Abstract
Loss of heterozygosity (LOH) at human chromosome 18q, which includes the gene Deleted in Colorectal Cancer (DCC), has been linked to colorectal and many other human cancers. DCC encodes the receptor for the axon guidance molecule Netrin (Net) and functions during neural development in a variety of organisms. However, since its discovery in the 1990s, the status of DCC as a tumor suppressor has been debated, primarily due to a lack of support for this hypothesis in animal models. A recent study from our laboratory capitalized on the genetic tractability of Drosophila melanogaster to demonstrate that this gene functions as an invasive tumor suppressor, thereby providing the first direct link between DCC loss and metastatic phenotypes in an animal model for cancer. Two subsequent studies from other laboratories have demonstrated that DCC suppresses tumor progression and metastasis in murine colorectal and mammary tumor models. Combined, these findings have prompted the rebirth of DCC as a tumor suppressor and highlighted the need for continued analysis of DCC function in animal models for human cancer.
Keywords: Apoptosis, axon guidance, cancer, DCC, Drosophila melanogaster, metastasis, netrin, tumor suppressor
LOH AT 18Q AND CANCER
LOH at chromosome 18q was identified in greater than 70% of human colorectal cancers [1, 2]. These findings triggered the search for a tumor suppressor gene in this chromosomal region, which led to the cloning of DCC. Expression of DCC, which encodes a cell surface receptor, was found to be altered in colorectal cancers, supporting the notion that DCC might be the 18q tumor suppressor [3]. Although it was initially hypothesized that DCC might function as a tumor suppressor gene, the tumor suppressor status of this gene has been debated since its discovery [4–7]. A chronological list of evidence both in support of and against this hypothesis is presented in (Table 1) and summarized below.
Table 1. DCC: Road to a Tumor Suppressor.
A chronological list of key findings that either supported or refuted the DCC tumor suppressor hypothesis is provided.
| Year | Finding | Referencesa |
|---|---|---|
| 1988 | LOH at 18q is implicated in colorectal cancer. | [1, 2] |
| 1990 | Cloning of DCC, found to be altered in colorectal cancers | [3] |
| 1996 | DCC functions as a Netrin receptor. | [18] |
| 1997 | Mouse DCC knockout lacks a colorectal cancer phenotype. | [9] |
| 1998 | Smad4 (which maps to 18q) functions as a tumor suppressor. | [8] |
| 1998 | DCC induces apoptosis. | [24] |
| 1999–2000 | Expression of DCC suppresses tumorigenic growth of cells in culture and following transplantation in nude mice. | [57, 58] |
| 2001 | Net-1 signaling through DCC promotes cell survival. | [25] |
| 2004 | Upregulated Net-1 induces tumor progression in the mouse intestine. | [27] |
| 2008–2009 | Upregulation of Net-1 in metastatic breast, non-small-cell lung, neuroblastoma, pancreatic adenocarcinoma, and IBD-associated cancers | [26, 30, 29], [55, 59] |
| 2011 | Fra/DCC functions as an invasive tumor suppressor in D. melanogaster. | [36] |
| 2012 | Mutation of DCC advances tumor progression in murine APC deficient colorectal and p53 deficient mammary tumor models. | [48, 49] |
Refer to the numbered references cited in the text.
DCC expression is reduced in a large percentage of colorectal tumors [3, 6]. However, point mutations in DCC are not often associated with tumors [5]. Furthermore, following cloning of DCC, Smad4, which is also located at 18q, was shown to function as a tumor suppressor gene, suggesting that this gene might in fact be the 18q locus of interest [8]. Moreover, the DCC knockout mouse did not demonstrate an increased incidence of tumor susceptibility [9]. This mouse did however exhibit axon guidance abnormalities [9], which have been associated with loss of DCC in a variety of other organisms, from C. elegans [10] to D. melanogaster [11], and more recently vector mosquitoes [12]. For these reasons, much of the focus of DCC research initially centered on its role as an axon guidance molecule [13].
DCC FUNCTIONS AS A RECEPTOR FOR NETRIN DURING AXON GUIDANCE
Similarities between the DCC and Net loss of function axon guidance phenotypes in C. elegans [10, 14], D. melanogaster [11, 15, 16], and mice [9, 17] suggested that DCC might function as a receptor for Net proteins, a notion that was confirmed by biochemical experiments [18]. Members of the Net family are laminin-related diffusible proteins that promote the outgrowth and guidance of commissural axons toward the midline in a variety of organisms [13, 19]. Upon their discovery in the chick [19], sequence comparisons indicated that chick net-1 and net-2 are related to C. elegans unc-6, which had previously been shown to direct the circumferential migration of axons to the midline in nematodes [10, 14]. NetA and B genes were thereafter isolated in Drosophila, where they regulate proper nerve cord formation by acting as short-range guidance cues that promote midline crossing of axons [15, 16, 20]. Likewise, inactivation of Net-1 in mice results in pathfinding defects that prevent most commissural axons from reaching the floor plate [17].
Axon guidance functions of Net proteins are mediated by various Net receptors, including members of the DCC family [21]. DCC family genes, including the C. elegans homolog unc-40 and the Drosophila homolog frazzled (fra), encode transmembrane proteins that have immunoglobulin domains and fibronectin type III repeats [7] (Fig. 1). DCC receptors are expressed on commissural axons and growth cones, where they function as cell-surface receptors for Net family proteins during axon guidance [22]. DCC has also been shown to regulate Net protein spatial distribution, and it has been suggested that this regulation of Net protein distribution guides neighboring axons through the creation of positional information for other Net receptors [23]. Mutation or inhibition of DCC receptors results in the failure of many commissural axons to reach the midline [9–11]. For example, the mouse DCC knockout exhibits axonal projection defects comparable to those observed in Net-1 deficient mice [9]. Although characterization of the phenotype of mice lacking DCC supported a role for this gene in Net-1-mediated axon guidance, it raised questions concerning the status of DCC as a colorectal tumor suppressor. Loss of murine DCC does not affect mouse intestinal growth, differentiation, or morphogenesis, and it does not result in increased tumorigenesis [9]. For this reason, although the axon guidance roles of DCC were intensely studied during the late 1990’s [13], its status as a tumor suppressor remained controversial at the turn of the century.
Fig. 1.
