Abstract
OBJECTIVES
The objective of this study was to measure the appropriateness of vancomycin monitoring in a pediatric tertiary care center and to evaluate the effectiveness of two interventions, autonomous pharmacy therapeutic drug monitoring and health care provider education, in reducing avoidable pediatric patient trauma and hospital cost.
METHODS
A retrospective chart review evaluating vancomycin therapeutic drug monitoring (TDM) in pediatric inpatients was performed before and after the introduction of an autonomous pharmacy TDM program and health care provider (HCP) education.
RESULTS
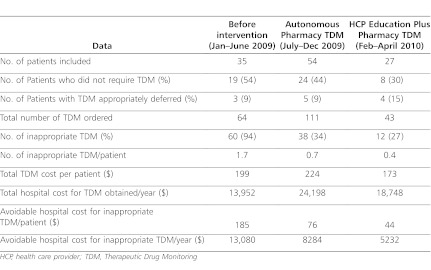
Thirty-five patients were included in our study, prior to any intervention. Of these, 9% of patients had trough concentrations appropriately deferred. Of the total of 64 trough concentrations obtained, 94% were considered to be inappropriate. After the start of the autonomous pharmacy TDM program, of the 54 eligible patients (111 troughs), 9% had trough concentrations appropriately deferred, and 34% were inappropriate. In the 3-month period following the introduction of HCP education in combination with pharmacy TDM, we identified 27 eligible patients. Among those, 15% of the patients had trough concentrations appropriately deferred. Of the 43 trough concentrations obtained, only 9% were considered to be inappropriate. The combination of pharmacy TDM with HCP education decreased annualized hospital cost by 60%, from $13,080 to $5232.
CONCLUSIONS
Inappropriate vancomycin TDM occurs commonly in our institution, resulting in unnecessary hospital cost and patient trauma. The combination of pharmacy TDM and HCP education significantly improved clinical practice; however, results were short-lived. Further interventions, such as computer based order entry, will likely be needed to reinforce and improve long-term TDM practice in pediatric patients.
INDEX TERMS: antibiotic stewardship, therapeutic drug monitoring, vancomycin
INTRODUCTION
The practice of therapeutic drug monitoring (TDM) was developed to maximize therapeutic effect while minimizing adverse effects of select medications.1,2 Increasing evidence suggests that the current use of TDM is suboptimal, with up to 70% to 80% of drug quantifications in hospitalized patients being inappropriately performed, primarily because of routine daily monitoring without pharmacological justification.3,4
Routine vancomycin TDM has been intensely debated,5–9 and scrutiny has resulted in the publication of consensus guidelines in January 2009 by the American Society of Health-System Pharmacists, the Infectious Disease Society of America, and the Society of Infectious Disease Pharmacists.1 These guidelines focused specifically on vancomycin TDM in adult patients. Guidelines recommend vancomycin TDM, with trough concentrations obtained after the fourth dose, for patients at high risk of nephrotoxicity (e.g., concurrent nephrotoxic drugs), those with unstable renal function, and those receiving more than 3 to 5 days of antibiotic. The goal of trough concentrations for complicated infections is between 15 and 20 mg/L, to improve drug penetration and clinical outcomes. Routine monitoring for a short course of vancomycin (3–5 days) in patients with stable renal function is not recommended.1
Appropriate TDM practice is particularly important in children, in whom blood draws lead to physical and emotional distress.10–12 Moreover, greater pain and anxiety responses are observed during subsequent procedures.10–12 Anxiety responses are not limited to the child, they also involve caregivers and medical staff members.10,13 In addition, frequent blood draws have resulted in anemia that requires blood transfusion in critically ill children.14
Effective TDM programs have significantly decreased antimicrobial use and annual hospital costs, as well as improved patient care.15–17 Surprisingly, there is limited pediatric data regarding the practice of vancomycin drug monitoring, with or without the use of antimicrobial stewardship programs.18 A review article by Bates et al19 addressed weaknesses of an autonomous pharmacy TDM alone and stressed the importance of a combined approach that incorporates physician behavior modification, autonomous TDM, and computerized physician ordering. These findings, along with the recent guidelines, prompted us to evaluate vancomycin TDM monitoring practice in our institution.
The primary objective of this study was to measure the appropriateness of vancomycin monitoring in a pediatric tertiary care center. The secondary objective was to evaluate the effectiveness of two interventions, autonomous pharmacy TDM as part of an antimicrobial stewardship program and health care provider (HCP) education, in reducing avoidable pediatric patient trauma and hospital costs.
