Abstract
Gene therapy of heart failure is gaining momentum as a result of the recent successful completion of phase II of the CUPID trial, which showed clinical safety and efficacy of an adeno-associated viral vector expressing SERCA2a. Resorting to gene therapy allows the manipulation of molecular targets not presently amenable to pharmacologic modulation. This review focuses on the molecular targets of heart failure gene therapy that have demonstrated translational potential. At present, most of these targets are related to calcium handling in the cardiomyocyte. They include SERCA2a, phospholamban, the S100A1 protein, the ryanodine receptor and the inhibitor of the protein phosphatase 1. Other targets, related to cAMP signaling, are reviewed, such as adenylyl cyclase. microRNAs are emerging as novel therapeutic targets and convenient vectors for gene therapy, particularly in heart disease. We propose a discussion of recent advances and controversies in key molecular targets of heart failure gene therapy.
Keywords: heart failure, gene therapy, calcium handling, phospholamban, adenylyl cyclase, protein phosphatase 1
Introduction
The emergence of gene therapy as a promising strategy to treat heart failure is a multifactorial phenomenon. First, there is an unmet need to develop effective treatments aimed at decreasing the morbidity and mortality of heart failure, a highly prevalent problem associated with a grim prognosis despite continuing therapeutic progress1,2. Second, attractive therapeutic targets were identified and validated, such as the sarco-endoplasmic reticulum calcium ATPase (SERCA2a)1 without available and effective pharmacologic modulators. The search for pharmacologic agents to modulate these targets was undertaken in parallel with the development of gene therapy tools. Isatroxime is a promising stimulator of SERCA2a recently evaluated in a clinical trial; of note, isatroxime is also an inhibitor of the sodium-potassium ATPase.3,4
However, the recent successful completion of a phase 2 clinical trial of gene therapy to enhance the myocardial expression of SERCA2a in patients with heart failure5 undoubtedly propels gene therapy in the clinical armamentarium as a safe and effective approach.
The subject of gene therapy in heart failure has been extensively reviewed.1,6,7 The purpose of this brief review is to provide a focused update on the current translational advances and controversies related to the molecular targets in the gene therapy of heart failure.
Multiple molecular mechanisms were targeted by gene therapy in animal models of heart failure.1,6,7 The common aim of these studies was to restore the function of cardiomyocytic signaling pathways consistently shown to be defective in heart failure, such as beta-adrenergic signaling and calcium handling. Other studies targeted distinct processes, like cell survival pathways.7 A common strategy of these studies was to use recombinant DNA-based vectors to modulate gene expression.
In this setting, it is important to note that vector-based modulation of molecular pathways is also a strategy for proof of concept studies in cardiovascular physiology and pathophysiology. Progression from vector-based gene expression modulation to clinical gene therapy is dependent both on the therapeutic potential of the target gene and on the lack of safe and effective pharmacological approaches. For example, proteine kinase C was modulated by viral vectors and pharmacologic agents in recent studies.8,9 Thus, our review will focus on therapeutic targets pertaining to gene therapy with translational potential.
Viral vectors
Viral vectors (reviewed by Kawase et al.7) have been very effective at infecting various cell types including cardiac myocytes. Recombinant adenoviral vectors were used early on to infect the heart with reasonable transgene expression; but the duration of expression was limited to weeks due to immune response generated against the remaining viral genes. Lentiviruses, which could infect a post mitotic cell, have also been used. However, their integration within the genome is concerning since they can lodge within a tumor suppressing or promoting area causing unchecked growth and division. Adeno-associated vectors (AAV) have emerged as ideal vectors for infecting the myocardium in the setting of heart failure. Their characteristics include long term transgene expression and minimal immune response; besides, recombinant AAV used for gene therapy do not integrate in the host genome (as opposed to wild-type AAV). Their small size is an advantage when infusing them through in the coronary arteries. One drawback of AAV is their inability to incorporate more than 4.7 kb of genetic material. Because of their transduction abilities and safety profile, AAV vectors have gained a strong foothold not only in cardiovascular diseases but also in other organ diseases.
Therapeutic targets related to calcium handling in the cardiomyocyte
The abnormal calcium handling in the failing cardiomyocyte is complex. It involves mainly sarcoplasmic (SR) Ca2+ leak through the Ryanodin Receptor (RyR), decreased SR Ca2+ uptake with a decline of SERCA2a expression and activity; all resulting in reduced SR Ca2+ loading6. This is a critical component of the impaired mechanical performance of failing hearts6, in addition to arrhythmogenesis10. Calcium handling proteins and their regulators are thus promising therapeutic targets in heart failure6.
SERCA2a overexpression
The overexpression of SERCA2a by gene therapy in heart failure represents a historical model, starting from the finding of defective calcium handling associated with reduced SERCA2a expression in failing cardiac myocytes, and progressing to a clinical trial where an adeno-associated virus (AAV) overexpressing SERCA2a was administered to heart failure patients.5,7
The multiple steps of this process, involving target validation, the development of gene therapy vectors and delivery methods along with the testing of animal models of heart failure have been reviewed elsewhere11. Along with the demonstration of improved myocardial mechanical function, the overexpression of SERCA2a had multiple effects including improved myocardial energetics, endothelial function and coronary flow.7 The antiarrhythmic effects of SERCA2a overexpression were demonstrated in acute ischemia-reperfusion.10 In chronic heart failure after myocardial infarction in rats, the overexpression of SERCA2a was associated with a reduction of spontaneous and provoked ventricular arrhythmia along with a reduction in calcium leak from the SR.12 The latter findings comfort the clinical safety of SERCA2a gene therapy in the arrhythmia-prone heart failure population.12
Phase II of the Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID) study was recently published.5 This trial confirmed the clinical safety of SERCA2a overexpression by gene therapy already demonstrated in phase I.13 Therapeutic efficacy was demonstrated in several clinical, biological and echocardiographic indicators of heart failure.5 In particular, patients receiving the highest dose of Adeno-associated virus 1 (AAV1)-SERCA2a demonstrated improvement in the six minutes walk test and the left ventricular end-systolic volume, along with a reduced hospitalization length and a reduced incidence of prespecified multiple cardiovascular events5.