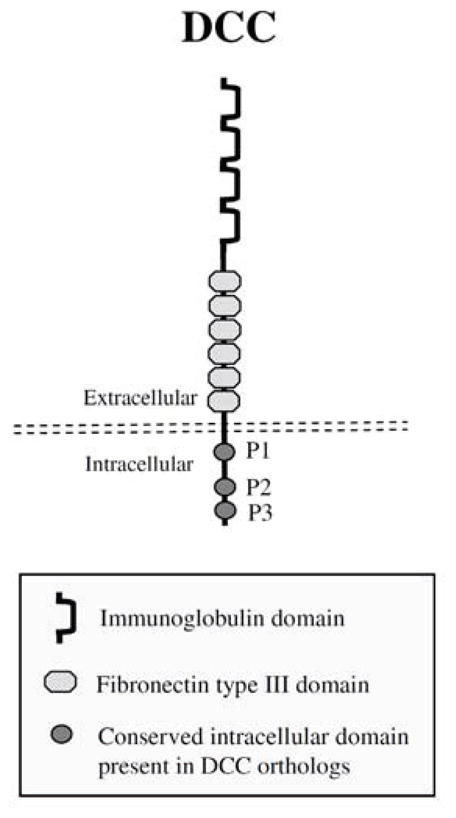
DCC protein structure. A schematic representation of DCC with the location of the immunoglobulin, fibronectin type III, transmembrane, as well as the conserved intracellular P1, P2, and P3 domains noted. The structure depicted is adapted from reference [7].
DCC: A DEPENDENCE RECEPTOR, BUT STILL NOT A TUMOR SUPPRESSOR?
The discovery that DCC functions as a dependence receptor (Fig. 2a, b) which induces cell survival in the presence of ligand prompted renewed interest in its putative functions in cancer [5]. Research demonstrated that in the absence of ligand, Caspase-9 is recruited into a complex with Caspase-3 and DCC. This in turn leads to caspase activation and cleavage of the death domain of DCC at position 1290, which exposes the DCC proapoptotic addiction dependence domain (ADD) and promotes cell death (Fig. 2b). Thus, in the absence of ligand, DCC promotes apoptosis. Conversely, in the presence of ligand, DCC-induced apoptosis is suppressed, and cells expressing DCC become dependent upon the presence of Net for their survival [24, 25]. It was proposed that this dependence receptor system could serve as a mechanism by which DCC functions as a tumor suppressor, as pretumorous or metastatic cells growing in regions of ligand unavailability would be eliminated by DCC-mediated apoptosis [5, 26].
Fig. 2.
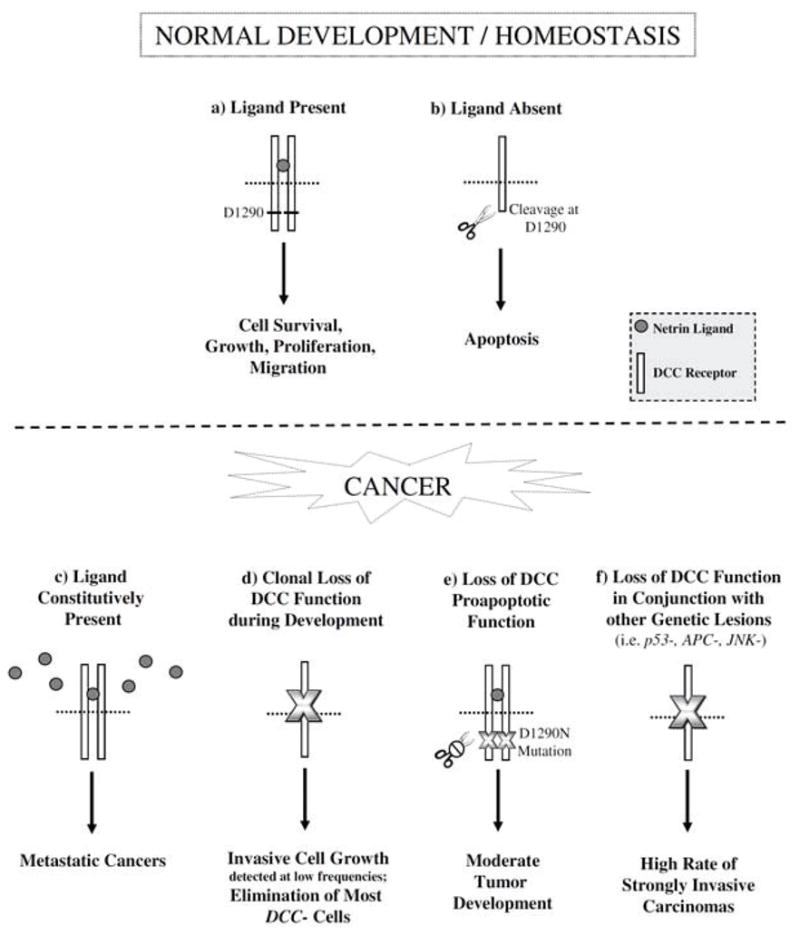
DCC tumor suppressing functions. Top: DCC functions as a dependence receptor during normal development and homeostasis. a) In the presence of Net ligand, DCC induces a variety of cellular responses including survival, growth, proliferation, and migration. b) However, in the absence of ligand, DCC is cleaved at D1290 and promotes cell death. c) In human cancers (bottom), the constitutive presence of Net ligand promotes metastatic cancer. (d–f) Recent studies in D. melanogaster and murine cancer models suggest that loss of DCC function also promotes cancer. d) During D. melanogaster development, although most somatic fra/DCC loss of function clones are eliminated, DCC deficiency can result in excess cell growth and invasion. e) The D1290N mutation results in loss of the proapoptotic function of DCC and moderate tumor formation in mice. f) In conjunction with other genetic lesions (i.e. mutation of P53, APC, or JNK), loss of DCC function results in highly invasive carcinomas. Such metastatic cancers may result from loss of the proapoptotic functions of DCC and direct stimulation of growth, proliferation, and invasion in response to changes in cell signaling (i.e. activated Rho signaling) that occur when DCC function is compromised. See text for details and references to studies supporting this model.
