Abstract
Background
The leading cause of asthma exacerbation is respiratory viral infection. Innate antiviral defense pathways are altered in the asthmatic epithelium, yet involvement of inflammasome signaling in virus-induced asthma exacerbation is not known.
Objective
To compare influenza-induced activation of inflammasome and innate immune signaling in human bronchial epithelial cells from asthmatics and non-asthmatics and investigate the role of caspase-1 in epithelial cell antiviral defense.
Methods
Differentiated primary human bronchial epithelial cells from asthmatics and non-asthmatics were infected with influenza A virus. An inflammasome-specific quantitative real-time polymerase chain reaction array was used to compare baseline and influenza-induced gene expression profiles. Cytokine secretion, innate immune gene expression, and viral replication were compared between human bronchial epithelial cells from asthmatics and non-asthmatics. Immunofluorescence microscopy was used to evaluate caspase-1 and PYCARD co-localization. Tracheal epithelial cells from caspase-1 deficient or wildtype mice were infected with influenza and assessed for antiviral gene expression and viral replication.
Results
Human bronchial epithelial cells from asthmatics had altered influenza-induced expression of inflammasome-related and innate immune signaling components, which correlated with enhanced production of interlukin-1β, interleukin-6, and tumor necrosis factor-α. Specifically, influenza-induced caspase-1 expression was enhanced and localization differed in human bronchial epithelial cells from asthmatics compared to non-asthmatics. Influenza-infected tracheal epithelial cells from caspase-1 deficient mice had reduced expression of antiviral genes and viral replication.
Conclusion
Caspase-1 plays an important role in the airway epithelial cell response to influenza infection, which is enhanced in asthmatics and may contribute to the enhanced influenza related pathogenesis observed in vivo.
Keywords: epithelial cell, asthma, influenza, antiviral, inflammasome, caspase-1, innate immunity
Introduction
Most acute asthma exacerbations are caused by respiratory viral infections and the resulting innate immune response (1–6). Epidemiological studies of the 2009 H1N1 influenza A virus (IAV) pandemic revealed an association between asthma diagnosis and increased morbidity and mortality from infection, yet the mechanisms by which underlying asthma enhances IAV-induced responses are poorly understood (7–10).
The primary sites for IAV infections are airway epithelial cells (AECs). The first step in the AEC response to viral infections is recognition of the virus by pattern recognition receptors (PRRs), such as NOD-like receptors (NLRs) and RIG-I- like receptors (RLRs), which activate downstream signaling cascades to initiate expression of cytokines and chemokines(11, 12). Several NLRs and RIG-I that oligomerize with caspase-1 and PYCARD to form the inflammasome complex have been implicated in the innate immune response to viruses, including IAV (12, 13). Formation of the inflammasome complex induces auto-activation of caspase-1, which catalyzes the proteolytic processing of pro-IL-1β and pro-IL-18. Active caspase-1 may also participate in several alternative mechanisms, including a pro-inflammatory form of programmed cell death termed pyroptosis (14). Alterations in inflammasome signaling are associated with inflammatory diseases including Crohns’ disease, gout, and atopic dermatitis (15); however, involvement of the inflammasome in virus-induced asthma exacerbation is unknown.
Human bronchial epithelial cells (HBEC) from asthmatics have modified innate immune responses to viral infection. A genome-wide expression study comparing rhinovirus (RV)-infected HBEC from asthmatics and non-asthmatics demonstrated that HBEC from asthmatics had altered RV-induced expression of immune response genes, including IL1B (16). In addition, HBEC from asthmatics have been shown to have deficient RV-induced interferon (IFN) production (17, 18) and increased production of the inflammatory cytokines IL-6, IL-8, and GM-CSF with RSV infection(19), correlating with the increased pro-inflammatory response to viral infections seen in vivo(20).
Since inflammasome signaling is important for antiviral response to IAV in AECs (21), and virus-induced IL-1β expression differs in HBEC from asthmatics and non-asthmatics (16), we hypothesized that in the asthmatic epithelium, altered expression of inflammasome and innate immune signaling components contributes to virus-induced asthma pathogenesis. Using differentiated primary HBEC, we compared IAV-induced activation of inflammasome and innate immune responses in HBEC from asthmatics and non-asthmatics. Our data show that IAV-induced expression of inflammasome and innate immune signaling components is enhanced in HBEC from asthmatics. Specifically, caspase-1 expression and localization differed in IAV-infected HBEC from asthmatics and associated with enhanced, albeit low levels, of IAV-induced IL-1β production. Using tracheal epithelial cells (MTEC) from caspase-1 deficient (Casp1 −/−) mice, we found that caspase-1 affects expression of several innate immunity genes and viral replication. Our results demonstrate an important role for caspase-1 in the response to IAV infection at the level of the epithelium, which may be independent of IL-1β production and is enhanced in HBEC from asthmatics.