Other ongoing clinical trials of overexpression of SERCA2a in heart failure were listed and reviewed recently7. They include (1) a trial in which AAV1. SERCA2a gene transfer will be performed one month after the placement of a left ventricular assist device (LVAD) with the endpoint being the ability to wean the LVAD, and (2) a trial where AAV1-SERCA2a gene transfer will be performed in patients with Class III/IV heart failure and cardiac structural parameters will be examined 6 months after gene transfer.
Enhancing activity of SERCA2a through modulation of phospholamban PLB
The therapeutic implications of the SERCA2a-PLB interaction in heart failure were first derived from studies on transgenic mice lacking PLB in which the progression of heart failure was abrogated14. Multiple gene therapy studies were conducted in vitro and in vivo using viral vectors with antisense RNA or shRNA to downregulate PLB.15,16 Other studies used dominant-negative forms of PLB, designed to remain in the inactive pentameric form of PLB.17 Since phosphorylation of PLB on the serin 16 residues lifts the inhibition on SERCA2a, a pseudo-phosphorylated form (the S16E PLB mutant) of the protein was overexpressed through gene therapy and demonstrated improved left ventricular function in animal models of heart failure, including a large animal model.18
Several challenges related to the modulation of PLB as a therapeutic target have emerged from recent studies.
First, it is known that PLB only modulates the calcium-dependence of SERCA2a activity, which is the affinity of the enzyme for calcium (KCa); therefore, PLB has no impact on the maximal SERCA2a activity at saturating calcium.19
Second, in one report, PLB knock-out mice progressed to heart failure after aortic constriction similarly to control animals.20 Although PLB ablation seems promising in rodents, complete ablation of PLB may not be beneficial in humans. Humans with PLB null mutations suffered from a lethal dilated cardiomyopathy.21 We must accompany our citation of this work21 by a word of caution, since the L39Stop mutation of PLB does not equate PLB ablation and may have generated a form of PLB exerting untoward effects.22 Moreover, the cardiac cellular toxicity of a therapy involving short hairpin RNA against PLB in dogs was recently reported and attributed to an adverse interference of shRNA with microRNA pathways.22
Third, the S16E mutant of PLB lacks the possibility of further phosphorylation at the serin 16 residue,19 which may limit its therapeutic applicability. Recently, a systematic approach was undertaken to identify mutants of PLB that possess an affinity to SERCA comparable to the wild-type PLB, while having less inhibitory potential and retaining the possibility of being phosphorylated.23 It has been shown that PLB phosphorylation does not lead to a dissociation of the SERCA-PLB complex; instead, PLB remains bound to SERCA while its inhibitory effect on SERCA is reduced by phosphorylation.19 This opens the possibility of using PLB mutants as a therapy by having a less inhibitory mutant act as a partial antagonist to wild type PLB, thus displacing wild type PLB from SERCA.19 The physiologic ratio of PLB/SERCA is 5/1, and a recent study showed a potential for increased SERCA activity with PLB mutants present in molar concentrations at least as abundant as wild type PLB19. Studies are underway to determine whether these in vitro ratios can be achieved in vivo in animal models of heart failure.
Finally, while overexpression of PLB mutants may be beneficial, this may lead to an increase in the total amount of PLB in the cardiomyocyte. Existing literature demonstrates reduction in cardiomyocyte function associated with the overexpression of PLB, wether transgenically or by adenoviral vector.21,24 Increase in PLB expression was found in diabetic cardiomyopathy and in the setting of resistin overexpression.25
Considering that PLB is only a 52 amino-acids peptide, one can expect, at least theoretically, to target pharmacologically the SERCA2a-PLB interaction.
Protein phosphatase 1, its endogenous inhibitor and regulator I1, and the activated form of I1, I1c
The PP1-I1 couple is a central and complex mechanism of regulation of phosphorylation and dephosphorylation in the cardiac myocyte and in other cell types, and was recently and extensively reviewed by Wittkopper et al.26 This couple has emerged as an attractive therapeutic target for heart failure, due to the increased levels and activity of PP1, together with reduced levels and activity of I1, in heart failure26. PP1 dephosphorylates PLB at the serin 16 residue (Fig. 1); thus, by enhancing PLB phosphorylation and SERCA2a activity, PP1 inhibition is expected to provide the therapeutic benefits of SERCA2a enhancement.