In mice, the ability of Net-1 to inhibit the proapoptotic function of DCC was shown to be important for tumor progression in the digestive tract [27]. During intestinal development in mice, Net-1 promotes maintenance, integrity, migration, and renewal of the intestinal epithelium by promoting cell survival in proliferating intestinal crypt progenitors that are exposed to high Net-1 levels. When mouse intestinal cells differentiate, they migrate to the surface of the intestinal luminal epithelium where lack of Net-1 results in DCC-induced apoptosis [5, 28]. Ectopic expression of Net-1 in the mouse gastrointestinal tract results in the spontaneous formation of hyperplastic and neoplastic lesions, and overexpression of Net-1 in an APC mutant background promotes intestinal tumor development. It was proposed that these tumors result from inhibition of DCC-induced apoptosis [27]. Likewise, elevated Net-1 levels have also been associated with metastatic breast cancer [26], aggressive neuroblastomas [29], and non-small cell lung cancer [30], suggesting that the abilities of Net-1 to prevent cell death and promote cell invasion may be critical in a number of different cancers. Additionally, Flannery et al. [31] demonstrated that Net-DCC signaling induces cellular growth and expression of key cellular growth regulators, including myc, cdk4, PCNA, and activated ERK. Thus, in addition to promoting cell survival and invasion, ectopic Net expression may also promote tumor cell growth (Fig. 2c).
Although a number of investigations suggested that DCC functions as a dependence receptor that promotes metastatic cancer when Net-1 ligand is present, these studies still failed to demonstrate that DCC functions as a classical tumor suppressor [5, 32]. While DCC had been shown to induce cell death when ligand is limiting (Fig. 2b), it was also found to promote tumor progression by mediating constitutive Net-1 signaling, which can confer a selective advantage on tumor cells (Fig. 2c). Furthermore, DCC deficiency had still not been shown to promote tumor progression in any animal model for cancer. For these reasons, many still deemed it to be a conditional tumor suppressor [5, 32].
ANALYSIS OF D. MELANOGASTER FRA/DCC PROVIDES THE FIRST DIRECT LINK BETWEEN LOSS OF DCC FUNCTION AND A METASTATIC PHENOTYPE IN AN ANIMAL MODEL FOR CANCER
D. melanogaster is an excellent system in which to study tumor suppressor gene function, as many of the hallmarks of human cancer, including self-sufficiency in growth and proliferative signals, insensitivity to anti-proliferative signals, evasion of apoptosis, and invasion/metastasis can be found in this animal model for cancer [13, 33, 34]. One advantage of this system is that it permits use of FLP/FRT recombination [35] to create clones of genetically distinct somatic cells that model human tumors. The genetic tractability of the Drosophila model also allows for multiple genetic manipulations to be performed simultaneously in such clones, a notable advantage given that multiple mutations typically cooperate to generate metastatic tumors in humans [13, 33].
In a recent investigation from our laboratory, Van-Zomeren-Dohm et al. [36] exploited the advantages of the D. melanogaster system to study the roles of Fra, the Drosophila homolog of DCC. It was anticipated that the genetic tractability of this system might shed light on the controversial status of DCC as a tumor suppressor. In these studies, the authors investigated the function of Fra/DCC during development of the eye-antennal imaginal disc, a tissue in which the functions of many tumor suppressors have been analyzed [33]. Recent investigations have highlighted the importance of studying cancer genes in a clonal context [37, 38], and for this reason the authors chose to generate somatic loss of function fra/DCC mutant clones. They found that most fra/DCC loss of function clones generated during imaginal disc development are eliminated by the end of the third larval instar. Although most fra/DCC mutant clones do not persist, in ~1% of adult flies in which fra/DCC loss of function mutant clones were generated, mutant clone cells formed overgrowths. These exciting observations suggested that it would be useful to develop a strategy that would permit more efficient analysis of fra/DCC loss of function clones.
The authors chose to use the mosaic analysis with a repressible cell marker (MARCM) system [39, 40] to drive expression of the caspase inhibitor P35 in fra/DCC loss of function mutant clones generated in the developing eye. Although the majority of these flies did not survive to adulthood, this strategy (which is often employed in Drosophila for cases in which loss of a gene results in cell lethality) permitted analysis of clones in third instar larval eye-antennal discs and occasional analysis of adults that escaped death. A striking phenotype was observed in these adult flies: the detection of red-pigmented fra/DCC mutant eye cells throughout their bodies (Fig. 3). In the most dramatic case, fra/DCC mutant clone cells generated in the developing eye could be detected on the adult wing (Fig. 3a). These fra/DCC mutant eye cells appeared to differentiate, at least in part, as they expressed the neural differentiation marker Elav in third instar eye discs and displayed red pigmentation in adults (Fig. 3). These observations led to the hypothesis that Fra/DCC may have invasive tumor-suppressing functions in Drosophila (Fig. 2d).
Fig. 3.
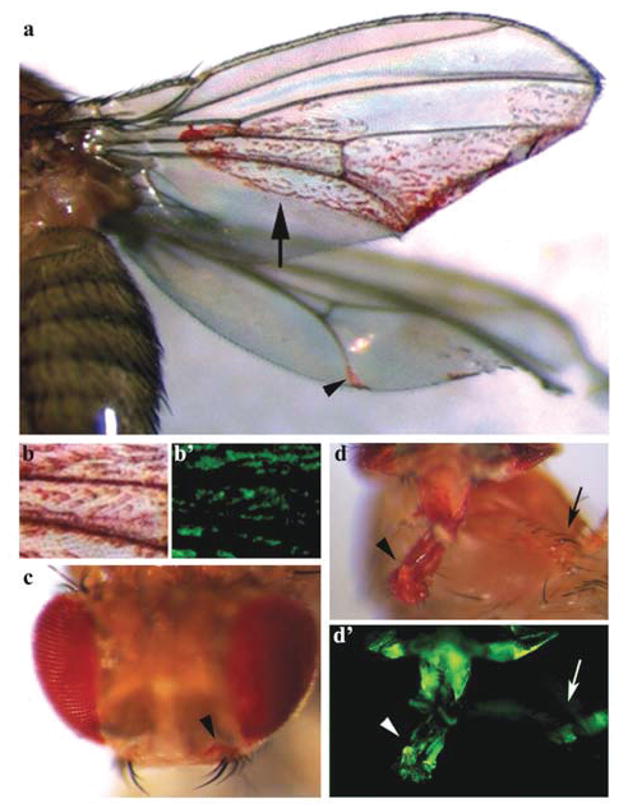
Loss of fra/DCC function during development of D. melanogaster results in metastatic phenotypes. Metastatic phenotypes resulting from somatic loss of function fra/DCC clones generated in the developing eye are shown. P35-rescued fra/DCC mutant red-pigmented eye cells generated in the developing eye have invaded the wing (arrow and arrowhead in a), head (arrowhead in c), proboscis (arrowhead in d) and leg (arrow in d). A high magnification view of the region marked by the arrow in a is shown in b. GFP expression (b′ and d′) used to positively mark mutant cells permitted live imaging of these invasive cells during development [36]. Panels a, b, and b′ were originally published in reference [36].
