Abstract
OBJECTIVES
Pharmacies encounter challenges when ensuring safe, timely medication dispensing to patients in the pediatric intensive care unit, when high-alert medications are needed in emergent situations. Removal of these medications from nursing stock presented challenges to providing timely administration to critical patients. The project's purpose was to develop a new method for reducing dispensing time while improving patient safety in pediatric intensive care units.
METHODS
A committee of physicians, nurses, a clinical pharmacist, and pharmacy administration collaborated for process development. The process established a list of compounded, ready-to-use infusions stored in the pharmacy, immediately available for dispensing. The dispensing mechanism includes ordering and dispensing processes using an “Urgent Drip Request” form. Most frequently ordered infusions (dopamine, epinephrine, norepinephrine) were added to automated dispensing cabinets in critical care units in concentrations that could be safely infused centrally or peripherally.
RESULTS
During the initial 4 months, 71 “Urgent Drip Request” sheets were processed. Drug utilization evaluation demonstrated a dispensing time of less than 1 minute for drip medications leaving the pharmacy after the form was received. No sheets processed exceeded the institutional 30-minute turnaround time, nor were errors or delays documented. Limited turnaround time data existed preimplementation but was not robust enough for analysis. It was not ethically feasible to perform a head-to-head comparison with the previous method, as it might have resulted in delay of therapy and negative patient outcomes.
CONCLUSIONS
This program allows high-alert medication infusion availability in an expedited manner, removes potential for compounding errors at the bedside, and assures clean room preparation. This has improved pharmacy efficiency in provision of safe patient care to critically ill pediatric patients.
INDEX TERMS: emergency medications, high-alert medications, medication safety, patient safety, pediatric
INTRODUCTION
Hospital pharmacies encounter many challenges when trying to ensure safe and timely medication dispensing to patients they serve. Processes must meet the challenge of providing the correct medication, prepared accurately and delivered in a time-frame suitable to the individual patient situation, without compromise to other patients. This is especially true in the pediatric intensive care unit (PICU), where a number of high-risk medications are needed immediately in emergent situations. The Joint Commission has identified a National Patient Safety Goal to “improve the safety of using high-alert medications.”1 The Institute for Safe Medication Practices has identified a number of medications as high-alert including adrenergic agents (epinephrine, norepinephrine) and concentrated electrolyte products (3% sodium chloride). In response to this Joint Commission goal, our institution removed these medications from all inpatient nursing units throughout the hospital so they could be obtained only from the pharmacy with a patient-specific order in the computerized physician order entry (CPOE) system. However, this presented a challenge to physicians, pharmacists, and nurses in providing timely administration of life-saving medications to critically ill patients. A new method that reduced the medication dispensing time while improving patient safety was necessary. The purpose of this project was to develop a process that would provide patients with high-alert, vasoactive medications in a timely and safe manner.
METHODS
This institution is a 296-bed pediatric hospital with a 36-bed PICU, a 12-bed cardiac intensive care unit (CICU), and a 31-bed neonatal intensive care unit (NICU). A multidisciplinary PICU leadership team identified the turnaround of vasoactive infusions to be an area in need of improvement, based upon limited data collected over a 1-week period. A task force was formed consisting of pharmacy administration, a clinical pharmacist, nurses, and critical care physicians. The team met on a weekly basis to work in partnership on the details of developing a process that maintained immediately available vasoactive infusions to the physician and nursing staff while improving patient safety and compliance with regulations. The new process would need to include medications to be compounded and secured by the pharmacy. A two-part approach was then taken to address the issue. First, compounded, ready-to-use infusions needed to be available for immediate dispensing and administration. The second step was to establish the mechanism by which the medications would be dispensed. The process was implemented after eight weekly, 1-hour collaborative sessions.