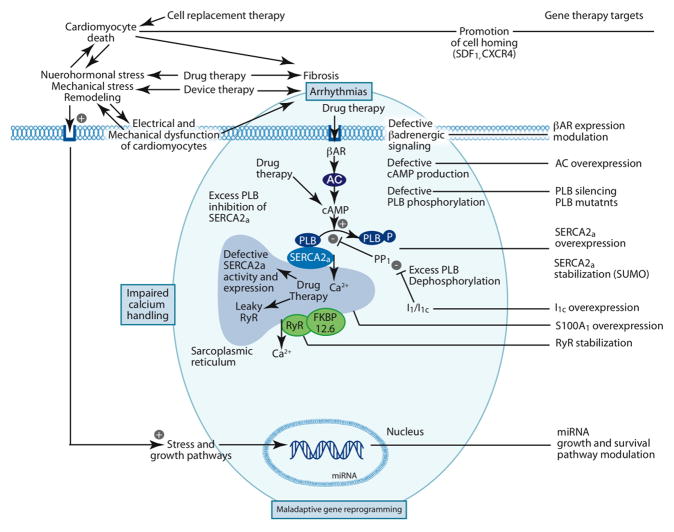
Figure 1.
Pathophysiologic processes in heart failure and corresponding therapeutic targets. The shaded area in blue emphasizes the relationship of impaired calcium handling and maladaptive gene reprogramming to the genesis of arrhythmias. For illustration purposes, the phopshorylated PLB was separated from SERCA2a; however, it is known to remain bound to SERCA2a with a lessened inhibition. SUMO1 was shown to stabilize SERCA2a. AC, adenylyl cyclase; βAR, beta-adrenergic receptor; CXCR4, chemokine (C-X-C motif) receptor 4; receptor for SDF1; FKBP12.6, FK506 binding protein 1B 12.6 kDa; I1, Inhibitor 1 of PP1; I1c, constitutively active I1; miRNA, microRNA; PLB, phospholamban; PP1, protein phosphatase 1; RyR, ryanodine receptor; SDF1, stromal cell-derived factor 1; SERCA2a, sarco-endoplasmic reticulum calcium ATPase; SUMO, small ubiquitin-related modifier.
Controversies have emerged in relation with this approach and are detailed in the review by Wittkopper et al.26 Mainly, excessive PP1 inhibition may lead to an unsafe hyperphosphorylation of the ryanodine receptor (RyR), which is arrhythmogenic; in the same vein, a cardioprotective effect of I1 ablation has been suggested26. Furthermore, PP1 is part of a network of protein phosphatases with multiple substrates; also, I1 is not a mere inhibitor but also a regulator and a “substrate-specifier” of PP1, and I1 is itself the target of regulating kinases and phosphatases.26
Thus far, the gene therapy efforts targeting the PP1-I1 complex in heart failure have focused on the overexpression of I1c, a truncated and pseudophosphorylated form of I1.26 The latter approach has shown beneficial effects on mechanical function of in a rat model of heart failure.27 I1c is expected to lack some of the drawbacks of I1, such as the hyperphosphorylation of RyR, although this latter fact is controversial26. Timing is also an issue, since the beneficial effect of PP1 inhibition was seen in younger animals while detrimental effects were observed in older animals26.
Last but not least, the relatively small size of I1c and its role as an inhibitor of PP1 opens the possibility of pharmacologic manipulation in addition to, or in replacement of, gene therapy.26
SUMO1 as a SERCA2a enhancing factor
A recent study has brought to light the interaction between SERCA2a and the small ubiquitin-related modifier 1 (SUMO1).28 SUMO1 was shown to preserve SERCA2a function and stability, and the overexpression of SUMO1 in a rodent model of heart failure had favorable effects on myocardial function.28
The ryanodine receptor as a therapeutic target in heart failure
Calcium leak from the SR through the ryanodine receptor (RyR) is a key pathophysiologic feature and therapeutic target in heart failure.4,6 As seen with impaired SR calcium uptake, RyR leak may lead to a systolic-diastolic calcium imbalance disrupting the mechanical function of the cardiac myocyte, in addition to arrhythmias.4
Surprisingly, a recent study has shown a reduction in RyR leak along with a reduction of RyR phosphorylation in rats with chronic heart failure overexpressing SERCA2a.12 The RyR itself was targeted by experimental gene therapy overexpressing the RyR modulator FKBP12.6 in isolated myocytes, leading to increased SR calcium content and improved myocyte shortening.4 Nevertheless, RyR leak is more likely to be directly targeted by pharmacologic agents shown to correct impaired cardiac function4,6 and prevent arrhthmias.6 Pharmacologic RyR modulation is currently evaluated in clinical trials.4
S100A1
S100A1 is a calcium-sensor protein that is thought to increase cardiomyocytic inotropy and lusitropy through the enhancement of SR calcium handling. In this regard, S100A1 appears to interact pleotropically with proteins related to calcium handling and excitation-contraction coupling.29,30 Also, S100A1 expression is reduced in heart failure.31 Restoring S100A1 expression through gene therapy had beneficial effects on myocardial mechanical function, calcium handling and energetics in small and large animal models of heart failure, and more recently in failing human cardiomyocytes.29–31 These results make S100A1 a promising therapeutic target for heart failure, although the precise mechanisms of its therapeutic effects remain to be defined.30
Therapeutic targets related to the cAMP signaling cascade in the cardiomyocyte
Beta-adrenergic receptor signaling
Chronic heart failure is associated with increased sympathetic outflow which may be compensatory early in the disease state, but long-term neurohormonal activation induces the desensitization of β-adrenergic signaling transduction, including β-adrenergic receptor (βAR) down-regulation; up-regulation of βARK (βAR kinase) and increased inhibitory G-protein alpha-subunit (GαI) function.32–34
Decreases in cAMP levels and production were reported in heart failure, although conflicting reports exist between rodent models and humans.35,36 Increasing cAMP through the administration of βAR agonists or through the inhibition of phosphodiesterases has proven detrimental in clinical heart failure4. On the other hand, genetic manipulation of the βAR signaling cascade, including β1/β2AR, Gαs overexpression or inhibition of βARKinase, resulted in transient improvements in contractile function, but lead to progressive cardiac hypertrophy and heart failure in aging animals.34,37
Adenylyl cyclases overexpression