To test this hypothesis, fra/DCC loss of function clones were studied in the developing eye-antennal disc, where clones were generated with an eye-specific driver. Although the eyeless promoter [41] used in the investigation was designed to be very tight, the investigators restricted all analyses to the eye-antennal disc in order to guard against any minor leakiness of the promoter and so that the point of clone origin (the eye) would be certain. Elav-positive fra/DCC mutant photoreceptor cells were detected outside of the developing eye field in the optic stalk and other regions of the eye-antennal disc that are not normally populated with photoreceptor cell bodies. In such cases, fra/DCC mutant Elav-positive foci were isolated and often located outside of mutant clone boundaries, suggesting that they had become invasive. Comparable results were obtained even in the absence of P35-rescue, and when loss of function clones were generated for two separate alleles of fra/DCC (fra3 or fra4, [11]). These findings indicated that fra/DCC mutant cells are invasive. However, one could alternatively argue that fra/DCC mutant cells located outside of the eye field had undergone a transformation toward an eye cell fate. The authors ruled out this possibility for a number of reasons (see [36] for details), mainly due to the fact that fra/DCC loss of function clones generated in the developing wing are not transformed into eye cells.
Immunohistochemical characterization of fra/DCC mutant cells in the third instar developing eye disc indicated that they express the tumor cell markers phospho-ERK and phospho-JNK. The clone cells were also found to exhibit changes in expression of E-cadherin, reorganization of the actin cytoskeleton, and loss of apical-basal polarity, characteristics typical of invasive tumor cells. Even though fra/DCC mutant cells retained expression of the neural differentiation marker Elav, a proportion of these cells were found to simultaneously express the mitotic marker phosphorylated histone H3. The authors also showed that loss of fra/DCC induces expression of Matrix metalloproteinase-1 (Mmp-1), basement membrane degradation, and invasion. Furthermore, by marking the fra/DCC mutant cells with GFP expression (Fig. 3), the investigators demonstrated through live imaging experiments that fra/DCC mutant cells are invasive. Their movies captured projections extending and retracting from motile fra/DCC mutant cells as they exited the eye disc. Combined, these data indicated that Fra/DCC functions as an invasive tumor suppressor during Drosophila development, suggesting that loss of DCC can promote metastatic cancer in humans [36] (Fig. 2d).
VanZomeren-Dohm et al. [36] then attempted to identify suppressors of the loss of function fra/DCC metastatic phenotype. They hypothesized that JNK, a mediator of metastasis [42] that is upregulated in fra/DCC mutant cells, might drive the invasion of these cells. However, inhibition of JNK signaling did not suppress basement membrane degradation or invasion of fra/DCC mutant cells. In fact, inhibition of JNK signaling in fra/DCC mutant cells appeared to block JNK-mediated cell death, thereby promoting enhanced overgrowth and invasion of fra/DCC mutant cells (Fig. 2f). These results were initially somewhat surprising, as JNK-induced Mmp1-dependent degradation of the basement membrane was previously determined to be critical for invasion of cells in other Drosophila metastatic cancer models [42]. However, others have shown that JNK activity is context dependent and does not always function to promote cell invasion [43, 44]. Furthermore, JNK-driven apoptosis has been observed in the context of other Drosophila tumor suppressor mutations [44], and it is therefore perhaps not unexpected that it would function in the apoptotic removal of fra/DCC mutant cells.
The authors then examined if inhibition of Rho1, which has also been implicated in the invasive behavior of wing and eye imaginal disc cells [37, 45], could block fra/DCC mutant cell invasion. They found that inhibition of Rho1 signaling through expression of dominant negative-Rho1 in fra/DCC mutant cells represses basement membrane degradation and invasion. The repression of basement membrane degradation and invasion by dominant negative-Rho1 was substantial, albeit incomplete, suggesting that this process may also be mediated by additional unknown factors. Our lab has recently pursued global analysis of gene expression in fra/DCC mutant cells (Sarro and Duman-Scheel, unpublished), as well as a genetic interaction screen for enhancers of the fra/DCC loss of function phenotype (Sarro, Tessier, and Duman-Scheel, unpublished). These studies are elucidating additional molecules that may modulate and mediate fra/DCC mutant cell metastasis.
It should be noted that comparable eye cell invasive metastatic adult phenotypes are not typically reported in Drosophila. An exception is the Ferres-Marco et al. [46] eyeful study, in which the combined manipulation of Delta, lola, and pipsqueak generated a metastatic eye phenotype. It is interesting that both the fra/DCC and eyeful studies resulted in the generation of invasive cells which were at least partially differentiated yet still dividing. Zhai et al. [47] discuss the delicate balance between differentiation, proliferation, cell death, and invasion in their recent analysis of Drosophila Cut, a transcription factor with tumor suppressing capabilities. Loss of Cut enhances the eyeful phenotype and contributes to a high frequency of long range eye cell metastasis. Their data argue that Cut normally functions to remove cells that are not able to fully differentiate, thereby preventing them from developing into cancer cells. Cut accomplishes this through coordinate regulation of multiple cellular processes, including apoptosis, differentiation, cell adhesion, and proliferation, the combined regulation of which is required for proper cell fate specification and maintenance of a differentiated state. The results of this investigation suggest that Cut functions in an evolutionarily conserved cancer prevention regulatory network [47]. It is possible that Fra/DCC functions in a comparable cancer-preventing regulatory network.
ASSESSING THE TUMOR-REPRESSING FUNCTIONS OF DCC IN MURINE CANCER MODELS
Following publication of the Drosophila frazzled/DCC study [36], two groups assessed the tumor-suppressor functions of DCC in mouse models for colorectal [48] and metastatic breast [49] cancers. These studies, which are reviewed below, also concluded that DCC functions as a tumor suppressor. To investigate the putative function of DCC as a colorectal cancer tumor suppressor, Castets et al. [48] constructed a mouse model in which aspartic acid residue 1290 of DCC was mutated [(DCC(D1290N)]. While mutation of this residue did not appear to impact Net-1 signaling through DCC, it did allow the authors to assess the importance of DCC pro-apoptotic activity, which was compromised by this mutation (Fig. 2e). In the mouse intestine, epithelial cell death is typically observed at the tips of the villi where Net-1 ligand is not abundant [5, 28]. In mice in which DCC proapoptotic activity was silenced, apoptosis in the intestinal epithelium was significantly decreased. This reduction in apoptosis was accompanied by limited spontaneous intestinal tumor formation, with nearly 15% of mutant mice (as compared to 0% of control animals) displaying neoplastic transformations, including adenomas and adenocarcinomas. Based on these results, the authors proposed that DCC-mediated cell death may normally function to limit the initiation of malignant transformations (Fig. 2e) [48].
