody-catalyzed activation of the prodrug. The prodrug itself was delivered systemically via i.p. injection. These studies revealed a dramatic reduction in s.c. tumor growth in animals that received both the catalytic antibody and prodrug 6 (Fig. 5). In contrast, administration of etoposide itself at the maximal tolerated dose of 40 mg/kg did not produce a substantial antitumor effect even though the animals demonstrated a 20% reduction in weight, symptomatic of systemic toxicity (21). No such systemic toxicity was observed in animals treated with prodrug 6 (1,250 mg/kg) at 30-fold the dosage of etoposide used. The lack of any obvious systemic toxicity of prodrug 6 at this high dosage is further evidence that the drug-masking linker is not readily removed by endogenous enzymes within the animals. These studies also demonstrated that localized activation of the prodrug is obviously superior to systemic application of etoposide, suggesting that the concentration of active etoposide at the tumor site generated by antibody-catalyzed activation of the prodrug 6 exceeds the concentration of etoposide attainable when it is administered systemically at the maximal tolerated dose.
In the present study, intratumoral injection of the catalyst directs localized activation of the prodrug, which is a key strategy for targeted catalytic antibody-mediated chemotherapy. However, this approach will not be applicable to disseminated metastatic disease that will require the development of targeting devices for catalytic antibody 38C2. In this regard, the treatment of neuroblastoma with antibodies directed against the disialoganglioside GD2 has been extensively studied in humans (12–15) and in the animal model used here (18, 19). Targeting of cytokines such as interleukin 2 to neuroblastoma with an anti-GD2 antibody fusion was also demonstrated to be effective in the neuroblastoma model used here. Consequently, a direct genetic fusion of the targeting antibody and the catalytic antibody may be a practical approach to reach this goal. Indeed, a number of approaches are currently available for the preparation of bispecific antibodies.
Application of this strategy to human therapy requires access to humanized catalytic antibodies. Recently, we have reported a phage display approach to the humanization of antibody 38C2 studied here (28). Using this approach, we have demonstrated that improvements in the catalytic activity of aldolase antibodies can also be achieved. Other murine aldolase catalytic antibodies have also recently become available that could be humanized by using this same method. These antibodies, 84G3 and 93F3, are the most efficient catalytic antibodies yet reported, providing rates up to 2 s−1, and rate accelerations (kcat/kuncat) exceeding 108 (29). Thus a range of humanized catalytic antibodies with variable kinetic properties should be available to test this approach, allowing for the effects of the catalytic efficiency of the activating enzyme to be evaluated in a systematic fashion.
In summary, we demonstrate in vivo efficacy by using a catalytic antibody-activated prodrug system. Mice bearing established neuroblastoma tumors showed no signs of toxicity when treated with an etoposide prodrug at 30-fold the maximum tolerated dose of etoposide. Activation of the prodrug by catalytic antibody at the tumor site produced a profound antitumor effect in a murine neuroblastoma model. This catalytic antibody-based prodrug activation system may provide for a new potentially nonimmunogenic approach to cancer chemotherapy and HIV-1 disease.
Acknowledgments
We thank B. List for helpful discussions. This study was supported by grants from the National Institutes of Health (CA27489 to C.F.B. and R.A.L., AI37470 to C.F.B., and CA42508 and 37641 to R.A.R. and H.N.L.) and from the Skaggs Institute for Chemical Biology to C.F.B. and R.A.L.