A promising way to increase bioavailability of cAMP and bypass the dysfunctional βAR cascade is the overexpression of adenylyl cyclase (AC).32 AC is the enzyme responsible for cAMP synthesis. As an effector of the βAR signaling cascade, AC seems to be the rate-limiting step in signal transduction, with stoichiometric ratios of only 3 AC molecules for 100 molecules of Gαs and 1 molecule of βAR.38
The two major cardiac isoforms of AC are AC5 and AC6; they are activated by Gαs and can be phosphorylated and inhibited by the protine kinase A (PKA), thus providing feedback regulation in the transduction cascade.39,40 These isoforms are differentially regulated by membrane receptors: purinergic receptors activate AC5,41 whereas AC6 is specifically activated by β1AR, but not by β2AR.42 AC5 protein is the most abundant in fetal hearts, it declines with development and increases with pressure-overload hypertrophy.43 By contrast, AC6 expression declines in heart failure models with chronic pressure overload and myocardial infarction, as a part of the desensitization of the βAR signaling cascade.43,44
Overexpression of AC5, despite an increase in basal and forskolin-stimulated cAMP production, does not constrain βAR signaling in cardiomyocytes or contractile function in young mice 45, but leads to the development of a dilated cardiomyopathy with aging (Lipskaia et al., unpublished). Moreover, targeted disruption of AC5 in mice was shown to prolong longevity and protect the heart against aging, pressure overload and catecholamine-induced stress.46,47
By contrast, overexpression of AC6 was reported to increase βAR-stimulated contractility, and improve cardiac function and survival in numerous animal models of cardiac dysfunction, most recently in an animal model of cardiac aging.48
The apparent contradiction between the cardiotoxic effects of β1AR signaling cascade stimulation and beneficial effect of AC6 overexpression was explained by the partially cytosolic localization of the AC6 molecule. This localization directly affects phospholmaban phosphorylation and increases sarcoplasmic reticumlum (SR) calcium transient.49,50 However, this hypothesis is disputed by studies of a mouse model with cardiac expression of AC type 8 (Refs. 51 and 52). AC8 is not coupled to the βAR signaling cascade and is stimulated by calcium-calmodulin. Young transgenic mice (2–4 months old) with cardiac AC8 overexpression showed an improved β-adrenergic reactivity that enhanced cAMP synthesis, PKA activity, SR caclium cycling and contractile function.51,53,54 Even so, increasing cAMP/PKA signaling by AC8 overexpression causes a progressive alteration of cardiac function leading to a dilated and hyperkinetic cardiomyopathy in aged mice (14 months old).52 Moreover, a recent report suggests that the beneficial effects of AC6 gene transfer on calcium cycling and contractile function might not be mediated by an increase in cAMP production and can be observed after gene transfer of an inactive mutant of AC6 (AC6mut).55
Amid the controversies surrounding the genetic manipulations of the cAMP signaling cascade, a clinical trial using AC6 as a target involving two phases was initiated (estimated study completion date is June 2012). Phase I is a dose escalation trial of intracoronary administration of Ad5.hAC6 (adenovirus serotype 5 encoding human adenylyl cyclase type 6) in patients with congestive heart failure, evaluating safety. Phase II is a randomized, double-blinded, placebo-controlled dose escalation trial evaluating efficacy (Clinical Trial.gov Identifier NCTOO787059). Safety and efficacy are evaluated 1- and 3-months post-injection. To assess response to therapy, global systolic and diastolic functions are analyzed by echocardiography during exercise treadmill and dobutamine infusion. Parameters measured also include left ventricular pressure development and decline.
This clinical trial may be able to address a few issues. The potential benefit of AC enhancement in heart failure cannot be clearly predicted from pre-clinical experiments; data obtained from several animal models are not consistent and a clear understanding of the cardiac AC/cAMP signaling pathway is still lacking. The vector used in this trial, recombinant adenovirus serotype 5 (Ad5), is used mostly in preclinical small animal models and mediates high-level cardiac transduction only in the short term (1 week). Furthermore, in animal models, Ad5 causes intense local inflammation and stimulate potent cellular and humoral immune responses.56
Stromal cell-derived factor 1 and its receptor CXCR4
These two molecules have emerged as a therapeutic target in ischemic heart failure57 due to the ability of the SDF-1-CXCR4 system to promote the homing of stem cells to infracted myocardium. A clinical trial is underway to investigate the therapeutic benefit of SDF-1 overexpression in ischemic cardiomyopathy.7 In parallel, existing literature highlights the direct effects of CXCR4 on the myocardium and the cardiac myocyte. SDF-1 was shown to decrease myocardial contractility ex vivo and on cardiac myocytes.58 One recent report has shown increased ischemia-reperfusion injury in rat hearts overexpressing CXCR4,59 while another report investigated the modulation of beta-adrenergic receptor signaling by SDF-1 and CXCR4,60 raising interrogations over the potential complex interaction between these chemokines and the cardiovascular system.