MATERIALS AND METHODS
This study was performed at the Upstate Golisano Children's Hospital in Syracuse, New York, a 71-pediatric bed, tertiary care teaching hospital. This study was considered exempt by the Institutional Review Board at SUNY Upstate Medical University. Patients eligible for the study were identified through SUNY Upstate Medical University pharmacy records of vancomycin use. Inclusion criteria for the retrospective chart review included patients <19 years old and hospitalization with vancomycin use for >24 hours. All pediatric patients, except premature neonates, were included in the study. The chart review was performed between January 2009 and April 2010 to evaluate whether these interventions improved general TDM practice. This review process included a preintervention period, from January 2009 to June 2009; a postintervention period of TDM, from July 2009 to December 2009; and a postintervention period of HCP education, from February 2010 to April 2010. We recorded information regarding vancomycin dose and interval, sample timing, and trough concentrations.
Prior to intervention, vancomycin dosing, frequency, and venipuncture-obtained trough concentrations were ordered by the pediatric resident physician, whereas the pediatric nurse was responsible for the timing of the sample collection. The resident and attending physician were then responsible for interpreting trough concentrations and determining appropriate medication changes with frequent but not universal pediatric pharmacist consultations. In July 2009, the pharmacy TDM program was initiated as part of our antimicrobial stewardship program on all pediatric floors, with the exception of the pediatric intensive care unit (PICU). The PICU faculty's resident education philosophy maintains that residents should receive input from consulting services but retain prescribing responsibilities for all aspects of patient care. Thus, while pharmacists participated in the care of PICU patients, the management differed from that of non-ICU patients.
The TDM program, which occurs 24 hours a day, 7 days a week, allowed for autonomous management of vancomycin regimens by pharmacists, according to an internal management protocol. Pharmacists used the following management principles: regimens with subtherapeutic trough concentrations were first optimized to a shorter dosing interval (i.e., every 8 hours to every 6 hours); for 6-hours regimens with subtherapeutic trough concentrations, the dose was increased proportionately to the desired trough concentration increase but in increments no greater than 5 mg/kg (i.e., 15 mg/kg every 6 hours was increased to 20 mg/kg every 6 hours); and regimens with supratherapeutic trough concentrations not attributable to sampling error in a renally stable patient involved either a reduction in dosing interval or reduction in dose, depending on the magnitude of the supratherapeutic concentration. “Pulse” dosing was used in patients with changing renal function at the clinical discretion of the pharmacist. In patients with impaired vancomycin clearance and evidence of worsening renal function for whom a fixed dosing schedule was felt not be reliable, pharmacists would use single doses of vancomycin followed by serum concentration monitoring to determine subsequent doses/intervals.
Pharmacists were previously trained and demonstrated competency in vancomycin TDM. Upon implementation of this program, the pediatric resident physician continued to order the initial vancomycin dose, frequency, and trough concentration, and the pediatric nurse was still responsible for the timing of the collection. Pediatric pharmacists prospectively reviewed prescribed regimens and trough concentration orders, advising changes for suboptimal regimens or rescheduling of trough concentrations. These changes were concomitantly communicated to the pediatric resident physician, and documented in the patient's medical record. However, in the PICU, the procedure remained the same, with the resident and attending physicians determining further vancomycin TDM and dose changes with consultations by pharmacists but without autonomous pharmacist management.
In January 2010, HCP education included a lecture to all pediatric and affiliated residents in training (postgraduate year 1 [PGY1]–PGY3) about appropriate vancomycin TDM. The lecture also included a pretest and posttest to document understanding of the material discussed. The pre- and posttests consisted of four identical questions (Figure 1). The proportions of correct answers before and after the educational intervention were calculated and compared.
Figure 1.
The Pre-Test and Post-Test Given to Health Care Providers at Time of Vancomycin Therapeutic Drug Monitoring Education.