Methods
Human bronchial epithelial cell (HBEC) culture
Primary HBEC were obtained from non-asthmatic (n=11) and asthmatic (n=13) adult volunteers by cytologic brushing during bronchoscopy using a protocol approved by the UNC-Chapel Hill School of Medicine Institutional Review Board. Refer to the online repository for subject characterization information (Table E1–E3). Mild asthma status was characterized by history of asthma symptoms (e.g. cough or wheeze) two times or less a week and no current use of inhaled steroids. All asthmatics were considered “mild” except for one, which was considered “moderately asthmatic” due to use of an oral steroid. Non-asthmatics had no history of asthma symptoms. HBEC were expanded to passage two in bronchial epithelial growth medium (BEGM; Cambrex Bioscience Walkersville, Inc., Walkersville, MD) and differentiated as described before (22).
Animals and murine tracheal epithelial cell (MTEC) isolation
C57BL/6 Casp1 −/− mice were purchased from Jackson Laboratories (Bar Harbor, ME). Female 6–8 weeks old Casp1 −/− or wildtype matched littermates were used throughout the study. All experimental procedures were approved by the University of North Carolina Institutional Animal Care and Use Committee. MTEC isolation and culture was performed as described by You et al. (23). MTEC were expanded to passage one in Ham’s F-12 medium (Invitrogen, Carlsbad, CA) before use.
Influenza infection
HBEC were infected with Influenza A/Bangkok/1/79 (H3N2)(24–25) diluted in Hank’s Buffered Saline Solution (HBSS, Invitrogen). MTEC were infected with mouse-adapted Influenza A/PR/8/34 (H1N1)(26–27) diluted in culture medium. Both viruses were obtained from Dr. Melinda Beck (Department of Nutrition, University of North Carolina, Chapel Hill), propagated in 10-day-old embryonated hens’ eggs, and collected from the allantoic fluid. For infection of HBEC and MTEC, 500,000 cells were infected with approximately 50 hemagglutination units (HAU). Control treated HBEC received HBSS alone, and MTEC received media alone.
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from HBEC from asthmatics (n=7) and non-asthmatics (n=8) using TRizol (Invitrogen) according to manufacturer instructions. First-strand cDNA synthesis and qRT-PCR were performed as previously described (28, 29). Refer to online repository for primer/probes. Differences in expression were determined using the Ct method and B-actin for normalization.
qRT-PCR array
Total RNA isolated from a subset of HBEC from asthmatics (n=3) and non-asthmatics (n=3) was purified using an RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA was prepared using a RT First Strand Kit (SABiosciences, Frederick, MD) and analyzed using the human inflammasome RT2 Profiler PCR Array System (SABiosciences). Gene expression results were normalized to GAPDH, analyzed using the ΔΔCt method, and probed for genes with ≤1.5-fold difference in expression.
Cytokine quantification
The apical surface of HBEC were washed with HBSS and analyzed for concentrations of IL-6, IL-1β, and TNFα using a commercially available ELISA kit (Meso Scale Discovery, Gaithersburg, MD). Lower limits of detection were: IL-1β= 0.5 pg/ml; IL-6= 0.22 pg/ml; TNFα= 0.49 pg/ml.
Immunofluorescence microscopy
HBEC from asthmatics (n=3) and non-asthmatics (n=3) were fixed with 4% paraformaldehyde (Sigma-Aldrich) and prepared for immunofluorescence as described previously (30). See online repository for caspase-1 and PYCARD antibody information. A Nikon C1si confocal microscope with a 60x oil lens and Nikon EZ-C1 3.8 software were used to acquire z-stack images (Nikon Instruments, Melville, NY), which were processed using the NIS-Elements software (Nikon). Images shown are composites of all z-stack slices. Equal adjustments were performed on all images..
Influenza virus titer
Influenza virus titers in apical washes (HBEC) or supernatants (MTEC) were determined by 50% tissue culture infections dose (TCID50) in Madin-Darby canine kidney cells (MDCK) and by hemagglutination as previously described (31).