MicroRNA as a gene therapy tool to target heart failure
MicroRNA are small non-coding RNA that modulate gene expression.61 Dysregulation of microRNA was demonstrated in cardiovascular and other diseases; circulating microRNA levels can be measured as diagnostic indicators; and soluble microRNA antagonists can be administered intravenously for therapeutic use.61 Finally, in a recent study, microRNA-1 (mir1) was expressed using an AAV serotype 9 in a rodent model of left ventricular hypertrophy and failure due to pressure overload, and this has resulted in regression of hypertrophy and improvements in ventricular function.62
Conclusions
Gene therapy was initially focused on treating monogenic diseases however the emergence of important targets associated with the failing myocardium along with the availability of safe vectors have rendered heart failure amenable to such a therapy. The subject is expanding, and it is not possible to explore all the molecules amenable to gene therapy in heart failure. The positive results from the CUPID trial will undoubtedly lead to other targets being tested by gene transfer.
Acknowledgments
This study was supported in part by AHA SDG 0930116N (LL), NIH RO1 HL083156, HL080498, HL093183 and P20HL100396 (RJH) and NIH-NHLBI T32HL007824 (ERC).
Footnotes
Conflicts of interest
Dr. Roger J. Hajjar is co-founder of Celladon and Nanocor Therapeutics.
References
- 1.Lipskaia L, Chemaly ER, Hadri L, Lompre AM, Hajjar RJ. Sarcoplasmic reticulum Ca(2+) ATPase as a therapeutic target for heart failure. Expert Opin Biol Ther. 2010;10:29–41. doi: 10.1517/14712590903321462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Ambrosy AP, Ferrandi M, Ferrari P. Combining SERCA2a activation and Na-K ATPase inhibition: a promising new approach to managing acute heart failure syndromes with low cardiac output. Discov Med. 2011;12:141–151. [PubMed] [Google Scholar]
- 4.Hasenfuss G, Teerlink JR. Cardiac inotropes: current agents and future directions. Eur Heart J. 2011;32:1838–1845. doi: 10.1093/eurheartj/ehr026. [DOI] [PubMed] [Google Scholar]
- 5.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H, Hajjar RJ. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lompre AM, Hajjar RJ, Harding SE, Kranias EG, Lohse MJ, Marks AR. Ca2+ cycling and new therapeutic approaches for heart failure. Circulation. 2010;121:822–830. doi: 10.1161/CIRCULATIONAHA.109.890954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawase Y, Ladage D, Hajjar RJ. Rescuing the failing heart by targeted gene transfer. J Am Coll Cardiol. 2011;57:1169–1180. doi: 10.1016/j.jacc.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hambleton M, Hahn H, Pleger ST, Kuhn MC, Klevitsky R, Carr AN, Kimball TF, Hewett TE, Dorn GW, 2nd, Koch WJ, Molkentin JD. Pharmacological- and gene therapy-based inhibition of protein kinase Calpha/beta enhances cardiac contractility and attenuates heart failure. Circulation. 2006;114:574–582. doi: 10.1161/CIRCULATIONAHA.105.592550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ladage D, Tilemann L, Ishikawa K, Correll RN, Kawase Y, Houser SR, Molkentin JD, Hajjar RJ. Inhibition of PKC{alpha}/{beta} With Ruboxistaurin Antagonizes Heart Failure in Pigs After Myocardial Infarction Injury. Circ Res. 2011 doi: 10.1161/CIRCRESAHA.111.255687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prunier F, Kawase Y, Gianni D, Scapin C, Danik SB, Ellinor PT, Hajjar RJ, Del Monte F. Prevention of ventricular arrhythmias with sarcoplasmic reticulum Ca2+ ATPase pump overexpression in a porcine model of ischemia reperfusion. Circulation. 2008;118:614–624. doi: 10.1161/CIRCULATIONAHA.108.770883. [DOI] [PubMed] [Google Scholar]
- 11.Gwathmey JK, Yerevanian AI, Hajjar RJ. Cardiac gene therapy with SERCA2a: from bench to bedside. J Mol Cell Cardiol. 2011;50:803–812. doi: 10.1016/j.yjmcc.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyon AR, Bannister ML, Collins T, Pearce E, Sepehripour AH, Dubb SS, Garcia E, O’Gara P, Liang L, Kohlbrenner E, Hajjar RJ, Peters NS, Poole-Wilson PA, Macleod KT, Harding SE. SERCA2a gene transfer decreases sarcoplasmic reticulum calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ Arrhythm Electrophysiol. 2011;4:362–372. doi: 10.1161/CIRCEP.110.961615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, Borow K, Dittrich H, Zsebo KM, Hajjar RJ. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minamisawa S, Hoshijima M, Chu G, Ward CA, Frank K, Gu Y, Martone ME, Wang Y, Ross J, Jr, Kranias EG, Giles WR, Chien KR. Chronic phospholamban-sarcoplasmic reticulum calcium ATPase interaction is the critical calcium cycling defect in dilated cardiomyopathy. Cell. 1999;99:313–322. doi: 10.1016/s0092-8674(00)81662-1. [DOI] [PubMed] [Google Scholar]