Because this is a pediatric facility, infusions are dispensed and administered in previously established, institution-standard concentrations that accommodate the type of intravenous access (central vs. peripheral) and size/weight of the patient. It was determined that the pharmacy department would need to stock a small supply (par level = 2) of each medication and concentration. Available options consisted of dobutamine, dopamine, epinephrine, esmolol, milrinone, nitroprusside, and norepinephrine. All infusions are prepared using aseptic technique in the horizontal laminar airflow hood located in the pharmacy's clean room, reducing the risk of a contaminated product. Syringes are stored in labeled bins in the main pharmacy refrigerators with consistent generic stock labeling that includes drug name, diluents, concentration, and expiration dating. When a syringe is dispensed, the dispensing pharmacist prepares replacement stock as soon as possible. The night-shift pharmacist is responsible for monitoring expiration dates and replacing outdated supplies to maintain par levels of these agents. (Expiration dating had been determined previously, depending upon medication stability and United States Pharmacopeia <797> regulations.)
Developing a process for dispensing vasoactive infusion syringes was more difficult. Because the infusions would be dispensed from the pharmacy department on a patient-specific basis instead of being dispensed as floor stock, an order was required legally. The logistics of communication of the order to the pharmacy department, labeling of the syringe, and transportation of the syringe to the bedside all needed to be ascertained.
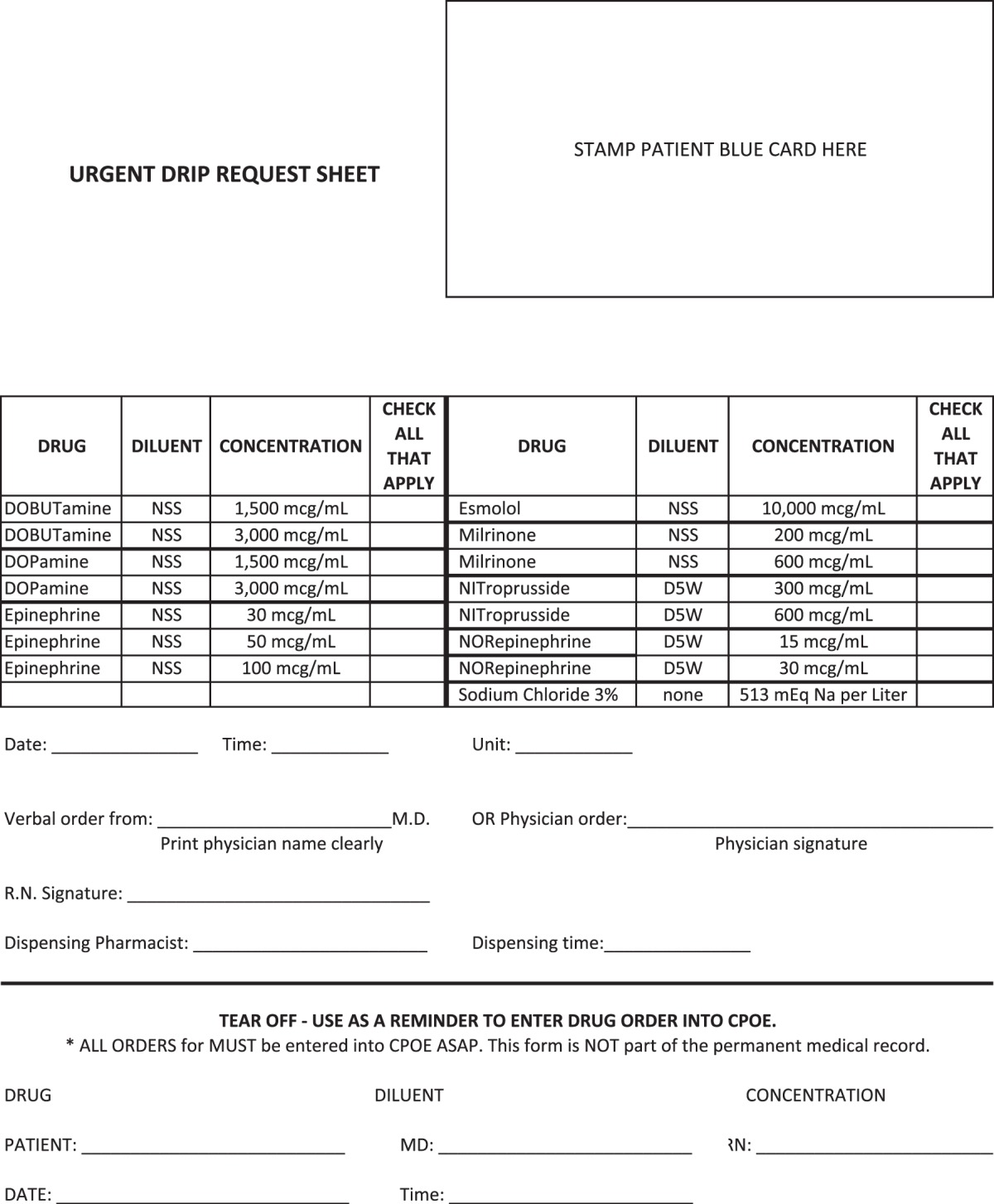
The process that was developed is described as follows. The critical care physician determines that there is an emergent situation (e.g., resuscitation from shock or a cardiac arrest) in which a patient requires one or more of the above-listed agents more quickly than usual pharmacy workflow would permit. The physician completes the “Urgent Drip Request” paper order form (Appendix), or the nursing staff is permitted to take verbal orders if needed. The form lists all of the available options. Completion of this form includes application of demographic addressograph sticker (includes patient name, medical record number, and date of birth), marking the box by the appropriate agent(s) and concentration(s), notation of date and time, and signature of ordering physician or designee. A phone call is placed to the pharmacy department to alert staff members that there is an order. The delivery method is arranged at that time. The form is then submitted to the pharmacy by a facsimile machine, pneumatic tube system, or by the pharmacy medication delivery assistant (MDA). The stock infusion is immediately dispensed to the intensive care unit either by pneumatic tube or delivery by the pharmacy MDA.