Statistical analysis
Array results were analyzed using the Comparative Marker Selection tool in GenePattern (www.broadinstitute.org/cancer/software/genepattern/)(32). Refer to online repository for additional array analysis. For gene expression, cytokine analysis, and viral titer data, individual differences between asthmatics and non-asthmatics or Casp1−/− and wildtype mice were assessed by analysis of variance (ANOVA) with a Tukey post-hoc test (*p < 0.05, ** p< 0.01, ***p < 0.001). Factorial ANOVA was used to determine the interaction between IAV infection and asthma status or mouse genotype (# p<0.05, ## p< 0.01, and ### p<0.001). Endpoints with no detection at baseline (MTEC IL-6 and M1 expression and viral titer) were assessed using unpaired T-test for individual differences between Casp1 −/− and wildtype MTEC 24 hours post-IAV infection (@ p<0.05). All graphs show mean ± SEM.
Results
HBEC from asthmatics have enhanced IAV-induced production of cytokines
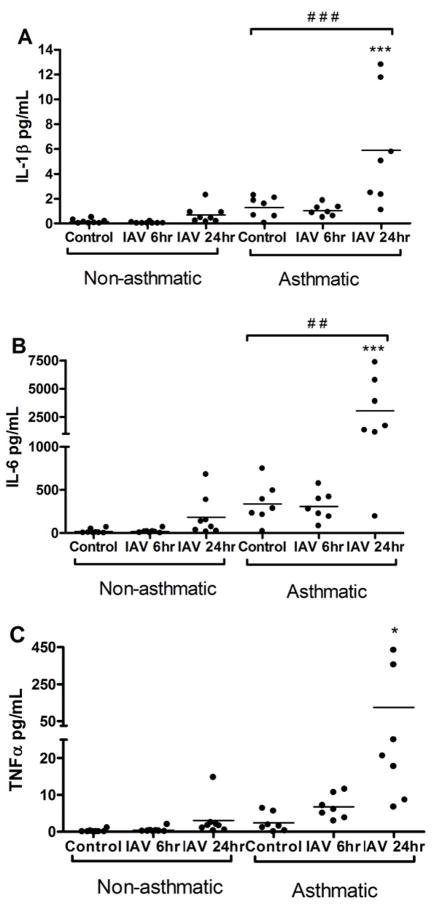
To determine whether HBEC from asthmatics have altered IAV-induced cytokine production, HBEC from asthmatics and non-asthmatics were infected with IAV for 6 or 24 hours and assessed for cytokine secretion (Figure 1A–C; Table E4–E5, Online Repository). HBEC from asthmatics secreted significantly more IAV-induced IL-6, TNFα, and IL-1β, with the greatest increase observed 24 hours post-infection. Notably, the subject characterized as “moderately asthmatic” (Table E1, Online Repository) had neither an ablated nor a particularly heightened response to IAV infection.
Figure 1.
HBEC from asthmatics have enhanced production of pro-inflammatory cytokines in response to IAV infection. Apical washes from HBEC from asthmatics (n=7) and non-asthmatics (n=8) were collected 6 or 24 hours post-IAV infection or 24 hours HBSS control treatment and analyzed for (A) IL-1β; (B) IL-6; and (C) TNFα concentrations by ELISA.
HBEC from asthmatics have differential expression of inflammasome- and innate immunity-related genes
Based on the elevated production of IL-1β by HBEC from asthmatics (Figure 1A), we investigated alterations in inflammasome signaling. Using an inflammasome-specific qRT-PCR array, encompassing 84 genes involved in innate immunity and inflammasome signaling, we compared baseline and IAV-induced gene expression between HBEC from asthmatics and non-asthmatics (Table 1 and Table E6, Online Repository for full array data). The expression of eight genes was significantly altered at baseline in HBEC from asthmatics (Table 1). Of these genes, all but CCL5 were down-regulated in HBEC from asthmatics. 24 hours post-IAV infection, HBEC from asthmatics had altered expression of seventeen genes, all of which were enhanced (Table 1).
Table I.