- 15.del Monte F, Harding SE, Dec GW, Gwathmey JK, Hajjar RJ. Targeting phospholamban by gene transfer in human heart failure. Circulation. 2002;105:904–907. doi: 10.1161/hc0802.105564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suckau L, Fechner H, Chemaly E, Krohn S, Hadri L, Kockskamper J, Westermann D, Bisping E, Ly H, Wang X, Kawase Y, Chen J, Liang L, Sipo I, Vetter R, Weger S, Kurreck J, Erdmann V, Tschope C, Pieske B, Lebeche D, Schultheiss HP, Hajjar RJ, Poller WC. Long-term cardiac-targeted RNA interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy. Circulation. 2009;119:1241–1252. doi: 10.1161/CIRCULATIONAHA.108.783852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziolo MT, Martin JL, Bossuyt J, Bers DM, Pogwizd SM. Adenoviral gene transfer of mutant phospholamban rescues contractile dysfunction in failing rabbit myocytes with relatively preserved SERCA function. Circ Res. 2005;96:815–817. doi: 10.1161/01.RES.0000163981.97262.3b. [DOI] [PubMed] [Google Scholar]
- 18.Kaye DM, Preovolos A, Marshall T, Byrne M, Hoshijima M, Hajjar R, Mariani JA, Pepe S, Chien KR, Power JM. Percutaneous cardiac recirculation-mediated gene transfer of an inhibitory phospholamban peptide reverses advanced heart failure in large animals. J Am Coll Cardiol. 2007;50:253–260. doi: 10.1016/j.jacc.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 19.Lockamy EL, Cornea RL, Karim CB, Thomas DD. Functional and physical competition between phospholamban and its mutants provides insight into the molecular mechanism of gene therapy for heart failure. Biochem Biophys Res Commun. 2011;408:388–392. doi: 10.1016/j.bbrc.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiriazis H, Sato Y, Kadambi VJ, Schmidt AG, Gerst MJ, Hoit BD, Kranias EG. Hypertrophy and functional alterations in hyperdynamic phospholamban-knockout mouse hearts under chronic aortic stenosis. Cardiovascular research. 2002;53:372–381. doi: 10.1016/s0008-6363(01)00487-4. [DOI] [PubMed] [Google Scholar]
- 21.Haghighi K, Kolokathis F, Pater L, Lynch RA, Asahi M, Gramolini AO, Fan GC, Tsiapras D, Hahn HS, Adamopoulos S, Liggett SB, Dorn GW, 2nd, MacLennan DH, Kremastinos DT, Kranias EG. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. The Journal of clinical investigation. 2003;111:869–876. doi: 10.1172/JCI17892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bish LT, Sleeper MM, Reynolds C, Gazzara J, Withnall E, Singletary GE, Buchlis G, Hui D, High KA, Gao G, Wilson JM, Sweeney HL. Cardiac gene transfer of short hairpin RNA directed against phospholamban effectively knocks down gene expression but causes cellular toxicity in canines. Hum Gene Ther. 2011;22:969–977. doi: 10.1089/hum.2011.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ha KN, Traaseth NJ, Verardi R, Zamoon J, Cembran A, Karim CB, Thomas DD, Veglia G. Controlling the inhibition of the sarcoplasmic Ca2+-ATPase by tuning phospholamban structural dynamics. J Biol Chem. 2007;282:37205–37214. doi: 10.1074/jbc.M704056200. [DOI] [PubMed] [Google Scholar]
- 24.Kadambi VJ, Ponniah S, Harrer JM, Hoit BD, Dorn GW, 2nd, Walsh RA, Kranias EG. Cardiac-specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocyte mechanics in transgenic mice. The Journal of clinical investigation. 1996;97:533–539. doi: 10.1172/JCI118446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chemaly ER, Hadri L, Zhang S, Kim M, Kohlbrenner E, Sheng J, Liang L, Chen J, PKR, Hajjar RJ, Lebeche D. Long-term in vivo resistin overexpression induces myocardial dysfunction and remodeling in rats. J Mol Cell Cardiol. 2011;51:144–155. doi: 10.1016/j.yjmcc.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wittkopper K, Dobrev D, Eschenhagen T, El-Armouche A. Phosphatase-1 inhibitor-1 in physiological and pathological beta-adrenoceptor signalling. Cardiovascular research. 2011;91:392–401. doi: 10.1093/cvr/cvr058. [DOI] [PubMed] [Google Scholar]
- 27.Pathak A, del Monte F, Zhao W, Schultz JE, Lorenz JN, Bodi I, Weiser D, Hahn H, Carr AN, Syed F, Mavila N, Jha L, Qian J, Marreez Y, Chen G, McGraw DW, Heist EK, Guerrero JL, DePaoli-Roach AA, Hajjar RJ, Kranias EG. Enhancement of cardiac function and suppression of heart failure progression by inhibition of protein phosphatase 1. Circ Res. 2005;96:756–766. doi: 10.1161/01.RES.0000161256.85833.fa. [DOI] [PubMed] [Google Scholar]
