Abstract
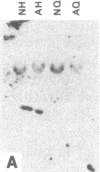
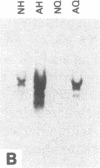
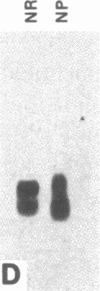
Nucleate and anucleate fragments of sea urchin eggs were prepared by centrifugation on sucrose step gradients. The amount of total RNA, poly(A)+ RNA, histone mRNA, actin mRNA, alpha-tubulin mRNA, and mitochondrial rRNA was determined for each fragment. Total RNA, poly(A)+ RNA, actin mRNA, and alpha-tubulin mRNA all distributed in the same ratio as the volume of the fragments. In contrast, the mitochondrial rRNA was found preferentially distributed in the anucleate fragments, coinciding with the distribution of the mitochondria. Histone mRNAs did not follow the fragment volume ratios, but rather were always found associated with the fragment containing the nucleus. To distinguish between nuclear association and possible artifacts associated with centrifugation, eggs were manually cut into nucleate and anucleate fragments and the amount of histone mRNA was determined for each set. Again only the fragments containing the nucleus had detectable amounts of histone mRNA. Although histone mRNAs were always associated with the nucleate fragment, very little histone mRNA was found associated with isolated egg nuclei prepared under gentle isotonic isolation conditions. Furthermore, embryos that have had first nuclear breakdown blocked with 6-dimethylaminopurine still initiated the recruitment of histone mRNAs into polysomes at the same time as control embryos, thus indicating that nuclear breakdown is not necessary for normal histone message utilization. These results demonstrate a message-specific sequestration of maternal histone mRNA which is physically different from that of other maternal mRNAs and which may govern the timing of maternal histone synthesis in sea urchin embryos.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. A cytological study of the centrifuged whole, half, and quarter eggs of the sea urchin, Arbacia punctulata. J Cell Biol. 1970 Dec;47(3):711–733. doi: 10.1083/jcb.47.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg D. A., Rebhun L. I. pH regulates the polymerization of actin in the sea urchin egg cortex. J Cell Biol. 1979 Oct;83(1):241–248. doi: 10.1083/jcb.83.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst B. P. Two-dimensional gel patterns of protein synthesis before and after fertilization of sea urchin eggs. Dev Biol. 1976 Sep;52(2):310–317. doi: 10.1016/0012-1606(76)90248-7. [DOI] [PubMed] [Google Scholar]
- Gross K. W., Jacobs-Lorena M., Baglioni C., Gross P. R. Cell-free translation of maternal messenger RNA from sea urchin eggs. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2614–2618. doi: 10.1073/pnas.70.9.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaumeyer J. F., Jenkins N. A., Raff R. A. Messenger ribonucleoprotein particles in unfertilized sea urchin eggs. Dev Biol. 1978 Apr;63(2):266–278. doi: 10.1016/0012-1606(78)90133-1. [DOI] [PubMed] [Google Scholar]
- Merlino G. T., Water R. D., Chamberlain J. P., Jackson D. A., El-Gewely M. R., Kleinsmith L. J. Cloning of sea urchin actin gene sequences for use in studying the regulation of actin gene transcription. Proc Natl Acad Sci U S A. 1980 Feb;77(2):765–769. doi: 10.1073/pnas.77.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischke D., Pardue M. L. Organization and expression of alpha-tubulin genes in Drosophila melanogaster. One member of the alpha-tubulin multigene family is transcribed in both oogenesis and later embryonic development. J Mol Biol. 1982 Apr 15;156(3):449–466. doi: 10.1016/0022-2836(82)90260-1. [DOI] [PubMed] [Google Scholar]
- Rebhun L. I., White D., Sander G., Ivy N. Cleavage inhibition in marine eggs by puromycin and 6-dimethylaminopurine. Exp Cell Res. 1973 Mar 15;77(1):312–318. doi: 10.1016/0014-4827(73)90582-x. [DOI] [PubMed] [Google Scholar]
- Skoultchi A., Gross P. R. Maternal histone messenger RNA: detection by molecular hybridization. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2840–2844. doi: 10.1073/pnas.70.10.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sures I., Lowry J., Kedes L. H. The DNA sequence of sea urchin (S. purpuratus) H2A, H2B and H3 histone coding and spacer regions. Cell. 1978 Nov;15(3):1033–1044. doi: 10.1016/0092-8674(78)90287-8. [DOI] [PubMed] [Google Scholar]
- Venezky D. L., Angerer L. M., Angerer R. C. Accumulation of histone repeat transcripts in the sea urchin egg pronucleus. Cell. 1981 May;24(2):385–391. doi: 10.1016/0092-8674(81)90328-7. [DOI] [PubMed] [Google Scholar]
- Wells D. E., Showman R. M., Klein W. H., Raff R. A. Delayed recruitment of maternal histone H3 mRNA in sea urchin embryos. Nature. 1981 Jul 30;292(5822):477–478. doi: 10.1038/292477a0. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Fitschen W. The mobilization of maternal histone messenger RNA after fertilization of the sea urchin egg. Cell Differ. 1978 Apr;7(1-2):103–114. doi: 10.1016/0045-6039(78)90011-8. [DOI] [PubMed] [Google Scholar]