The physician must enter an order into the CPOE system as soon as the situation permits. The dispensing pharmacist verifies the order and dispenses a new infusion syringe by using the patient-specific infusion label generated. If the dispensing pharmacist determines for any reason that an order will not be placed by the physician (verbal communication, patient demise, or other reason) or that the medication will not continue beyond the emergency situation, then an order is entered for the limited purposes of documentation and generation of a charge on the patient's account. The entry is short-dated in order to avoid an active order on the patient's profile. Table 1 summarizes the existing policy.
Table 1.
Summary of Pharmacy Department Policy and Procedure
RESULTS
This program has been successful in the improvement of emergency medication turnaround time. Current hospital policy requires that any medication ordered through the CPOE system designated as statum (STAT) at this institution must have a promised time of 30 minutes from time of order placement to delivery to patient location. Prior to implementation of the new process, a limited turnaround time audit was performed over a 7-day period of STAT vasoactive infusion orders (n = 12). The time between order entry and product delivery exceeded 30 minutes for 25% of ordered infusions and exceeded 60 minutes for 16.7%. Only 58.4% of orders audited were delivered within the institution standard of 30 minutes.
More extensive drug use evaluation was conducted after commencement of the program. In the first 4 months after program inception, a total of 71 “Urgent Drip Request” sheets were processed by the pharmacy. An audit of these requests was performed, and it was observed that it took less than 1 minute for all drip medications to leave the pharmacy after the “Urgent Drip Request” sheet was received by pharmacy personnel (time was recorded on the sheet when the sheet was received by pharmacy and again when infusions left the pharmacy). Because of the emergent nature of these situations, there has not been a way to consistently and effectively record the exact time the infusion reached the patient's bedside. No medication errors or delays in therapy have been reported since the program's initiation in August 2007.