- 28.Kho C, Lee A, Jeong D, Oh JG, Chaanine AH, Kizana E, Park WJ, Hajjar RJ. SUMO1-dependent modulation of SERCA2a in heart failure. Nature. 2011;477:601–605. doi: 10.1038/nature10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pleger ST, Shan C, Ksienzyk J, Bekeredjian R, Boekstegers P, Hinkel R, Schinkel S, Leuchs B, Ludwig J, Qiu G, Weber C, Raake P, Koch WJ, Katus HA, Muller OJ, Most P. Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci Transl Med. 2011;3:92ra64. doi: 10.1126/scitranslmed.3002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belmonte SL, Margulies KB, Blaxall BC. S100A1: Another Step Toward Therapeutic Development for Heart Failure. J Am Coll Cardiol. 2011;58:974–976. doi: 10.1016/j.jacc.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 31.Brinks H, Rohde D, Voelkers M, Qiu G, Pleger ST, Herzog N, Rabinowitz J, Ruhparwar A, Silvestry S, Lerchenmuller C, Mather PJ, Eckhart AD, Katus HA, Carrel T, Koch WJ, Most P. S100A1 genetically targeted therapy reverses dysfunction of human failing cardiomyocytes. J Am Coll Cardiol. 2011;58:966–973. doi: 10.1016/j.jacc.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feldman DS, Carnes CA, Abraham WT, Bristow MR. Mechanisms of disease: beta-adrenergic receptors--alterations in signal transduction and pharmacogenomics in heart failure. Nature clinical practice. 2005;2:475–483. doi: 10.1038/ncpcardio0309. [DOI] [PubMed] [Google Scholar]
- 33.Lipskaia L, Ly H, Kawase Y, Hajjar RJ, Lompre AM. Treatment of heart failure by calcium cycling gene therapy. Future cardiology. 2007;3:413–423. doi: 10.2217/14796678.3.4.413. [DOI] [PubMed] [Google Scholar]
- 34.Vinge LE, Raake PW, Koch WJ. Gene therapy in heart failure. Circulation research. 2008;102:1458–1470. doi: 10.1161/CIRCRESAHA.108.173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao MH, Lai NC, Roth DM, Zhou J, Zhu J, Anzai T, Dalton N, Hammond HK. Adenylylcyclase increases responsiveness to catecholamine stimulation in transgenic mice. Circulation. 1999;99:1618–1622. doi: 10.1161/01.cir.99.12.1618. [DOI] [PubMed] [Google Scholar]
- 36.Regitz-Zagrosek V, Hertrampf R, Steffen C, Hildebrandt A, Fleck E. Myocardial cyclic AMP and norepinephrine content in human heart failure. Eur Heart J. 1994;15(Suppl D):7–13. doi: 10.1093/eurheartj/15.suppl_d.7. [DOI] [PubMed] [Google Scholar]
- 37.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circulation research. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 38.Ostrom RS, Post SR, Insel PA. Stoichiometry and compartmentation in G protein-coupled receptor signaling: implications for therapeutic interventions involving G(s) The Journal of pharmacology and experimental therapeutics. 2000;294:407–412. [PubMed] [Google Scholar]
- 39.Defer N, Best-Belpomme M, Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. American journal of physiology. 2000;279:F400–416. doi: 10.1152/ajprenal.2000.279.3.F400. [DOI] [PubMed] [Google Scholar]
- 40.Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annual review of pharmacology and toxicology. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- 41.Puceat M, Bony C, Jaconi M, Vassort G. Specific activation of adenylyl cyclase V by a purinergic agonist. FEBS letters. 1998;431:189–194. doi: 10.1016/s0014-5793(98)00747-9. [DOI] [PubMed] [Google Scholar]
- 42.Stark JC, Haydock SF, Foo R, Brown MJ, Harding SE. Effect of overexpressed adenylyl cyclase VI on beta 1- and beta 2-adrenoceptor responses in adult rat ventricular myocytes. British journal of pharmacology. 2004;143:465–476. doi: 10.1038/sj.bjp.0705976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu CL, Chandra R, Ge H, Pain J, Yan L, Babu G, Depre C, Iwatsubo K, Ishikawa Y, Sadoshima J, Vatner SF, Vatner DE. Adenylyl cyclase type 5 protein expression during cardiac development and stress. Am J Physiol Heart Circ Physiol. 2009;297:H1776–1782. doi: 10.1152/ajpheart.00050.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Espinasse I, Iourgenko V, Richer C, Heimburger M, Defer N, Bourin MC, Samson F, Pussard E, Giudicelli JF, Michel JB, Hanoune J, Mercadier JJ. Decreased type VI adenylyl cyclase mRNA concentration and Mg(2+)-dependent adenylyl cyclase activities and unchanged type V adenylyl cyclase mRNA concentration and Mn(2+)-dependent adenylyl cyclase activities in the left ventricle of rats with myocardial infarction and longstanding heart failure. Cardiovascular research. 1999;42:87–98. doi: 10.1016/s0008-6363(98)00283-1. [DOI] [PubMed] [Google Scholar]
- 45.Tepe NM, Lorenz JN, Yatani A, Dash R, Kranias EG, Dorn GW, 2nd, Liggett SB. Altering the receptor-effector ratio by transgenic overexpression of type V adenylyl cyclase: enhanced basal catalytic activity and function without increased cardiomyocyte beta-adrenergic signalling. Biochemistry. 1999;38:16706–16713. doi: 10.1021/bi991619k. [DOI] [PubMed] [Google Scholar]
- 46.Vatner SF, Yan L, Ishikawa Y, Vatner DE, Sadoshima J. Adenylyl cyclase type 5 disruption prolongs longevity and protects the heart against stress. Circ J. 2009;73:195–200. doi: 10.1253/circj.cj-08-0957. [DOI] [PubMed] [Google Scholar]