Abbreviations
- ADEPT
antibody-directed enzyme prodrug therapy
- TFA
trifluoroacetic acid
References
- 1.Niculescu-Duvaz I, Springer C J. Adv Drug Delivery Rev. 1997;26:151–172. doi: 10.1016/s0169-409x(97)00032-x. [DOI] [PubMed] [Google Scholar]
- 2.Syrigos K N, Epenetos A A. Anticancer Res. 1999;19:605–614. [PubMed] [Google Scholar]
- 3.Aghi M, Hochberg F, Beakefield X O. J Gene Med. 2000;2:148–164. doi: 10.1002/(SICI)1521-2254(200005/06)2:3<148::AID-JGM105>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn G M, Datta A, Denham H, Wentworth P., Jr Adv Phys Org Chem. 1998;31:249–392. [Google Scholar]
- 5.Wagner J, Lerner R A, Barbas C F., III Science. 1995;270:1797–1800. doi: 10.1126/science.270.5243.1797. [DOI] [PubMed] [Google Scholar]
- 6.Barbas C F, III, Heine A, Zhong G, Hoffmann T, Gramatikova S, Björnestedt R, List B, Anderson J, Stura E A, Wilson E A, Lerner R A. Science. 1997;278:2085–2092. doi: 10.1126/science.278.5346.2085. [DOI] [PubMed] [Google Scholar]
- 7.Shabat D, Rader C, List B, Lerner R A, Barbas C F., III Proc Natl Acad Sci, USA. 1999;96:6925–6930. doi: 10.1073/pnas.96.12.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wentworth P, Datta A, Blakey D, Blakey D, Boyle T, Partridge L J, Blackburn G M. Proc Natl Acad Sci USA. 1996;93:799–803. doi: 10.1073/pnas.93.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell D A, Gong B, Kochersperger L M, Yonkovich S, Gallop M A, Schultz P G. J Am Chem Soc. 1994;116:2165–2166. [Google Scholar]
- 10.Hande K R. Eur J Cancer. 1998;34:1514–1521. doi: 10.1016/s0959-8049(98)00228-7. [DOI] [PubMed] [Google Scholar]
- 11.Niethammer D, Handgretinger R. Eur J Cancer. 1995;31A:568–571. doi: 10.1016/0959-8049(95)00032-e. [DOI] [PubMed] [Google Scholar]
- 12.Handgretinger R, Anderson K, Lang P, Dopfer R, Klingebiel T, Schrappe M, Reuland P, Gillies S D, Reisfeld R A, Neithammer D. Eur J Cancer. 1995;31A:261–267. doi: 10.1016/0959-8049(94)00413-y. [DOI] [PubMed] [Google Scholar]
- 13.Handgretinger R, Baader P, Dopfer R, Klingebiel T, Reuland P, Treuner J, Reisfeld R A, Neithammer D. Cancer Immunol Immunother. 1992;35:199–204. doi: 10.1007/BF01756188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung N K, Cheung I Y, Canete A, Yeh S J, Kushner B, Bonilla M A, Heller G, Larson S M. Cancer Res. 1994;54:2228–2233. [PubMed] [Google Scholar]
- 15.Uttenreuther-Fischer M M, Huang C S, Reisfeld R A, Yu A L. Cancer Immunol Immunother. 1995;41:29–36. doi: 10.1007/BF01788957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Senter P D, Saulnier M G, Schreiber G J, Hirschberg D L, Brown J P, Hellstrom I, Hellstrom K E. Proc Natl Acad Sci USA. 1988;85:4842–4846. doi: 10.1073/pnas.85.13.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senter P D, Schreiber G J, Hirschberg D L, Ashe S A, Hellstrom K E, Hellstrom I. Cancer Res. 1989;49:5789–5792. [PubMed] [Google Scholar]
- 18.Lode H N, Xiang R, Varki N M, Dolman C S, Gillies S D, Reisfeld R A. J Natl Cancer Inst. 1997;89:1586–1594. doi: 10.1093/jnci/89.21.1586. [DOI] [PubMed] [Google Scholar]
- 19.Lode H N, Xiang R, Dreier T, Varki N M, Gillies S D, Reisfeld R A. Blood. 1998;91:1706–1715. [PubMed] [Google Scholar]
- 20.Saari W S, Schwering J E, Lyle P A, Smith S J, Engelhardt E L. J Med Chem. 1990;33:97–101. doi: 10.1021/jm00163a016. [DOI] [PubMed] [Google Scholar]
- 21.Linden C J. In Vivo. 1989;3:259–262. [PubMed] [Google Scholar]
- 22.Bagshawe K D, Sharma S K, Burke P J, Melton R G, Knox R J. Curr Opin Immunol. 1999;11:579–583. doi: 10.1016/s0952-7915(99)00004-7. [DOI] [PubMed] [Google Scholar]
- 23.Martin J, Stribbling S M, Poon G K, Begent R H J, Napier M, Sharma S K, Springer C J. Cancer Chemother Pharmacol. 1997;40:189–201. doi: 10.1007/s002800050646. [DOI] [PubMed] [Google Scholar]
- 24.Bagshawe K D, Sharma S K. Transplant Proc. 1996;28:3156–3158. [PubMed] [Google Scholar]
- 25.Abuchowski A, Davis F F, Davis S. Cancer Treat Rep. 1981;65:1077–1081. [PubMed] [Google Scholar]
- 26.Bagshawe K D. Br J Cancer. 1989;60:275–281. doi: 10.1038/bjc.1989.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.List B, Shabat D, Zhong G f, Turner J M, Li A, Bui T, Anderson J, Lerner R A, Barbas C F., III J Am Chem Soc. 1999;121:7283–7291. [Google Scholar]
- 28.Tanaka F, Lerner R A, Barbas C F., III J Am Chem Soc. 2000;122:4835–4836. [Google Scholar]
- 29.Zhong G, Lerner R A, Barbas C F., III Angew Chem. 1999;111:3957–3960. [Google Scholar]