Abstract
The size of a cell’s nucleus is usually proportional to the size of the cell itself. How are the two linked? The answer lies, at least in part, in the import of one or more cytoplasmic cargoes into the nucleus.
It is rare to come across a basic question in cell biology that is almost entirely unresolved, but what determines the size of a cell or of an organelle is one such question1. Does the cell use a ‘molecular ruler’ to directly assess the size of its compartments, or does it use a surrogate, such as protein concentration, to determine how big its structures are? Reporting in Cell, Levy and Heald2 provide evidence that at least partly answers these questions in regard to the size of the cell nucleus.
Regulation of nuclear size is perhaps one of the most striking, and enigmatic, examples of organelle-size control, because it is tightly linked to cell size3. Indeed, there is a constant ratio between nuclear and cell volumes (the N/C volume ratio), and deviations from it are associated with disease4. But how is this ratio regulated? To address this question, researchers have attempted5,6 to perturb the N/C volume ratio in yeast, but to no avail: neither nuclear-DNA content, nor varied growth conditions, nor drug treatments, could alter the ratio.
Another question is what aspect of cell volume affects nuclear size: is it the cell’s entire volume; the volume of only the cytoplasm; or perhaps that of another organelle? In multinucleated fission yeast, the size of each nucleus is proportional to its surrounding cytoplasm6, but how the cytoplasm affects nuclear size, if at all, has remained unknown.
Levy and Heald2 study regulation of nuclear size in two related frog species — Xenopus laevis and Xenopus tropicalis — that differ in both body size and the number of chromosome copies per cell (ploidy). Xenopus laevis is larger and its cells are tetraploid, whereas X. tropicalis is smaller and its cells are diploid. The two species also differ in another aspect: the cells and nuclei of X. laevis are larger.
An advantage of Xenopus as an experimental model is that its nuclei can be assembled in a test tube using the chromatin (DNA–protein complexes) and extracts of its egg cytoplasm. This allowed Levy and Heald2 to ask, what is the main determinant of nuclear size in Xenopus: the DNA or a cytoplasmic factor?
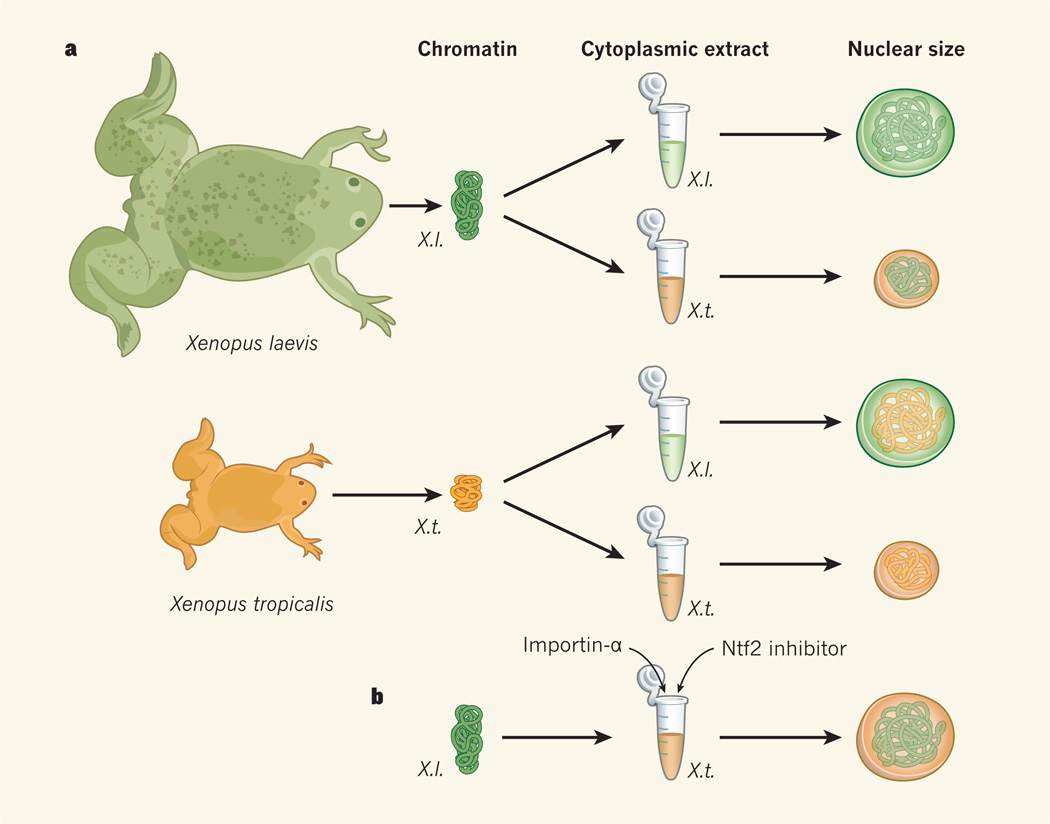
The authors added sperm chromatin from either X. laevis or X. tropicalis to egg extracts from either X. laevis or X. tropicalis (Fig. 1a). They found that, although both extracts can trigger assembly of the nuclear envelope around the chromatin, the X. laevis extract forms larger nuclei than the X. tropicalis extract, regardless of the DNA used. This indicates that one or more cytoplasmic factors determine nuclear size.