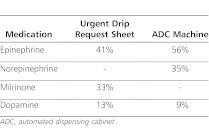
As the program has evolved, further evaluation has occurred. Since the program's inception, a modification has been made to improve rapid availability of dopamine, epinephrine, and norepinephrine through the addition of these ready-to-use infusions to the automated dispensing cabinets (ADCs) in all critical care units in the hospital. In 2009, our institution relocated to a newer, much larger campus. To ensure that the expanded sizes of the hospital building and critical care units would not negatively affect the efficient delivery of drips from the pharmacy to the patient's bedside, the program was reevaluated 6 months prior to the hospital move and for the first 6 months at the new campus. The program continues to be used frequently. In the 12- month period from August 1, 2010, to August 1, 2011, there were a total of 535 high-alert infusions dispensed, combined from ADCs and the pharmacy department stock. The pharmacy received 22 “Urgent Drip Request” sheets and supplied patients with 31 infusions. In this same time period, 504 high-alert infusions were removed from ADCs in the critical care units. Of these, 447 were removed from the PICU, 34 were removed from the NICU, and 23 were removed from the CICU. The medications most frequently requested are listed in Table 2.
Table 2.
Most Frequently Requested Infusions During August 2010 Through August 2011 Audit
An informal survey in 2010 of the Medical Director of the PICU and the nursing administrative staff revealed continued satisfaction with the current process. The pharmacists and pharmacy director are enthusiastic about the program. There has been no further process improvement opportunities suggested to this point.
DISCUSSION
The creation of the “Urgent Drip Request” sheet provides patients with high-alert, vasoactive medications in emergency situations. This form is available on the intensive care units and can be completed by the physician. All infusions listed on the sheet are already prepared and stored in the refrigerator in the pharmacy for immediate dispensing upon receipt of the drip request sheet. Through implementation of this process, the need for nurse preparation of these drips in the unit has been eliminated, stocks of high-risk medications have been removed from the intensive care units, and these agents are reliably prepared at standard concentrations in an environment that is compliant with United States Pharmacopeia regulations <797>.
In the time since program inception, a modification has been made to improve rapid availability of emergent infusions in the intensive care units. The most frequently ordered infusions were determined to be dopamine, epinephrine, and norepinephrine. These three agents have been made available in the ADCs in all critical care units in the hospital. All three agents are prepared at concentrations that can be safely infused through a central or peripheral line. When one of these agents is removed from the ADC for a patient, a medication order and a charge are automatically generated in that patient's profile in the CPOE system. The pharmacy technicians assigned to refill the ADCs are charged with maintaining par levels, auditing expiration dating, and restocking syringes as necessary.
As discussed earlier, the three most commonly used vasoactive infusions, dopamine, epinephrine, and norepinephrine, have been made available in the ADCs in all critical care units in the hospital. A problem resulted from variation in the method of restocking the ADC with these infusions. All epinephrine syringes in the PICU were set to expire on the same day; the technician responsible for restocking the machines removed all epinephrine syringes from all ADCs located in the PICU with the intention of replacing them later that day. Shortly afterward, a PICU patient required one of these syringes immediately and there was none available in the unit. However, the “Urgent Drip Request” sheet was still available, and the syringe was supplied from pharmacy's premade stock with no significant delay in therapy. To remedy this situation, technicians were instructed never to remove syringes from ADCs without having replacements with them for immediate restocking. Now assessment and replenishing of stock of these infusions is performed several times daily at preset times and this type of incident has not recurred.
In addition to moving the most commonly used vasoactive infusions to the ADCs, an additional modification has occurred. The PICU serves neurotrauma patients who may require an immediate bolus or infusion of 3% sodium chloride for prevention of secondary brain injury. Our institutional practice is to dispense all 3% sodium chloride intravenous orders in compounded 50-ml syringes for use on infusion pumps as a patient safety measure. However, the neurotrauma physicians determined that the time required for preparation of these syringes may be potentially harmful to their patients. To rectify this problem, a stock infusion of 3% sodium chloride was added to the “Urgent Drip Request” sheet to provide rapid availability of this electrolyte.
