Abstract
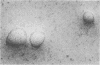
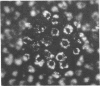
Fluid and electrolyte transport by epithelial cells in vitro can be recognized by the ability of cultured cells to form domes and by the electrical properties of monolayer cultures. Pulmonary alveolar epithelial cells are thought to be partially responsible for fluid movement in the fetal lung, but their role in electrolyte transport in the adult lung is not known. We isolated alveolar type II cells from adult rat lung and maintained them on plastic culture dishes alone, on plastic culture dishes coated with an extracellular matrix, and on collagen-coated Millipore filters. Numerous large domes were formed on culture dishes coated with the extracellular matrix; smaller domes were formed on uncoated plastic culture dishes. Sodium butyrate (3 mM) stimulated dome formation. Transmission electron microscopy showed that the epithelial cells had flattened but still retained lamellar inclusions and that the cells were polarized with microvilli on the apical surface facing the culture medium. The electrical properties of the monolayers maintained on collagen-coated Millipore filters were tested in two laboratories. The transepithelial potential differences were 0.7 +/- 0.1 mV (24 filters, seven experiments) and 1.3 +/- 0.1 mV (13 filters, two experiments) apical side negative, and the corresponding resistances were 217 +/- 11 ohm X cm2 and 233 +/- 12 ohm X cm2. Terbutaline (10 microM) produced a biphasic response with a transient decrease and then a sustained increase in potential difference. Amiloride (0.1 mM) completely abolished the potential difference when it was added to the apical side but not when it was added to the basal side, whereas 1 mM ouabain inhibited the potential difference more effectively from the basal side. Thus, type II cells form a polarized epithelium in culture, and these cells actively transport electrolytes in vitro.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson T. M., Boyd R. D., Platt H. S., Strang L. B. Composition of alveolar liquid in the foetal lamb. J Physiol. 1969 Sep;204(1):159–168. doi: 10.1113/jphysiol.1969.sp008905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert R. K., Lakshminarayan S., Hildebrandt J., Kirk W., Butler J. Increased surface tension favors pulmonary edema formation in anesthetized dogs' lungs. J Clin Invest. 1979 May;63(5):1015–1018. doi: 10.1172/JCI109369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisbee C. A., Machen T. E., Bern H. A. Mouse mammary epithelial cells on floating collagen gels: transepithelial ion transport and effects of prolactin. Proc Natl Acad Sci U S A. 1979 Jan;76(1):536–540. doi: 10.1073/pnas.76.1.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLEMENTS J. A. Pulmonary edema and permeability of alveolar membranes. Arch Environ Health. 1961 Mar;2:280–283. [PubMed] [Google Scholar]
- Cereijido M., Ehrenfeld J., Meza I., Martínez-Palomo A. Structural and functional membrane polarity in cultured monolayers of MDCK cells. J Membr Biol. 1980;52(2):147–159. doi: 10.1007/BF01869120. [DOI] [PubMed] [Google Scholar]
- Cereijido M., Meza I., Martínez-Palomo A. Occluding junctions in cultured epithelial monolayers. Am J Physiol. 1981 Mar;240(3):C96–102. doi: 10.1152/ajpcell.1981.240.3.C96. [DOI] [PubMed] [Google Scholar]
- Cereijido M., Robbins E. S., Dolan W. J., Rotunno C. A., Sabatini D. D. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J Cell Biol. 1978 Jun;77(3):853–880. doi: 10.1083/jcb.77.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs L. G., Geppert E. F., Williams M. C., Greenleaf R. D., Mason R. J. Metabolic properties and ultrastructure of alveolar type II cells isolated with elastase. Biochim Biophys Acta. 1980 Jun 23;618(3):510–523. doi: 10.1016/0005-2760(80)90270-2. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Cabral L. J., Stephens R. J., Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975 Feb;22(1):142–150. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- Geppert E. F., Williams M. C., Mason R. J. Primary culture of rat alveolar type II Cells on floating collagen membranes. Morphological and biochemical observations. Exp Cell Res. 1980 Aug;128(2):363–374. doi: 10.1016/0014-4827(80)90072-5. [DOI] [PubMed] [Google Scholar]
- Gil J., Bachofen H., Gehr P., Weibel E. R. Alveolar volume-surface area relation in air- and saline-filled lungs fixed by vascular perfusion. J Appl Physiol Respir Environ Exerc Physiol. 1979 Nov;47(5):990–1001. doi: 10.1152/jappl.1979.47.5.990. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. R. Do plasma and serum have different abilities to promote cell growth? Proc Natl Acad Sci U S A. 1980 May;77(5):2726–2730. doi: 10.1073/pnas.77.5.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. Extracellular matrix and control of proliferation of vascular endothelial cells. J Clin Invest. 1980 Jun;65(6):1351–1364. doi: 10.1172/JCI109799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler J. S., Perkins F. M., Johnson J. P. Studies of renal cell function using cell culture techniques. Am J Physiol. 1980 Jan;238(1):F1–F9. doi: 10.1152/ajprenal.1980.238.1.F1. [DOI] [PubMed] [Google Scholar]