Genes with significantly different expression in HBEC from asthmatics vs. non-asthmatics at baseline (control treatment) and 24 hours post-IAV infection
| Gene Symbol | Gene Name | Average Non-Asthmatic 2−ΔΔCt 1 | Average Asthmatic 2−ΔΔCt 1 | Average FD3 | p-value2 |
|---|---|---|---|---|---|
| Baseline (control treatment) | |||||
| BCL2 | B-cell CLL/lymphoma 2 | 0.89 | 0.33 | 0.39 | 0.050 |
| CCL5 | Chemokine (C-C motif) ligand 5 | 0.68 | 3.04 | 4.50 | 0.002 |
| IRAK1 | Interleukin-1 receptor-associated kinase 1 | 1.23 | 0.60 | 0.49 | 0.050 |
| MAP3K7IP1 | Mitogen-activated protein kinase kinase kinase 7 interacting protein 1 | 0.96 | 0.63 | 0.66 | 0.050 |
| MAPK13 | Mitogen-activated protein kinase 13 | 1.12 | 0.54 | 0.48 | 0.050 |
| NFKBIB | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, β | 1.14 | 0.61 | 0.54 | 0.050 |
| NLRP1 | NLR family, pyrin domain containing 1 | 1.49 | 0.67 | 0.45 | 0.050 |
| PYCARD | PYD and CARD domain containing | 1.15 | 0.65 | 0.57 | 0.050 |
|
| |||||
| 24 hours post- IAV infection | |||||
| BIRC2 | Baculoviral IAP repeat-containing 2 | 0.88 | 2.41 | 2.75 | 0.002 |
| BIRC3 | Baculoviral IAP repeat-containing 3 | 0.56 | 4.89 | 8.72 | 0.002 |
| CASP1 | Caspase 1, apoptosis-related cysteine peptidase (interlekin 1β, convertase) | 0.75 | 2.16 | 2.86 | 0.002 |
| CASP4 | Caspase 4, apoptosis-relted cysteine peptidase | 0.73 | 1.85 | 2.54 | 0.002 |
| CASP5 | Caspase 5, apoptosis-related cysteine peptidase | 0.60 | 10.90 | 18.13 | 0.002 |
| CCL5 | Chemokine (C-C motif) ligand 5 | 0.38 | 5.56 | 14.80 | 0.002 |
| CFLAR | CASP8 and FADD-like apoptosis regulator | 1.19 | 3.11 | 2.61 | 0.002 |
| IL12A | Interleukin 12A | 0.42 | 3.44 | 8.29 | 0.002 |
| IRF1 | Interferon regulatory factor 1 | 0.74 | 1.51 | 2.04 | 0.002 |
| IRF2 | Interferon regulatory factor 2 | 1.04 | 1.84 | 1.77 | 0.002 |
| MYD88 | Myeloid differentiation primary response gene (88) | 0.84 | 1.67 | 1.99 | 0.002 |
| NLRC5 | NLR family, CARD domain containing 5 | 0.65 | 1.49 | 2.30 | 0.002 |
| NOD2 | Nucleotide-binding oligomerization domain containing 2 | 0.92 | 2.48 | 2.71 | 0.002 |
| PANX1 | Pannexin 1 | 0.95 | 2.92 | 3.07 | 0.002 |
| RIPK2 | Receptor-interacting serine-threonine kinase 2 | 0.71 | 3.14 | 4.44 | 0.002 |
| TNF | Tumor necrosis factor (TNF superfamily, member 2) | 0.55 | 7.67 | 13.86 | 0.002 |
| TXNIP | Thioredoxin interacting protein | 0.94 | 1.62 | 1.73 | 0.002 |
IAV, influenza A virus; HBEC, human bronchial epithelial cell; FD, fold difference
Differences in gene expression between HBEC from asthmatics (n=3) and non-asthmatics (n=3) were determined using the ΔΔCt method. 2−ΔΔCt represents the fold change in gene expression.
Significantly different gene expression (p ≤ 0.05) was determined using signal-to-noise ratio (SNR) analysis.
The fold difference (FD) in expression between HBEC from asthmatics and non-asthmatics was determined by dividing the average asthmatic 2−ΔΔCt by the average non-asthmatic 2−ΔΔCt. Only genes with greater than 1.5 times higher or lower expression were considered “significantly different.”
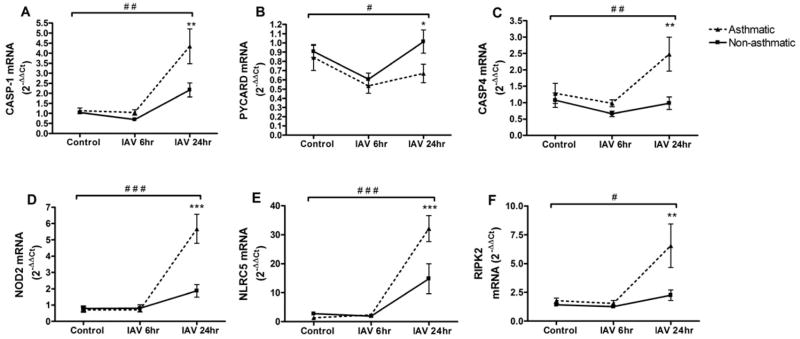
To confirm and expand upon the array results, we performed gene-specific qRT-PCR analysis of several genes with significant differences in HBEC from asthmatics using an increased number of subjects and expanded time course. Similar to the array results, HBEC from asthmatics had significantly increased expression of CASP1, CASP4, RIPK2, NLRC5, and NOD2 after IAV infection (Figure 2A, C–F). The greatest increase was observed 24 hours post-IAV infection, correlating with the highest production of cytokines. In both HBEC from asthmatics and non-asthmatics, the expression of PYCARD, an adaptor protein which links caspase-1 to one of several inflammasome receptors, was initially reduced with IAV infection, and then returned to baseline levels by 24 hours post-infection (Figure 2B). The expression of PYCARD was slightly decreased in HBEC from asthmatics throughout the time course, correlating with the array results.