Castets et al. [48] hypothesized that given the high incidence of DCC loss observed in late-stage tumors, DCC may predominantly function as a tumor suppressor in late stages of tumorigenesis. To test this, they examined the effect of the DCC(D1290) mutation in an APC+/1638N background. APC+/1638N mice are known to develop a moderate level of intestinal tumors [50], but in the DCC(D1290) homozygous background, the incidence and aggressiveness of the adenocarcinomas significantly increased (Fig. 2f). A reduced incidence of apoptosis was also observed in these tumors. Based on their observations, the authors argued that inhibition of DCC-induced apoptosis may disrupt the balance between proliferation and death in low grade tumors and stimulate tumor progression by increasing the likelihood for additional mutations to accumulate over time. The authors concluded that the results of their investigation demonstrate that DCC functions as a tumor suppressor in the intestinal tract by acting as a gatekeeper that limits tumor progression through its ability to promote apoptosis.
Krimpenfort et al. [49] examined the putative tumor-suppressing functions of DCC in a p53 deficient mouse mammary tumor model. They introduced a Cre/loxP conditional DCC mutant lacking a transmembrane domain into a background in which the cytokeratin 14 promoter was used to ablate both copies of p53 in mammary epithelial tissue. They chose this well-established model because it yields well-encapsulated tumors that do not typically metastasize [51, 52]. Furthermore, as expression of Unc5h (which also functions as a Net-1 receptor) is known to be reduced in p53 deficient conditions [53], the authors argued that use of this model would guard against the potential for Unc5h to mask the effects of DCC loss. This was an important concern given that multiple Net receptors are found in mice [6, 7]. Their results suggested that DCC loss does not increase the incidence of p53 deficient primary tumors, but that the additional loss of DCC promotes metastasis of p53 deficient mammary tumors (Fig. 2f). The authors speculated that this increase in metastatic tumors might relate to the ability of DCC to induce cell death when Net-1 ligand is limiting, for example in cases where cells have disseminated from the primary tumor site. Through cell culture experiments with p53 deficient mouse mammary tumor cells, they next demonstrated that DCC promotes Net-1-dependent cell survival. Consistent with these in vitro data, the authors found that mice intravenously injected with DCC deficient p53 deleted cells had significantly more tumor cell clusters in the lungs as compared to those injected with DCC proficient p53 deleted cells. The authors concluded that DCC functions as a tumor suppressor which induces the death of cells that disseminate from the primary tumor mass [49].
FROM FLIES TO MICE
Together, our initial study in the developing Drosophila eye [36] and two subsequent studies in mice [48, 49] have provided convincing new evidence that DCC functions as a tumor suppressor in three animal models for cancer (Fig. 2d–f). These critical findings have prompted the rebirth of DCC as a tumor suppressor. While the three studies have certainly quieted a 15-year old debate, they raise new questions about the functions of DCC in cancer.
The Drosophila study [36] provided evidence that loss of function mutations in DCC/fra alone could result in tumor-like outgrowths, albeit at a very low frequency (~1% of the animals in which mutant clones had been induced). This result differs from the initial DCC mouse knockout study [9], as well as the Krimpenfort et al. investigation [49] in which no increased incidence of primary tumors was found to be associated with loss of DCC. In addition to DCC, several other proteins, including Unc-5 and Neogenin, function as Net-1 receptors in mice [32]. Such redundancy, which is perhaps not an issue in the Drosophila eye given the metastatic phenotypes observed (Fig. 3), could explain the lack of cancer phenotypes in the DCC knockout mouse [9]. The Krimpenfort et al. [49] mammary tumor study partially addresses the receptor redundancy issue through choice of a model in which Unc-5 should not be a confounding factor, but it was not addressed in the Castets et al. [48] investigation. Furthermore, redundancy with additional Net-1 receptors [i.e. Down’s syndrome cell adhesion molecule (DSCAM), Neogenin, A2b] could still be an issue in these and other mouse DCC deficient tumor studies.
The Drosophila study also differs from the Castets et al. [48] investigation where the authors used a mutant in which the proapoptotic functions of DCC were specifically disrupted. As discussed above, the authors found that mice homozyogous for this mutation had a moderately increased incidence of spontaneous neoplastic transformations in the intestinal tract. However, they argued that the DCC(D1290N) mutation impacts only the proapoptotic functions of DCC while permitting Net-1-DCC signaling (Fig. 2e), as indicated by the viability of these animals and their lack of obvious brain defects. If Net-1-DCC signaling is in tact in these animals, then it is difficult to directly compare these experiments to the Drosophila study [36] in which loss of function fra/DCC mutations were utilized. A comparable analysis of the proapoptotic functions of Fra/DCC have not yet been performed in Drosophila, but pursuing this line of experiments might prove useful. An interesting observation in the VanZomeren-Dohm [36] investigation is that most fra/DCC mutant clones generated in the Drosophila eye-antennal disc do not persist beyond the third larval instar (Fig. 2d). Based on the dependence receptor model (Fig. 2a, b), and in light of the two murine studies [48, 49], one might have expected that loss of function mutations in fra/DCC would result in increased cell viability. One possible interpretation of the Drosophila result is that Net ligand, which is expressed in the eye-antennal disc, provides a survival cue for developing cells in this tissue. fra/DCC mutant cells lacking the ability to receive this survival cue could ultimately die, presumably through activation of JNK signaling [36].
Another critical difference between the three recent fra/DCC investigations discussed herein is that only the Drosophila study involved production of somatic clones of mutant cells (Figs. 2d, 3). Recent studies have highlighted the importance of studying cancer genes in a clonal context [37, 38]. In support of this notion, expression of fra-RNAi throughout the developing Drosophila eye does not generate metastatic eye phenotypes (although fra-RNAi clones, like fra loss of function clones, do not typically persist during development; C. Tessier and M. Duman-Scheel, unpublished observation). It is possible that using an experimental design more comparable to the one employed by VanZomeren-Dohm et al. [36], one in which clones of DCC loss of function cells are generated (and perhaps also rescued from death), could generate primary tumor phenotypes in mice. However, if a comparable somatic clonal study were to be performed in mice, it may be very difficult to score enough adult animals to observe this effect. Even in the fly model, rescue of DCC mutant clones with P35 expression was employed to facilitate completion of the investigation. This has consequently raised the question of whether expression of P35 influenced the results. However, VanZomeren-Dohm et al. [36] controlled for use of P35. Moreover, they demonstrated that use of P35 did not appear to be a confounding factor in the investigation, as basement membrane degradation, invasion, and tumor-like overgrowths could all be observed in the absence of P35 rescue, albeit at a low frequency (Fig. 2d).