- Handler J. S., Steele R. E., Sahib M. K., Wade J. B., Preston A. S., Lawson N. L., Johnson J. P. Toad urinary bladder epithelial cells in culture: maintenance of epithelial structure, sodium transport, and response to hormones. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4151–4155. doi: 10.1073/pnas.76.8.4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey C. D., Pittman F. E. A simple methylene blue-azure II-basic fuchsin stain for epoxy-embedded tissue sections. Stain Technol. 1974 Jan;49(1):9–14. doi: 10.3109/10520297409116929. [DOI] [PubMed] [Google Scholar]
- Leighton J., Brada Z., Estes L. W., Justh G. Secretory activity and oncogenicity of a cell line (MDCK) derived from canine kidney. Science. 1969 Jan 31;163(3866):472–473. doi: 10.1126/science.163.3866.472. [DOI] [PubMed] [Google Scholar]
- Lever J. E. Inducers of mammalian cell differentiation stimulate dome formation in a differentiated kidney epithelial cell line (MDCK). Proc Natl Acad Sci U S A. 1979 Mar;76(3):1323–1327. doi: 10.1073/pnas.76.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever J. E. Regulation of dome formation in differentiated epithelial cell cultures. J Supramol Struct. 1979;12(2):259–272. doi: 10.1002/jss.400120210. [DOI] [PubMed] [Google Scholar]
- MACKLIN C. C. The pulmonary alveolar mucoid film and the pneumonocytes. Lancet. 1954 May 29;266(6822):1099–1104. doi: 10.1016/s0140-6736(54)92154-6. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Williams M. C. Phospholipid composition and ultrastructure of A549 cells and other cultured pulmonary epithelial cells of presumed type II cell origin. Biochim Biophys Acta. 1980 Jan 18;617(1):36–50. doi: 10.1016/0005-2760(80)90222-2. [DOI] [PubMed] [Google Scholar]
- Matthay M. A., Landolt C. C., Staub N. C. Differential liquid and protein clearance from the alveoli of anesthetized sheep. J Appl Physiol Respir Environ Exerc Physiol. 1982 Jul;53(1):96–104. doi: 10.1152/jappl.1982.53.1.96. [DOI] [PubMed] [Google Scholar]
- Mills J. W., Macknight A. D., Dayer J. M., Ausiello D. A. Localization of [3H]ouabain-sensitive Na+ pump sites in cultured pig kidney cells. Am J Physiol. 1979 Mar;236(3):C157–C162. doi: 10.1152/ajpcell.1979.236.3.C157. [DOI] [PubMed] [Google Scholar]
- Misfeldt D. S., Hamamoto S. T., Pitelka D. R. Transepithelial transport in cell culture. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1212–1216. doi: 10.1073/pnas.73.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt D. S., Sanders M. J. Transepithelial transport in cell culture: D-glucose transport by a pig kidney cell line (LLC-PK1). J Membr Biol. 1981 Mar 15;59(1):13–18. doi: 10.1007/BF01870816. [DOI] [PubMed] [Google Scholar]
- Nielson D. W., Goerke J., Clements J. A. Alveolar subphase pH in the lungs of anesthetized rabbits. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7119–7123. doi: 10.1073/pnas.78.11.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojakian G. K. Tumor promoter-induced changes in the permeability of epithelial cell tight junctions. Cell. 1981 Jan;23(1):95–103. doi: 10.1016/0092-8674(81)90274-9. [DOI] [PubMed] [Google Scholar]
- PATTLE R. E. Properties, function, and origin of the alveolar lining layer. Proc R Soc Lond B Biol Sci. 1958 Feb 18;148(931):217–240. doi: 10.1098/rspb.1958.0015. [DOI] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M. Galloylglucoses of low molecular weight as mordant in electron microscopy. I. Procedure, and evidence for mordanting effect. J Cell Biol. 1976 Sep;70(3):608–621. doi: 10.1083/jcb.70.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snashall P. D., Hughes J. M. Lung water balance. Rev Physiol Biochem Pharmacol. 1981;89:5–62. doi: 10.1007/BFb0035264. [DOI] [PubMed] [Google Scholar]
- Staub N. C. Pulmonary edema. Physiol Rev. 1974 Jul;54(3):678–811. doi: 10.1152/physrev.1974.54.3.678. [DOI] [PubMed] [Google Scholar]
- Staub N. C. The pathogenesis of pulmonary edema. Prog Cardiovasc Dis. 1980 Jul-Aug;23(1):53–80. doi: 10.1016/0033-0620(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Walters D. V., Olver R. E. The role of catecholamines in lung liquid absorption at birth. Pediatr Res. 1978 Mar;12(3):239–242. doi: 10.1203/00006450-197803000-00017. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H., Welsh M. J. Anion selectivity of the chloride-transport process in dog tracheal epithelium. Am J Physiol. 1980 Sep;239(3):C112–C117. doi: 10.1152/ajpcell.1980.239.3.C112. [DOI] [PubMed] [Google Scholar]
- Williams M. C. Conversion of lamellar body membranes into tubular myelin in alveoli of fetal rat lungs. J Cell Biol. 1977 Feb;72(2):260–277. doi: 10.1083/jcb.72.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T. A. Effect of alveolar wall shape on alveolar water stability. J Appl Physiol Respir Environ Exerc Physiol. 1981 Jan;50(1):222–224. doi: 10.1152/jappl.1981.50.1.222. [DOI] [PubMed] [Google Scholar]