Vancomycin trough monitoring was considered appropriate when the following criteria were met: serum trough concentrations were obtained with or after the fourth dose for infections with documented culture results and susceptibilities requiring the use of vancomycin for >72 hours; patients with renal dysfunction, patients concurrently receiving nephrotoxic drugs, clinically unstable patients who had a minimum of two consecutive documented increases in serum creatinine concentration of 0.5 mg/dL or of ≥50% increase from baseline; and once weekly for hemodynamically stable patients on vancomycin for more than 3 days. Clinically stable inpatients receiving empirical vancomycin therapy for less than 72 hours who did not have TDM were considered to have trough concentrations appropriately deferred. Inappropriate monitoring included samples obtained for peak concentrations, trough concentrations obtained within 24 hours of antibiotic discontinuation, trough concentrations obtained prior to culture results in clinically stable patients, trough concentrations collected prior to one hour before the fourth dose, and trough concentrations collected within a week of a therapeutic trough concentration without change in renal function or addition of a nephrotoxic medication.
The vancomycin TDM cost of $109 was estimated by including the cost of phlebotomy ($40 per draw) and cost of vancomycin trough measurement ($69 per concentration measurement). Analyses included a descriptive phase to assess the frequencies of all variables and an analytic phase to evaluate associations between the two interventions (autonomous pharmacy monitoring and HCP education) and vancomycin trough monitoring practice. The presence of appropriately collected trough concentrations was expressed as proportions. The Pearson χ2 test was used to assess categorical variable associations; all hypotheses were two-tailed with a p value of ≤0.05 considered statistically significant.
RESULTS
Vancomycin TDM Data for Hospitalized Pediatric Patients (Excluding Those in the PICU)
In the 6 months prior to any intervention, a total of 64 trough concentrations were obtained from the 35 patients who met inclusion criteria for this study. Inappropriate TDM was documented in 19 patients (54%), and 3 patients (9%) had trough concentrations appropriately deferred. After the start of the autonomous pharmacy TDM program, a total of 111 trough concentrations were obtained from 54 patients. Twenty-four patients (44%) had inappropriate TDM, while 5 patients (9%) had TDM appropriately deferred. After HCP education was added to the autonomous pharmacy TDM program, 43 trough concentrations were obtained from 27 patients included in the study. Of these, 8 patients (30%) had an inappropriate TDM, and 4 (15%) patients had trough concentrations appropriately deferred (Table 1).
Table 1.
Results from Vancomycin TDM Among Pediatric Patients Admitted to the Pediatric Inpatient Unit at Upstate Golisano Children's Hospital in Syracuse, NY
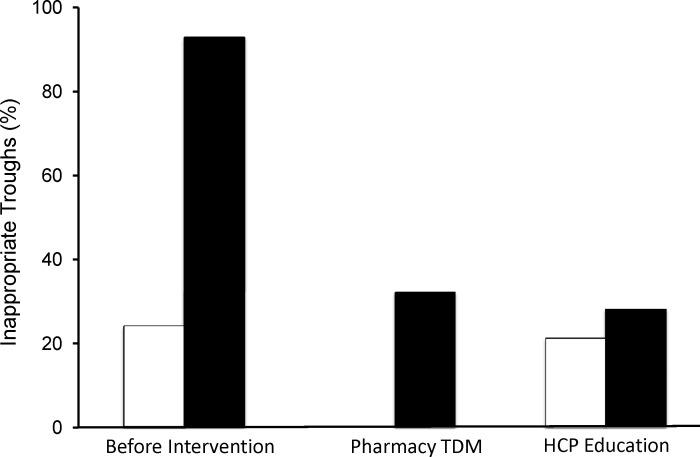
Prior to any intervention, 94% of all trough concentrations obtained were inappropriate. This proportion decreased to 34% after the initiation of the autonomous pharmacy TDM (p<0.001) (Figure 1). The combination of HCP education and pharmacy TDM further reduced the proportion of inappropriate trough concentrations to 27% (p<0.001), thereby significantly decreasing inappropriate venipunctures and trauma to the patient (Figure 2). Prior to intervention, the cost of inappropriate TDM performed was $199 per patient, which decreased to $173 per patient with the combination of autonomous pharmacy TDM program and HCP education. With these interventions, the annualized avoidable hospital cost due to inappropriate vancomycin TDM decreased 60%, from $13,080 to $5,232.
Figure 2.
Proportion of Inappropriate Vancomycin Therapeutic Drug Monitoring.