Since publication of the VanZomeren-Dohm et al. [36] manuscript, we have initiated a genetic interaction screen in the Drosophila eye that examines whether lesions in other signaling pathways, in the absence of P35 rescue, will enhance the fra/DCC loss of function phenotype. We have found that expression of fra-RNAi in combination with other genetic lesions results in a variety of eye abnormalities, ranging from rough eyes to dramatic metastatic adult eye phenotypes (J. Sarro, C. Tessier, M. Duman-Scheel, unpublished). The results of this screen, as well as the published observation that inhibition of JNK signaling enhances the fra/DCC loss of function phenotype [36], are in agreement with the recent murine studies, both of which illustrated that loss of DCC in conjunction with other mutations (APC, p53), can result in the formation of highly invasive carcinomas (Fig. 2f). Interestingly, interactions between fra and APC were uncovered in the Drosophila screen. Furthermore, recent microarray experiments in which changes in gene expression in response to gain and loss of Net-Fra/DCC signaling were assessed have uncovered multiple hits in the Wnt pathway, including APC (J. Sarro and M. Duman-Scheel, unpublished). These results are interesting given the Castets et al. [48] finding that silencing DCC-induced apoptosis is associated with increases in the number and aggressiveness of intestinal tumors in an APC mutant context [48].
Finally, it is interesting to consider the results of the Krimpenfort et al. [49] mammary tumor study in the context of the Drosophila [36] investigation. Based on their analysis of p53 deficient mammary tumors, Krimpenfort et al. [49] suggest that DCC limits survival of disseminated tumor cells, as supported by both their cell culture and intravenous injection experiments. However, in light of the fra/DCC investigation [36], it is also seems likely that loss of DCC in p53 deficient mammary tumor cells more directly contributes to the invasive properties of these cells (Fig. 2f). For example, given that inhibition of Rho signaling suppresses invasion of fra/DCC mutant cells [36], it is plausible that altered Rho signaling, which is known to correlate with breast tumor progression, metastasis, and poor prognosis [54], could contribute to the enhanced metastatic capacity of p53-deficient mammary tumors lacking DCC.
In addition to Rho signaling, microarray experiments recently performed in our laboratory suggest that components of a number of other cell signaling pathways that regulate development and cancer are differentially expressed in response to fra/DCC deletion (J. Sarro and M. Duman-Scheel, unpublished). We are in the process of determining if these signaling pathways function in invasive growth of DCC mutant cells. In light of the Krimpenfort et al. [49] study, it is anticipated that this work will promote a better understanding of metastatic breast cancers in which DCC is deficient. In support of this notion, bioinformatic analysis of the fra/DCC loss of function microarray hits uncovered significant breast neoplasm human disease gene networks. Colorectal neoplasm disease networks were also identified, further supporting the use of Drosophila to model the roles of Fra/DCC in colorectal cancer. Furthermore, these studies uncovered significant prostatic and lung cancer disease networks (J. Sarro and M. Duman-Scheel, unpublished), suggesting that DCC may also function as a tumor suppressor in these tissues.
IMPLICATIONS FOR THERAPIES
It is well established for a number of cancers that gain of autocrine Net-1 signaling (Fig. 2c) confers a selective advantage on tumor cells and promotes metastatic cancers [32]. For this reason, therapy development to date has primarily focused on disrupting the interaction of Net-1 with its receptors. Studies in metastatic breast cancer cells [26], many of which are known to have elevated Net-1 levels, suggest that targeting Net-1 signaling has good therapeutic potential. Experimental reduction of Net-1 levels through small interfering siRNA (siRNA) has been shown to promote cell death in metastatic breast [26] and lung [30] cancers. siRNA silencing of Netrin-1 (Ntn1) was also found to inhibit tumor cell invasion of pancreatic ductal adenocarcinoma cells in a chick model [55]. Comparable strategies could be employed to combat the myriad of other human cancers that have been linked to activated Net-1 signaling [32].
Drug development has concentrated on biological agents that mimic the interaction of DCC with Net-1 [32]. For example, the fifth fibronectin domain of DCC, DCC-5fbn, is known to interact with Net-1. Although DCC-5fbn does not appear to block Net-1/receptor interactions, it prevents receptor multimerization which is required for Net-1 inhibition of apoptosis [56] and possibly for induction of other Net-1-DCC signaling responses [32]. While DCC-5fbn shows promise for treating both metastatic breast [26] and lung [30] cancers, little is known about the toxicity of this agent [32], a concern that must be addressed in future studies. Although autocrine Net-1 signaling has been linked to cancer, Net and DCC have many normal biological functions [13]. For this reason, if therapies targeting Net-1 or DCC are to be utilized, it may be critical to employ delivery strategies, such as nanoparticles, that limit delivery of the therapeutics to the tumor site.
The three recent investigations of the tumor suppressing functions of DCC highlighted in this review make it clear that loss of DCC can promote tumor progression (Fig. 2d–f). These studies indicate that targeting DCC, rather than the ability of Net-1 to interact with DCC, may in fact advance tumorigenesis in some contexts. In such cases, it may instead be more appropriate to activate the proapoptotic functions of DCC. In support of this, expression of DCC has been found to suppress tumorigenic growth of cells in culture and following transplantation of such cells in nude mice [57, 58]. Studying how signaling is altered in response to cellular loss of DCC may also reveal novel therapeutic approaches. In this regard, given that the genetic tractability of the Drosophila system was vital in providing the first direct link between fra/DCC deficiency and a metastatic phenotype in an animal model for cancer, it is likely that the genetic interaction screen and microarray approaches we are employing to study fra/DCC in the fly will be fruitful.