Figure 2.
Gene- specific qRT-PCR confirmation of inflammasome-related gene expression. Total RNA from HBEC from asthmatics (n=7) and non-asthmatics (n=8) at 6 or 24 hours post-IAV infection or 24 hours HBSS control treatment was analyzed for expression of (A) CASP1; (B) PYCARD; (C) CASP4; (D) NOD2; (E) NLRC5; and (F) RIPK2 by qRT-PCR. Ct values were normalized to β-actin.
Caspase-1 and PYCARD immunofluorescence microscopy
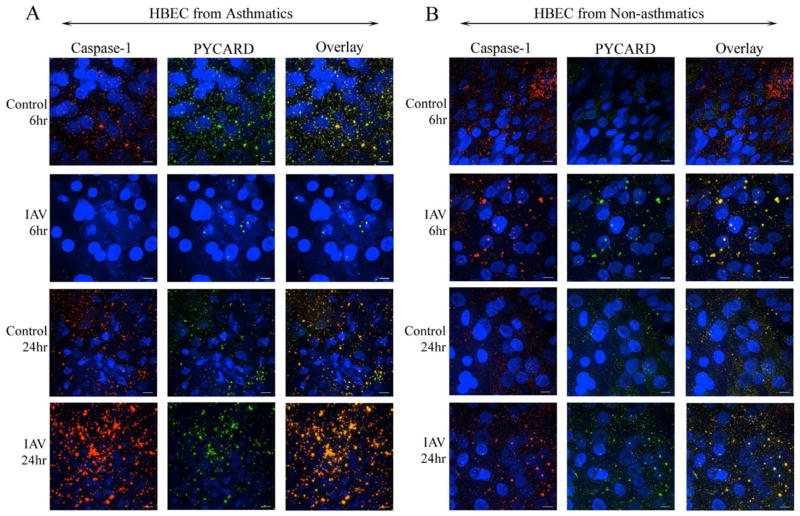
Inflammasome signaling involves the oligomerization of caspase-1, PYCARD, and a PRR to form the inflammasome complex. To determine whether IAV- induced expression of caspase-1 translates to caspase-1/PYCARD co-localization, we used immunofluorescence microscopy to qualitatively assess the localization of PYCARD and caspase-1 in HBEC from asthmatics and non-asthmatics 6 or 24 hours post-IAV infection (Figure 3, and Figures E2–E3, Online Repository). In both groups at baseline, caspase-1 and PYCARD were located diffusely throughout the cell (Figure 3A–B 1st and 3rd rows, E2A-B 1st row). With IAV infection, we observed the formation of co-localized caspase-1/PYCARD foci, especially in the asthmatics, and particularly at 24 hours post-IAV infection (Figure 3A–B 2nd and 4th rows, E2A–B 2nd row). The HBEC from asthmatics had large, intense foci of co-localized PYCARD and caspase-1 at 24 hours post-IAV infection, (Figure 3A 4th row, E2A 2nd row), correlating with the enhanced caspase-1 expression 24 hours post-IAV infection (Figure 2A). The co-localization of caspase-1 and PYCARD did not correlate with cytotoxicity, as we did not detect any differences in cytotoxicity throughout the infection time course (Figure E1, Online Repository). Our data show that PYCARD and caspase-1 co-localized in response to IAV infection in HBEC, and suggests that the intensity of co-localization appears to differ in HBEC from asthmatics.
Figure 3.
Caspase-1 and PYCARD co-localize with IAV-infection. HBEC from (A) asthmatics and (B) non-asthmatics 6 or 24 hours post-IAV infection or HBSS control treatment were probed for Caspase-1 (red) and PYCARD (green). DAPI stain identified nucleic acid (blue). Images are representative of 3 asthmatic and 3 non-asthmatic isolates. White bars=10μm.