White bar = patients in the Pediatric Intensive Care Unit (Before Intervention and After HCP Education)
Black bar = patient in the Pediatric Inpatient Units (Before Interventions, After the Start of Autonomous Pharmacy
TDM, and then After HCP Education Was Added to the Pharmacy TDM program)
Vancomycin TDM Data for PICU Patients
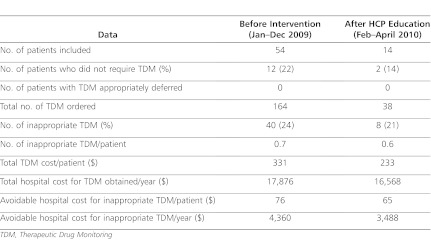
Prior to the interventions, a total of 164 trough concentrations were obtained from the 54 PICU patients who met inclusion criteria and were included in the study (Table 2). After HCP education was performed, there were 38 trough concentrations obtained from 14 PICU patients included in the study. For the entire study period, there were 14 patients who did not require vancomycin TDM (12 prior to HCP education and 2 after intervention). There were no PICU patients who had trough concentrations appropriately deferred.
Table 2.
Results from Vancomycin TDM Among PICU Patients Admitted to the Upstate Golisano Children's Hospital in Syracuse, NY
Prior to intervention, 24% of all trough concentrations obtained were inappropriate. This proportion decreased to 21% after HCP education was performed (p=0.6). Prior to intervention, the cost of inappropriate TDM performed was $331 per patient, which decreased to $233 per patient with the combination of autonomous pharmacy TDM program with HCP education. With these interventions, the annualized avoidable hospital cost due to unnecessary vancomycin TDM decreased by 20%, from $4,360 to $3,488, after the intervention of HCP education. The proportion of avoidable cost for vancomycin TDM also decreased significantly from 24% to 21% (p<0.0002).
Health Care Provider Education
HCP education was performed in January 2010, and comprehension of the material was documented by pre- and post-test evaluation (Figure 1). The cumulative score of correct answers, prior to the education of 28 HCP was 52%. After the intervention, this score significantly increased by 45% (95% confidence interval, 35%–55%) to 97% after the educational intervention.
DISCUSSION
In hospitalized pediatric patients, inappropriate vancomycin TDM increased pediatric trauma and hospital costs in our institution. Our data have shown that up to 94% of vancomycin TDM at our institution was inappropriate prior to any intervention, resulting in avoidable patient trauma and hospital costs of $13,080 per year. Education of HCP alone has little effect in changing provider's prescribing patterns which was demonstrated by the data collected from the patients admitted to the PICU. However, education in combination with an autonomous pharmacy TDM program significantly reduced inappropriate vancomycin TDM to 27% and avoidable hospital cost by 60%, to $5,232.
Bates et al19 described strategies for improving TDM practices, including HCP education, which was found to be effective in decreasing the frequency of vancomycin TDM by approximately 60%. The concern with implementing HCP education alone is that while the physician prescribing change is effective, it is only short term, and the effect of education wanes over time.15,19 This is especially true at training hospitals, such as ours, due to the frequent turnovers of HCPs.
Multispecialty antimicrobial stewardship programs have been developed to optimize clinical outcomes and decrease unwanted effects of antimicrobials, including excess cost.15 Effective programs have been shown to decrease annual hospital costs of between $200,000 and $900,000.15 Similarly, the autonomous pharmacy TDM, a major aspect of the antimicrobial stewardship program in our institution, has been demonstrated to decrease hospital costs associated with vancomycin TDM. However, there is still improvement to be made.
In a time of advancing technology, the computerized physician order entry systems used by many facilities may be a more effective method of intervention at the time of antibiotic prescribing. Bates et al19 found that the use of a reminder on the computer screen when ordering vancomycin was enough to maintain appropriate TDM practice 1 year after HCP education.
While TDM is both clinically and economically beneficial, there is increasing evidence that current practice is inappropriate, as a significant proportion of serum drug concentrations are obtained without pharmacologic justification.19 In a time of significant financial burden, it is imperative to optimize the cost-effectiveness of practices such as TDM. We have shown that there is an excessive amount of vancomycin TDM in our hospital. Reduction of inappropriate TDM, through a combination of HCP education and pharmacy TDM, decreases the number of venipunctures performed thus reducing pediatric patient trauma as well as hospital cost. Previously reported data suggest that the effect of HCP education is temporary and requires further reinforcement, but this has not been formally evaluated at our institution. Further long-term interventions, such as computer-based changes, should be used to reinforce and optimize appropriate TDM practice.