Acknowledgments
Thanks to members of the lab, especially to Joe Sarro, Charles Tessier, and Ellen Flannery for providing preliminary data and useful comments that helped shape the manuscript. Thank you to Tracy Vargo-Gogola and Zongzhao Zhai for stimulating conversations. Netrin-Fra/DCC research in the Duman-Scheel laboratory has been funded by NIH/NIAID Award R01 AI 081795, and by IUSM Research Enhancement, Notre Dame Genomics and Bioinformatics Core Pilot, and IUPUI Research Support Funds Grant awards to MDS.
Footnotes
CONFLICTS OF INTEREST
The authors confirm that this article content has no conflicts of interest.
References
- 1.Bos JL, Fearon ER, Hamilton SR, et al. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293–7. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–32. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 3.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 4.Roush W. Putative cancer gene shows up in development instead. Science. 1997;276:534–5. doi: 10.1126/science.276.5312.534. [DOI] [PubMed] [Google Scholar]
- 5.Mehlen P, Fearon ER. Role of the dependence receptor DCC in colorectal cancer pathogenesis. J Clin Oncol. 2004;22:3420–8. doi: 10.1200/JCO.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Mehlen P, Furne C. Netrin-1: when a neuronal guidance cue turns out to be a regulator of tumorigenesis. Cell Mol Life Sci. 2005;62:2599–616. doi: 10.1007/s00018-005-5191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chedotal A, Kerjan G, Moreau-Fauvarque C. The brain within the tumor: new roles for axon guidance molecules in cancers. Cell Death Differ. 2005;12:1044–56. doi: 10.1038/sj.cdd.4401707. [DOI] [PubMed] [Google Scholar]
- 8.Takaku K, Oshima M, Miyoshi H, Matsui M, Seldin MF, Taketo MM. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell. 1998;92:645–56. doi: 10.1016/s0092-8674(00)81132-0. [DOI] [PubMed] [Google Scholar]
- 9.Fazeli A, Dickinson SL, Hermiston ML, et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- 10.Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- 11.Kolodziej PA, Timpe LC, Mitchell KJ, et al. frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell. 1996;87:197–204. doi: 10.1016/s0092-8674(00)81338-0. [DOI] [PubMed] [Google Scholar]
- 12.Clemons A, Haugen M, Le C, et al. siRNA-mediated gene targeting in Aedes aegypti embryos reveals that frazzled regulates vector mosquito CNS development. PLoS One. 2011;6:e16730. doi: 10.1371/journal.pone.0016730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duman-Scheel M. Netrin and DCC: axon guidance regulators at the intersection of nervous system development and cancer. Curr Drug Targets. 2009;10:602–10. doi: 10.2174/138945009788680428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishii N, Wadsworth WG, Stern BD, Culotti JG, Hedgecock EM. UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron. 1992;9:873–81. doi: 10.1016/0896-6273(92)90240-e. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell KJ, Doyle JL, Serafini T, et al. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron. 1996;17:203–15. doi: 10.1016/s0896-6273(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 16.Harris R, Sabatelli LM, Seeger MA. Guidance cues at the Drosophila CNS midline: identification and characterization of two Drosophila Netrin/UNC-6 homologs. Neuron. 1996;17:217–28. doi: 10.1016/s0896-6273(00)80154-3. [DOI] [PubMed] [Google Scholar]
- 17.Serafini T, Colamarino SA, Leonardo ED, et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–14. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 18.Keino-Masu K, Masu M, Hinck L, et al. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–85. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- 19.Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans. UNC-6 Cell. 1994;78:409–24. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 20.Brankatschk M, Dickson BJ. Netrins guide Drosophila commissural axons at short range. Nat Neurosci. 2006;9:188–94. doi: 10.1038/nn1625. [DOI] [PubMed] [Google Scholar]
- 21.Cirulli V, Yebra M. Netrins: beyond the brain. Nat Rev Mol Cell Biol. 2007;8:296–306. doi: 10.1038/nrm2142. [DOI] [PubMed] [Google Scholar]
- 22.Kaprielian Z, Runko E, Imondi R. Axon guidance at the midline choice point. Dev Dyn. 2001;221:154–81. doi: 10.1002/dvdy.1143. [DOI] [PubMed] [Google Scholar]
- 23.Hiramoto M, Hiromi Y, Giniger E, Hotta Y. The Drosophila Netrin receptor Frazzled guides axons by controlling Netrin distribution. Nature. 2007;406:886–9. doi: 10.1038/35022571. [DOI] [PubMed] [Google Scholar]
- 24.Mehlen P, Rabizadeh S, Snipas SJ, Assa-Munt N, Salvesen GS, Bredesen DE. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature. 1998;395:801–4. doi: 10.1038/27441. [DOI] [PubMed] [Google Scholar]
- 25.Llambi F, Causeret F, Bloch-Gallego E, Mehlen P. Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. EMBO J. 2001;20:2715–22. doi: 10.1093/emboj/20.11.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitamant J, Guenebeaud C, Coissieux MM, et al. Netrin-1 expression confers a selective advantage for tumor cell survival in metastatic breast cancer. Proc Natl Acad Sci USA. 2008;105:4850–5. doi: 10.1073/pnas.0709810105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazelin L, Bernet A, Bonod-Bidaud C, et al. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature. 2004;431:80–4. doi: 10.1038/nature02788. [DOI] [PubMed] [Google Scholar]
- 28.Bernet A, Fitamant J. Netrin-1 and its receptors in tumour growth promotion. Expert Opin Ther Targets. 2008;12:995–1007. doi: 10.1517/14728222.12.8.995. [DOI] [PubMed] [Google Scholar]
- 29.Delloye-Bourgeois C, Fitamant J, Paradisi A, et al. Netrin-1 acts as a survival factor for aggressive neuroblastoma. J Exp Med. 2009;206:833–47. doi: 10.1084/jem.20082299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delloye-Bourgeois C, Brambilla E, Coissieux MM, et al. Interference with netrin-1 and tumor cell death in non-small cell lung cancer. J Natl Cancer Inst. 2009;101:237–47. doi: 10.1093/jnci/djn491. [DOI] [PubMed] [Google Scholar]
- 31.Flannery E, VanZomeren-Dohm A, Beach P, Holland WS, Duman-Scheel M. Induction of cellular growth by the axon guidance regulators Netrin A and Semaphorin-1a. Dev Neurobiol. 2010;70:473–84. doi: 10.1002/dneu.20788. [DOI] [PubMed] [Google Scholar]