This new emergency infusion process has shifted the responsibility of drug preparation from the nursing staff to the pharmacy, which benefits patient safety. Avoidance of preparation of adrenergic infusions on the units eliminates the chance of calculation and compounding errors by nursing staff and physicians. It has been demonstrated that any time nurses are forced to rush through medication preparation, they are more prone to calculation errors,2 especially in critical care units, where patients are prescribed twice as many medications as those outside of the intensive care unit.3 Pharmacists have the opportunity to improve patient safety by preparing all intravenous medications within the pharmacy department in a routine time frame. As pharmacy personnel have received specialized training in medication compounding, preparation of stock infusion replacements within the pharmacy represents a lower risk for compounding errors compared to preparation of infusions on the patient care unit. Pharmacy technicians are able to focus their attention on working at a reasonable pace, using standard compounding instructions. Use of standardized processes and standardized medication concentrations has been shown to improve safety.3 Preparation of drugs in the pharmacy clean room using aseptic technique reduces the risk of contaminated parenteral solutions.
This method of providing critical care units with premade vasoactive infusions for emergent situations is a truly innovative program. No published literature exists on this type of process. The vasoactive medications needed in these emergent situations are often available only in concentrations formulated primarily for adults.4 Patients at this institution range from large adult size to neonates that weigh less than one kilogram. This specialized patient population requires extra calculations and dilutions that significantly increase the possibility of error,4 and by removing these responsibilities from physicians and nurses, it may be concluded improvements have been made to patient safety and care through usage of standardized medication processes as described and recommended in the literature. It is also thought that the improvement in turnaround time demonstrated by the data collected in this project could have a positive effect on clinical outcomes.
While it is difficult to quantify medication errors due to inconsistencies in reporting, it has been long demonstrated that error rates in pediatric patients are even higher than those in adults, and have even been reported to be as high as 1 in 6.4 orders.5 A number of factors contribute to these higher error rates, including the complexity of calculating pediatric dosing, the extra steps required in medication preparation, and the inability of children, especially those that are very young, to effectively communicate adverse drug events to healthcare providers.4 Because these children are still developing, they often have fewer internal reserves to tolerate even the smallest errors.6 Process change recommendations that have been demonstrated to improve patient safety include use of CPOE and involvement of clinical pharmacists in patient care decisions,3 all of which have been implemented in this institution. In addition, there are recommendations to limit and standardize concentrations of high alert medications, which are being accomplished by this program.
Although data are scarce regarding errors made during critical care emergency medical situations, Kozer et al7 demonstrated the high potential for medication errors during resuscitation in a pediatric emergency department. A number of mock resuscitations were simulated by teams of doctors and nurses who frequently participated in real resuscitations. Sessions were recorded, and then observed for potential medication errors. Syringes prepared during simulation were also collected and analyzed for concentration and drug amounts. Medication errors were identified in seven of the eight mock resuscitations.7 Errors varied to include incomplete orders by physicians (i.e., medications verbally ordered without a specific dose), dosing errors, errors in preparation and administration, and variations in drug concentration in syringes prepared during the simulation.7 This study once again demonstrates the increased probability for errors in a high-pressure, high-stress environment that exists during a medical emergency. In this particular study, 16% of syringes analyzed deviated at least 20% from the expected dose.7 It can be assumed that in an actual emergency situation, there is an even higher incidence of medication errors than previously estimated because concentrations of drugs administered are never analyzed. It has been estimated that between 80 and 200 steps may be associated with the administration of a single dose of medication in a hospital.8 Providing these vasoactive medications at a variety of ready-to-use concentrations eliminates a series of steps where errors frequently occur.
Limitations associated with this project include a lack of preliminary data collected prior to the implementation of the program. When the turnaround time data regarding STAT infusions was identified as an issue in the aforementioned 7-day audit, the collaborative group felt that efforts were best served by focusing on the process change in an expedited manner rather than delving further into more extensive data collection studies, due to the nature of the medications. It is not ethically feasible to measure and compare the previous method of providing vasoactive medications to patients in a head-to-head trial, as it could result in delay of therapy and negative patient outcomes; however, it is suspected that the availability of additional preimplementation data could have demonstrated a greater impact of the process change and program value.
Another notable limitation is that the improvement in patient safety as discussed can only be described as a theoretical one. The actual patient risks incurred with nurses preparing syringes at the bedside in an emergency situation was not evaluated during this project. There have been no reported errors in our institution involving the preparation of infusions by the nurse under urgent conditions; however, this is most likely because the error may have not been realized due to the nature of the critical situation occurring at the time. There was no process in place to analyze the composition of those preparations.