ABBREVIATIONS
- HCP
health care provider
- PICU
pediatric intensive care unit
- TDM
therapeutic drug monitoring
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health-Syst Pharm. 2009;66(1):82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 2.Iwamoto T, Kagawa Y, Kojima M. Clinical efficacy of therapeutic drug monitoring in patients receiving vancomycin. Biol Pharm Bull. 2003;26(6):876–879. doi: 10.1248/bpb.26.876. [DOI] [PubMed] [Google Scholar]
- 3.Schoenenberger RA, Tanasijevic MJ, Jha A, et al. Appropriateness of antiepileptic drug level monitoring. JAMA. 1995;274(20):1622–1626. [PubMed] [Google Scholar]
- 4.Canas F, Tanasijevic M, Ma'Luf N, et al. Evaluating the appropriateness of digoxin level monitoring. Arch Intern Med. 1999;159(4):363–368. doi: 10.1001/archinte.159.4.363. [DOI] [PubMed] [Google Scholar]
- 5.Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006:S35–S39. doi: 10.1086/491712. 429SUPPL 1) [DOI] [PubMed] [Google Scholar]
- 6.Hammett-Stabler CA, Johns T. Laboratory guidelines for monitoring of antimicrobial drugs. Clin Chem. 1998;44(5):1129–1140. [PubMed] [Google Scholar]
- 7.Moellering RC., Jr Monitoring serum vancomycin levels: climbing the mountain because it is there? Clin Infect Dis. 1994;18(4):544–546. doi: 10.1093/clinids/18.4.544. [DOI] [PubMed] [Google Scholar]
- 8.Stevens DL. The role of vancomycin in the treatment paradigm. Clin Infect Dis. 2006;42(Suppl 1):S51–S57. doi: 10.1086/491714. [DOI] [PubMed] [Google Scholar]
- 9.Darko W, Medicis JJ, Smith A. Mississippi mud no more: cost-effectiveness of pharmacokinetic dosage adjustment of vancomycin to prevent nephrotoxicity. Pharmacotherapy. 2003;23(5):643–650. doi: 10.1592/phco.23.5.643.32199. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy RM, Luhmann J, Zempsky WT. Clinical implications of unmanaged needle insertion pain and distress in children. Pediatrics. 2008;122:S130–S133. doi: 10.1542/peds.2008-1055e. (Suppl 3) [DOI] [PubMed] [Google Scholar]
- 11.Fradet C, McGrath PJ, Kay J, et al. A prospective survey of reactions to blood tests by children and adolescents. Pain. 1990;40(1):53–60. doi: 10.1016/0304-3959(90)91050-S. [DOI] [PubMed] [Google Scholar]
- 12.Humphrey GB, Boon CM. van Linden van denHeuvell GF, van de Wiel HB. The occurrence of high levels of acute behavioral distress in children and adolescents undergoing routine venipunctures. Pediatrics. 1992;90(1):87–91. [PubMed] [Google Scholar]
- 13.Smith RW, Shah V, Goldman RD, Taddio A. Caregivers' responses to pain in their children in the emergency department. Arch Pediatr Adolesc Med. 2007;161(6):578–582. doi: 10.1001/archpedi.161.6.578. [DOI] [PubMed] [Google Scholar]
- 14.Bateman ST, Lacroix J, Boven K. et al for the Pediatric Acute Lung Injury and Sepsis Investigators Network. Anemia, blood loss, and blood transfusions in North American children in the intensive care unit. Am J Respir Crit Care Med. 2008;178(1):26–33. doi: 10.1164/rccm.200711-1637OC. [DOI] [PubMed] [Google Scholar]
- 15.Dellit TH, Owens RC, McGowan JE, Jr, et al. Infectious Disease Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 16.Welty TE. Impact of vancomycin therapeutic drug monitoring on patient care. Ann Pharmacother. 1994;28(12):1335–1339. doi: 10.1177/106002809402801201. [DOI] [PubMed] [Google Scholar]
- 17.Bond CA, Raehl CL. Clinical and economic outcomes of pharmacist-managed aminoglycoside or vancomycin therapy. Am J Health-Syst Pharm. 2005;62(15):1596–1605. doi: 10.2146/ajhp040555. [DOI] [PubMed] [Google Scholar]
- 18.Chicella M, Adkins J, Mancao MY, et al. Impact of pediatric specific guidelines for vancomycin serum concentration monitoring on patient care. J Pediatr Pharm Pract. 1999;4:146–151. [Google Scholar]
- 19.Bates DW, Soldin SJ, Rainey PM, Micelli JN. Strategies for physician education in therapeutic drug monitoring. Clin Chem. 1998;44(2):401–407. [PubMed] [Google Scholar]