Figure 1. The cytoplasm regulates nuclear size.
a, To examine factors affecting nuclear size, Levy and Heald2 mixed sperm chromatin from either Xenopus laevis (X.l.) or X. tropicalis (X.t.) with cytoplasmic extracts from the eggs of either X. laevis or X. tropicalis. They find that, regardless of the chromatin used, X. laevis extracts promote formation of large nuclei, whereas X. tropicalis extracts promote formation of smaller nuclei. (The drawings of X. laevis and X. tropicalis are to scale). b, Two proteins, importin-α and Ntf2, account for the differences between the X. laevis and X. tropicalis extracts: addition of importin-α and inhibition of Ntf2 activity in X. tropicalis extracts lead to formation of larger nuclei.
A hint of the underlying difference between the extracts from the two frog species came from the authors’ analyses of nuclear import — the process by which proteins are transported into the nucleus7. Both the rate of nuclear import and the maximum size of the imported cargo were greater in the nuclei reconstituted with X. laevis extracts. The capacity for nuclear import therefore might be a regulator of nuclear size.
The two extracts differed in two proteins that mediate nuclear import — importin-α and Ntf2. In X. laevis extracts, the levels of importin-α were higher and those of Ntf2 lower than in X. tropicalis extracts. Indeed, when Levy and Heald added active (phosphorylated) importin-α to X. tropicalis extracts they observed an increase in nuclear size. The role of Ntf2 in regulating nuclear size is less straightforward. Nonetheless, X. tropicalis extracts supplemented with both active importin-α and an inhibitor of Ntf2 activity form nuclei that are similar in size to those formed in X. laevis extracts2 (Fig. 1b).
That nuclear import affects nuclear size is perhaps not surprising, as a previous paper8 showed that, in the absence of import, the nuclear envelope — which forms around the chromosomes at the end of mitotic cell division — fails to expand. But in showing that, at least in vitro, import is a limiting factor for nuclear growth, Levy and Heald’s report provides a specific step that could be targeted for regulating nuclear size in vivo.
Which of the cargoes carried by importin-α are crucial for controlling nuclear size? From an engineering standpoint, enlarging a structure could require an extension of its underlying framework. Whether the nucleus has an internal framework — the ‘nuclear matrix’ — is debatable, but there is no doubt that the nuclear lamina serves as a framework supporting the nuclear envelope. Indeed, Levy and Heald found that the addition of lamin B3, a component of the nuclear lamina, to X. tropicalis extracts resulted in increased nuclear size. Thus, nuclear import may regulate nuclear size through controlling the availability of nuclear-lamina components.
Neither yeast nor plants have lamins, which raises the question of how general is regulation of nuclear size by lamin import. Whether nuclear import itself affects nuclear size in yeast is unresolved5,6, although at least one study6 found that a prolonged inhibition of nuclear export increases nuclear volume by 50%. It could be, therefore, that nuclear size in yeast and plants also depends on nuclear import, not of lamin but of some other cargo.
The cell-free system Levy and Heald describe is remarkably useful for identifying proteins and processes that affect nuclear size. The next step will be to determine how such processes affect nuclear size within the cell, and whether they contribute to the N/C volume ratio. Levy and Heald did address this question by injecting importin-α into developing X. laevis embryos. They observed a transient increase in nuclear size in early stages of development, but whether cell volume is affected in any way remains unknown.
When considering the scaling of nuclear size with cell size, one must take into account the increase not only in volume, but also in surface area4. An influx of material through nuclear import could lead to physical stress that signals for an increase in the surface area of the nuclear envelope. Alternatively, because the nuclear membrane is continuous with that of another organelle, the endoplasmic reticulum, the cell might regulate nuclear size by controlling the amount of endoplasmic-reticulum membrane that is allocated to the nucleus.
Nevertheless, geometry tells us that surface area increases at a slower rate than volume (surface area is a function of the radius squared, whereas volume is a function of the radius cubed). Intriguingly, a recent study9 on the scaling of transcription with cell size in yeast revealed that, whereas expression of most genes increases in proportion to the increase in cell size, the expression of genes encoding cell-surface proteins lags behind. How this size-sensing mechanism works isn’t clear, but it would be interesting to examine whether the abundance of proteins associated with nuclear-envelope expansion is also ‘size-sensitive’.
Levy and Heald’s results2 uncover a process that affects nuclear size, providing a glimpse into a mechanism that may couple nuclear volume to cell volume. To fully understand the N/C volume ratio, researchers need biological tools — mutants and/or RNAi knockdowns — that perturb this ratio. So to anyone who thinks that all the interesting basic cell-biologi cal questions have been answered, here is one that is still wide open.
References
- 1.Chan Y-HM, Marshall WF. Organogenesis. 2010;6:88–96. doi: 10.4161/org.6.2.11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy DL, Heald R. Cell. 2010;143:288–298. doi: 10.1016/j.cell.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson EB. The Cell in Development and Heredity. 1925 (Macmillan) [Google Scholar]
- 4.Webster M, Witkin KL, Cohen-Fix O. J. Cell Sci. 2009;122:1477–1486. doi: 10.1242/jcs.037333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jorgensen P, et al. Mol. Biol. Cell. 2007;18:3523–3532. doi: 10.1091/mbc.E06-10-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neumann FR, Nurse P. J. Cell Biol. 2007;179:593–600. doi: 10.1083/jcb.200708054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terry LJ, Shows EB, Wente SR. Science. 2007;318:1412–1416. doi: 10.1126/science.1142204. [DOI] [PubMed] [Google Scholar]
- 8.Newport JW, Wilson KL, Dunphy WG. J. Cell Biol. 1990;111:2247–2259. doi: 10.1083/jcb.111.6.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu C-Y, Rolfe PA, Gifford DK, Fink GR. PLoS Biol. 2010;8:e1000523. doi: 10.1371/journal.pbio.1000523. [DOI] [PMC free article] [PubMed] [Google Scholar]