HBEC from asthmatics have increased expression of antiviral genes, which correlates with enhanced IAV replication
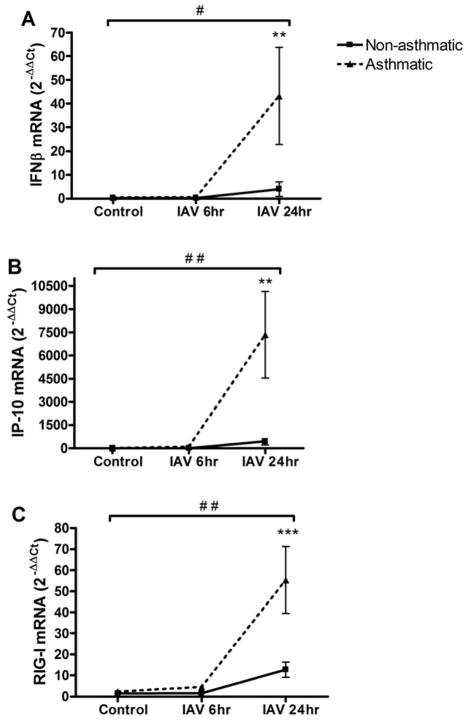
We next investigated whether HBEC from asthmatics also had enhanced expression of known antiviral genes, particularly interferon-γ induced protein 10 (IP-10), interferon β (IFNβ) and retinoic acid inducible-gene I (RIG-I). Our data show that HBEC from asthmatics had elevated expression of these genes 24 hours post-IAV infection (Figures 4A–C).
Figure 4.
HBEC from asthmatics have enhanced expression of antiviral genes. Total RNA from HBEC from asthmatics ( n=7) and non-asthmatics ( n=8) at 6 or 24 hours post-IAV infection or 24 hours HBSS control treatment was analyzed for expression of (A) IFNβ; (B) IP-10; and (C) RIG-I by qRT-PCR. Ct values were normalized to β-actin.
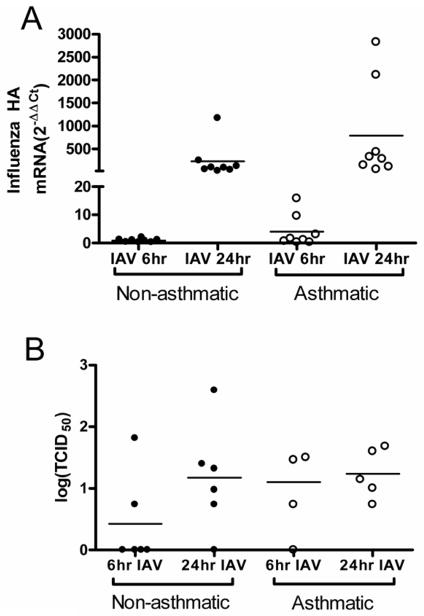
To determine whether the enhanced innate immune response was due to increased viral replication, we assessed influenza hemagglutinin (HA) transcripts and TCID50 viral titers of HBEC from asthmatics and non-asthmatics(24, 33). Though HBEC from asthmatics had no significant overall differences compared to non-asthmatics (Figures 5), there was significant correlation between the expression of IP-10, IFNβ, and RIG-I and influenza HA transcript number, suggesting that the enhanced innate immune response in the HBEC from asthmatics was correlated with greater viral replication (Figure E4A–C, Online Repository). Likewise, caspase-1 expression was correlated with influenza HA mRNA levels, suggesting that caspase-1 is involved in the antiviral response (Figure E4D, Online Repository).
Figure 5.
Viral replication is not significantly increased in HBEC from asthmatics. (A) Total RNA from IAV-infected HBEC from asthmatics (n=7) and non-asthmatics (n=8) were analyzed for IAV hemagglutinin (HA) RNA by qRT-PCR. Ct values were normalized to β-actin. (B) Vial titer of apical washes from HBEC from asthmatics (n=4–5) and non-asthmatics (n=6) at 6 and 24 hours post-IAV infection.
MTEC from Casp1−/− mice have diminished antiviral response to IAV infection and reduced viral replication
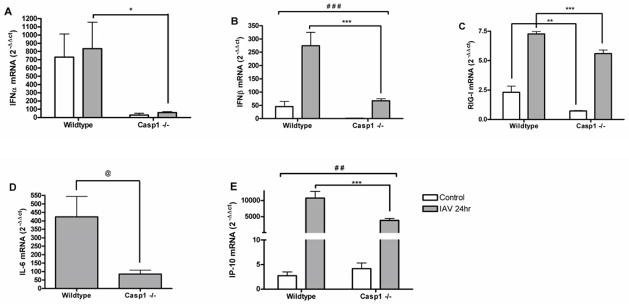
Our findings in HBEC from asthmatics indicated the enhanced expression of caspase-1 was associated with heightened innate immune response to IAV. To further assess whether caspase-1 was causally linked to antiviral defense against IAV infection, we examined the expression of innate immune genes by MTEC from Casp1 −/− and wildtype mice, which were cultured and infected with IAV ex vivo. Our results show that Casp1 −/− MTEC had reduced IAV-induced expression of IFNα, IFNβ, IL-6, and IP-10 compared to wildtype (Figure 6), suggesting that the presence of caspase-1 is necessary for optimal expression of these genes. The baseline and IAV-induced expression of RIG-I was decreased to a similar extent in Casp1 −/− MTEC, suggesting that caspase-1 expression may regulate baseline expression of RIG-I, and that these effects persist during IAV infection.