- 47.Okumura S, Takagi G, Kawabe J, Yang G, Lee MC, Hong C, Liu J, Vatner DE, Sadoshima J, Vatner SF, Ishikawa Y. Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9986–9990. doi: 10.1073/pnas.1733772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang T, Hammond HK, Firth A, Yang Y, Gao MH, Yuan JX, Lai NC. Adenylyl cyclase 6 improves calcium uptake and left ventricular function in aged hearts. J Am Coll Cardiol. 2011;57:1846–1855. doi: 10.1016/j.jacc.2010.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao MH, Tang T, Guo T, Miyanohara A, Yajima T, Pestonjamasp K, Feramisco JR, Hammond HK. Adenylyl cyclase type VI increases Akt activity and phospholamban phosphorylation in cardiac myocytes. The Journal of biological chemistry. 2008;283:33527–33535. doi: 10.1074/jbc.M805825200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao MH, Tang T, Miyanohara A, Feramisco JR, Hammond HK. beta(1)-Adrenergic receptor vs adenylyl cyclase 6 expression in cardiac myocytes: differences in transgene localization and intracellular signaling. Cellular signalling. 2010;22:584–589. doi: 10.1016/j.cellsig.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lipskaia L, Defer N, Esposito G, Hajar I, Garel MC, Rockman HA, Hanoune J. Enhanced cardiac function in transgenic mice expressing a Ca(2+)-stimulated adenylyl cyclase. Circulation research. 2000;86:795–801. doi: 10.1161/01.res.86.7.795. [DOI] [PubMed] [Google Scholar]
- 52.Lipskaia L, Mougenot N, Jacquet A, Atassi F, Hajjar RJ, Hatem SN, Limon I. Compartmentalization of cAMP increase at the level of sarcoplasmic reticulum results in dilated and hypertrophic cardiomyopathy in aged transgenic mice. Eur Heart J. 2011;32(suppl 1):999. [Google Scholar]
- 53.Georget M, Mateo P, Vandecasteele G, Jurevicius J, Lipskaia L, Defer N, Hanoune J, Hoerter J, Fischmeister R. Augmentation of cardiac contractility with no change in L-type Ca2+ current in transgenic mice with a cardiac-directed expression of the human adenylyl cyclase type 8 (AC8) Faseb J. 2002;16:1636–1638. doi: 10.1096/fj.02-0292fje. [DOI] [PubMed] [Google Scholar]
- 54.Georget M, Mateo P, Vandecasteele G, Lipskaia L, Defer N, Hanoune J, Hoerter J, Lugnier C, Fischmeister R. Cyclic AMP compartmentation due to increased cAMP-phosphodiesterase activity in transgenic mice with a cardiac-directed expression of the human adenylyl cyclase type 8 (AC8) Faseb J. 2003;17:1380–1391. doi: 10.1096/fj.02-0784com. [DOI] [PubMed] [Google Scholar]
- 55.Levin SD, Taft DW, Brandt CS, Bucher C, Howard ED, Chadwick EM, Johnston J, Hammond A, Bontadelli K, Ardourel D, Hebb L, Wolf A, Bukowski TR, Rixon MW, Kuijper JL, Ostrander CD, West JW, Bilsborough J, Fox B, Gao Z, Xu W, Ramsdell F, Blazar BR, Lewis KE. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur J Immunol. 2011;41:902–915. doi: 10.1002/eji.201041136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lyon AR, Sato M, Hajjar RJ, Samulski RJ, Harding SE. Gene therapy: targeting the myocardium. Heart (British Cardiac Society) 2008;94:89–99. doi: 10.1136/hrt.2007.116483. [DOI] [PubMed] [Google Scholar]
- 57.Ghadge SK, Muhlstedt S, Ozcelik C, Bader M. SDF-1alpha as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol Ther. 2011;129:97–108. doi: 10.1016/j.pharmthera.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 58.Pyo RT, Sui J, Dhume A, Palomeque J, Blaxall BC, Diaz G, Tunstead J, Logothetis DE, Hajjar RJ, Schecter AD. CXCR4 modulates contractility in adult cardiac myocytes. J Mol Cell Cardiol. 2006;41:834–844. doi: 10.1016/j.yjmcc.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen J, Chemaly E, Liang L, Kho C, Lee A, Park J, Altman P, Schecter AD, Hajjar RJ, Tarzami ST. Effects of CXCR4 gene transfer on cardiac function after ischemia-reperfusion injury. Am J Pathol. 2010;176:1705–1715. doi: 10.2353/ajpath.2010.090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.LaRocca TJ, Schwarzkopf M, Altman P, Zhang S, Gupta A, Gomes I, Alvin Z, Champion HC, Haddad G, Hajjar RJ, Devi LA, Schecter AD, Tarzami ST. beta2-Adrenergic receptor signaling in the cardiac myocyte is modulated by interactions with CXCR4. J Cardiovasc Pharmacol. 2010;56:548–559. doi: 10.1097/FJC.0b013e3181f713fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Rooij E. The art of microRNA research. Circ Res. 2011;108:219–234. doi: 10.1161/CIRCRESAHA.110.227496. [DOI] [PubMed] [Google Scholar]
- 62.Karakikes I, Chaanine A, Kim J, Lebeche D, Hajjar R. Abstract 20916: Therapeutic Cardiac-Targeted Delivery of Mir-1 Reverses Hypertrophy and Preserves Cardiac Function in a Pressure Overload Animal Model. Circulation. 2010;122:A20916. [Google Scholar]