The last limitation was the instance when expiring infusion syringes were removed from the ADC machines prior to their replacement. This type of situation had never been previously encountered. While the nursing personnel and pharmacists received education and training, the pharmacy technicians received minimal instruction regarding the restocking process. This was resolved with additional training.
CONCLUSIONS
This innovative pharmacy program addresses a challenge that hospital pharmacies often encounter. It allows for high-alert medication infusions to be available in an expedited manner upon receipt of an “Urgent Drip Request” sheet and removes the potential for a medication compounding error in a high-stress environment by eliminating nurse preparation of infusions at the patient's bedside. As a final point, it ensures that the product is prepared in a clean environment. This process is prospectively monitored and modifications are made as warranted. Changes that have been implemented since the program's inception include the addition of 3% sodium chloride syringes to the “Urgent Drip Request” sheet and the addition of frequently used agents (epinephrine, norepinephrine, and dopamine) to ADCs in all critical care units.
The program has been successful because it addresses the physician's desire for the immediate availability of urgently needed medications while at the same time maintaining the patient safety goals set by the hospital and regulatory agencies. This new process uses compounded, ready-to use vasoactive infusions at various concentrations and a mechanism for dispensing them. This program has improved pharmacy efficiency in the provision of safe patient care to critically ill pediatric patients.
ACKNOWLEDGMENT
We would like to posthumously acknowledge Marc Fry, BScPharm, for his efforts as a Lead Pharmacist during the design and implementation of this process.
ABBREVIATIONS
- ADC
Automated Dispensing Cabinet
- CICU
Cardiac Intensive Care Unit
- CPOE
Computerized Physician Order Entry
- ICU
Intensive Care Unit
- MDA
Medication Delivery Assistant
- NICU
Neonatal Intensive Care Unit
- PAR
the level at which stock is replenished
- PICU
Pediatric Intensive Care Unit
- STAT
statum
Appendix. Urgent Drip Request Form
Appendix.
Urgent Drip Request Form
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.VA National Center for Patient Safety – TIPS website. 2012 http://www.patientsafety.gov/TIPS/tips.html. Accessed July 24, (Discussed in Volume 2 Issue 5; Volume 3 Issue 4; Volume 5 Issue 1; Volume 6 Issue 1) [Google Scholar]
- 2.Ozkan S, Kocaman G, Ozturk C, et al. Frequency of pediatric medication administration errors and contributing factors. J Nurs Care Qual. 2011;26(2):136–143. doi: 10.1097/NCQ.0b013e3182031006. [DOI] [PubMed] [Google Scholar]
- 3.Moyen E, Camiré E, Stelfox HT. Clinical review: medication errors in critical care. Crit Care. 2008;12(2):208–214. doi: 10.1186/cc6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sentinel event alert: preventing pediatric medication errors. The Joint Commission. (39) Issue. April 11, 2008. http://www.jointcommission.org/assets/1/18/SEA_39.PDF. Accessed July 14, 2012. [PubMed] [Google Scholar]
- 5.Stucky ER. American Academy of Pediatrics Committee on Drugs; American Academy of Pediatric Committee on Hospital Care. Prevention of medication errors in the pediatric inpatient setting. Pediatrics. 2003;112(2):431–436. doi: 10.1542/peds.112.2.431. [DOI] [PubMed] [Google Scholar]
- 6.Kaushal R, Bates D, Landrigan C, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285(16):2114–2120. doi: 10.1001/jama.285.16.2114. [DOI] [PubMed] [Google Scholar]
- 7.Kozer E, Seto W, Verjee Z, et al. Prospective observational study on the incidence of medication errors during simulated resuscitation in a paediatric emergency department. BMJ. 2004;329(7478):1321–1326. doi: 10.1136/bmj.38244.607083.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pharmacy-nursing shared vision for safe medication use in hospitals: Executive session summary. Am J Health Syst Pharm. 2003;60(10):1046–1052. doi: 10.1093/ajhp/60.10.1046. [DOI] [PubMed] [Google Scholar]