Figure 6.
Casp1 −/− MTEC have diminished antiviral gene expression in response to IAV infection. RNA from wildtype (n=8) and Casp1 −/− (n=9) MTEC 24 hours post-IAV infection or control were analyzed for expression of (A) IFNα; (B) IFNβ; (C) RIG-I; (D) IL-6; (E) IP-10 by qRT-PCR. Ct values were normalized to β-actin. @ p<0.05 Student’s T-test Casp1 −/− vs. wildtype, 24hrs post-IAV infection only; * p<0.05, **p<0.01; ***p<0.001 ANOVA and Tukey post-hoc test, Casp1 −/− vs. wildtype 24hrs post-IAV and control infection; ##p<0.01, ### p<0.001 Factorial ANOVA, interaction between genotype and infection.
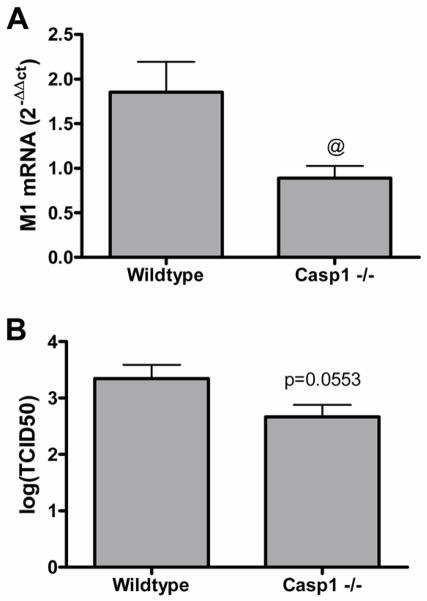
To determine whether decreased viral replication was associated with the diminished innate immune gene expression response in Casp1−/− MTEC, we assessed viral replication 24 hours post-IAV infection. Compared to wildtype, the Casp1 −/− MTEC had reduced levels of influenza matrix 1 (M1) RNA and lower viral titers (Figure 7). To determine if viral replication was necessary for the diminished innate immune gene expression response in Casp1 −/− MTEC, wildtype and Casp1 −/− MTEC were infected with UV-inactivated (replication deficient) IAV, wildtype IAV, or media control and assessed for expression of IP-10, which had the most robust IAV-induced expression (Figure 6), and influenza M1 RNA (Figure E5, Online Repository). No M1 transcripts were detected from cells infected with UV-inactivated IAV. As observed in Figure 6, wildtype-IAV infected Casp1 −/− MTEC had significantly lower IP-10 expression compared to wildtype MTEC. When infected with UV-inactivated virus, the Casp1 −/− and wildtype MTEC had similar expression levels of IP-10, suggesting that viral replication was necessary for the diminished innate immune gene expression in Casp1 −/− MTEC.
Figure 7.
CASP1 −/− MTEC have diminished viral replication. (A) Total RNA from Casp1 −/− (n=9) and wildtype (n=8) MTEC 24 hours post-IAV infection or media control treatment were analyzed for IAV matrix 1 (M1) RNA by qRT-PCR. Ct values were normalized to β-actin. (B) Vial titer was assessed using media supernatents from Casp1 −/− (n=9) and wildtype (n=8) MTEC 24 hours post-IAV infection.
Discussion
Respiratory viral infections are the leading cause of asthma exacerbations. Innate antiviral defense pathways are altered in the asthmatic epithelium, yet involvement of the inflammasome in virus-induced asthma exacerbations is unknown. We compared IAV-induced activation of inflammasome and innate immune signaling between differentiated HBEC from asthmatics and non-asthmatics, and found that HBEC from asthmatics had modified baseline and IAV-induced expression of genes involved in innate immune and inflammasome signaling. In particular, we showed that caspase-1 expression was enhanced in HBEC from asthmatics, correlating with enhanced, albeit low levels, of IAV-induced IL-1β production. Using MTEC from Casp1 −/− and wildtype mice, we found that IAV-infected Casp1 −/− MTEC had decreased expression of innate immunity genes and viral replication compared to wildtype. These results establish an important role for caspase-1 in the AEC response to IAV, which may involve inflammasome-independent functions and is enhanced in the asthmatic epithelium.