- 32.Mehlen P, Delloye-Bourgeois C, Chedotal A. Novel roles for Slits and netrins: axon guidance cues as anticancer targets? Nat Rev Cancer. 2011;11:188–97. doi: 10.1038/nrc3005. [DOI] [PubMed] [Google Scholar]
- 33.Brumby AM, Richardson HE. Using Drosophila melanogaster to map human cancer pathways. Nat Rev Cancer. 2005;5:626–39. doi: 10.1038/nrc1671. [DOI] [PubMed] [Google Scholar]
- 34.Stefanatos RK, Vidal M. Tumor invasion and metastasis in Drosophila: a bold past, a bright future. J Genet Genomics. 2011;38:431–38. doi: 10.1016/j.jgg.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–37. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 36.VanZomeren-Dohm A, Sarro J, Flannery E, Duman-Scheel M. The Drosophila Netrin receptor frazzled/DCC functions as an invasive tumor suppressor. BMC Dev Biol. 2011;11:41. doi: 10.1186/1471-213X-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vidal M, Warner S, Read R, Cagan RL. Differing Src signaling levels have distinct outcomes in Drosophila. Cancer Res. 2007;67:10278–85. doi: 10.1158/0008-5472.CAN-07-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohsawa S, Sugimura K, Takino K, Xu T, Miyawaki A, Igaki T. Elimination of oncogenic neighbors by JNK-mediated engulfment in Drosophila. Dev Cell. 2011;20:315–28. doi: 10.1016/j.devcel.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2011;24:251–4. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- 40.Cabernard C, Affolter M. Distinct roles for two receptor tyrosine kinases in epithelial branching morphogenesis in Drosophila. Dev Cell. 2005;9:831–42. doi: 10.1016/j.devcel.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–60. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- 42.Srivastava A, Pastor-Pareja JC, Igaki T, Pagliarini R, Xu T. Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proc Natl Acad Sci USA. 2007;104:2721–6. doi: 10.1073/pnas.0611666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bossuyt W, De Geest N, Aerts S, Leenaerts I, Marynen P, Hassan BA. The atonal proneural transcription factor links differentiation and tumor formation in Drosophila. PLoS Biol. 2009;7:e40. doi: 10.1371/journal.pbio.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grzeschik NA, Parsons LM, Richardson HE. Lgl, the SWH pathway and tumorigenesis: It’s a matter of context & competition! Cell Cycle. 2010;9:3202–12. doi: 10.4161/cc.9.16.12633. [DOI] [PubMed] [Google Scholar]
- 45.Speck O, Hughes SC, Noren NK, Kulikauskas RM, Fehon RG. Moesin functions antagonistically to the Rho pathway to maintain epithelial integrity. Nature. 2003;421:83–7. doi: 10.1038/nature01295. [DOI] [PubMed] [Google Scholar]
- 46.Ferres-Marco D, Gutierrez-Garcia I, Vallejo DM, Bolivar J, Gutierrez-Avino FJ, Dominguez M. Epigenetic silencers and Notch collaborate to promote malignant tumours by Rb silencing. Nature. 2006;439:430–6. doi: 10.1038/nature04376. [DOI] [PubMed] [Google Scholar]
- 47.Zhai ZHN, Papgiannouli F, Hamacher-Brady A, Brady N, Sorge S, Bezdan D, Lohmann I. Antagonistic regulation of apoptosis and differentiation by the Cut transcription factor represents a tumor suppressing mechanism in Drosophila. PLoS Genetics. 2012:e1002582. doi: 10.1371/journal.pgen.1002582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castets M, Broutier L, Molin Y, et al. DCC constrains tumour progression via its dependence receptor activity. Nature. 2012;482:534–7. doi: 10.1038/nature10708. [DOI] [PubMed] [Google Scholar]
- 49.Krimpenfort P, Song JY, Proost N, Zevenhoven J, Jonkers J, Berns A. Deleted in colorectal carcinoma suppresses metastasis in p53-deficient mammary tumours. Nature. 2012;482:538–41. doi: 10.1038/nature10790. [DOI] [PubMed] [Google Scholar]
- 50.Fodde R, Smits R, Hofland N, Kielman M, Meera KP. Mechanisms of APC-driven tumorigenesis: lessons from mouse models. Cytogenet Cell Genet. 1999;86:105–11. doi: 10.1159/000015361. [DOI] [PubMed] [Google Scholar]
- 51.Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–25. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 52.Liu X, Holstege H, van der Gulden H, et al. Somatic loss of BRCA1 and p53 in mice induces mammary tumors with features of human BRCA1-mutated basal-like breast cancer. Proc Natl Acad Sci USA. 2007;104:12111–6. doi: 10.1073/pnas.0702969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyamoto Y, Futamura M, Kitamura N, Nakamura Y, Baba H, Arakawa H. Identification of UNC5A as a novel transcriptional target of tumor suppressor p53 and a regulator of apoptosis. Int J Oncol. 2010;36:1253–60. doi: 10.3892/ijo_00000609. [DOI] [PubMed] [Google Scholar]
- 54.McHenry PR, Vargo-Gogola T. Pleiotropic functions of Rho GTPase signaling: a Trojan horse or Achilles’ heel for breast cancer treatment? Curr Drug Targets. 2010;11:1043–58. doi: 10.2174/138945010792006852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dumartin L, Quemener C, Laklai H, et al. Netrin-1 mediates early events in pancreatic adenocarcinoma progression, acting on tumor and endothelial cells. Gastroenterology. 2010;138:1595–606. doi: 10.1053/j.gastro.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 56.Mille F, Llambi F, Guix C, et al. Interfering with multimerization of netrin-1 receptors triggers tumor cell death. Cell Death Differ. 2009;16:1344–51. doi: 10.1038/cdd.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Velcich A, Corner G, Palumbo L, Augenlicht L. Altered phenotype of HT29 colonic adenocarcinoma cells following expression of the DCC gene. Oncogene. 1999;18:2599–606. doi: 10.1038/sj.onc.1202610. [DOI] [PubMed] [Google Scholar]
- 58.Kato H, Zhou Y, Asanoma K, et al. Suppressed tumorigenicity of human endometrial cancer cells by the restored expression of the DCC gene. Br J Cancer. 2000;82:459–66. doi: 10.1054/bjoc.1999.0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paradisi A, Maisse C, Coissieux MM, et al. Netrin-1 up-regulation in inflammatory bowel diseases is required for colorectal cancer progression. Proc Natl Acad Sci USA. 2009;106:17146–51. doi: 10.1073/pnas.0901767106. [DOI] [PMC free article] [PubMed] [Google Scholar]