AEC-derived cytokines and chemokines contribute to the inflammatory cell influx and airway hyperresponsiveness associated with asthma exacerbation. AECs from asthmatics have altered production of cytokines and chemokines in response to RV and RSV (16, 17, 19). Consistent with these observations, we found that baseline and/or IAV-induced levels of innate immune cytokines and chemokines (IL-1β, TNFα, IL-6, CCL5, IP-10, and β) were enhanced inIFN HBEC from asthmatics. Previous studies have shown that these cytokines are increased in asthmatic airways in vivo either at baseline or following challenge and contribute to virus-induced asthma exacerbation (34–39). Our findings add to the literature suggesting that baseline differences in HBEC from asthmatics may prime the epithelium for an exaggerated innate immune response to viral infection.
Many of the cytokines and chemokines with enhanced expression in HBEC from asthmatics are under NF-kB regulation. NF-kB activity has previously been shown to be enhanced in AECs from asthmatics(40, 41). We found that expression of NFKBIB, an inhibitor of NF-kB signaling, was reduced in HBEC from asthmatics at baseline (Table 1), which may contribute to the enhanced innate immune response to IAV infection. Interestingly, caspase-1 has been shown to activate NF-kB via RIPK2, a CARD-containing kinase(42) that, similar to caspase-1, had enhanced IAV-induced expression in HBEC from asthmatics (Table 1, Figure 2E). Additionally, Nod2 activation of NF-kB signaling has been shown to induce CCL5 release in murine macrophages(43). Our findings indicate that IAV-induced expression of Nod2 was enhanced in HBEC from asthmatics (Table 1, Figure 2D), suggesting that Nod2 may be a regulator of IAV-induced CCL5 release that is enhanced in HBEC from asthmatics.
The enhanced IAV-induced production of IL-1β suggested that HBEC from asthmatics may have modified inflammasome activity. Other studies have demonstrated involvement of the inflammasome and, more specifically, NOD-like receptor protein 3 (NLRP3), in defense against IAV infection (21, 44, 45). Allen et al. found that AECs secrete IL-1β, albeit at much lower concentrations than monocytes (21). We similarly observed low levels of IL-1β secretion, suggesting that AECs are not a major source of this cytokine. Though the canonical function of the inflammasome is activation of caspase-1 for the proteolytic processing of pro-IL-1β and pro-IL-18, caspase-1 has alternative functions involved in the activation of NF-kB signaling, cell death, cellular metabolism, and cell repair (14). Therefore, caspase-1 may play alternative roles in non-myeloid cells, such as AECs, which do not produce high levels of IL-1β.
Based on this knowledge, we used a less biased approach to identify inflammasome-related pathways activated by IAV infection. The most consistent differences in IAV-induced gene expression were the increased expression of CASP1 and CASP4 in HBEC from asthmatics. Caspase-4, a member of the caspase-1 subfamily, is involved in inflammatory responses to IAV infection (46–48). The murine homolog of caspase-4, caspase-11, may be involved in caspase-1 activation and inflammasome-mediated cell death (49, 50).
Activation of the inflammasome pathway involves oligomerization of caspase-1 and PYCARD with a PRR to form the inflammasome complex. The formation of large caspase-1/PYCARD foci has previously been shown in macrophages and monocytes (51–53). In resting monocytes and macrophages, PYCARD and caspase-1 exist diffusely throughout the cell (50, 52). When stimulated by an agonist, PYCARD forms large cytosolic foci, which are often associated with NLRs and caspase-1 (50). Using immunofluorescence microscopy, we demonstrated IAV-induced co-localization of caspase-1 and PYCARD in HBEC. In agreement with the gene expression and cytokine data, we observed large and intense caspase-1 and PYCARD co-localization with IAV infection especially in asthmatics at 24 hours post-IAV infection.
Our findings in HBEC indicate that caspase-1 is involved in the innate immune response to IAV infection. To further define the role of caspase-1 in antiviral defense, we infected MTEC from Casp1 −/− and wildtype mice with IAV. Notably, the mouse-adapted influenza virus influenza A/PR/8/34 strain was used to infect MTEC and may differ in infectivity compared the human influenza A/Bangkok/1/79 strain. Our results indicate that IAV-infected MTEC and HBEC had similar patterns of innate immune gene expression. We found that 24 hours post-IAV infection, Casp1 −/− MTEC had decreased viral replication, correlating with reduced expression of several innate immune genes. Interestingly, enhanced caspase-1 expression in HBEC was correlated with increased influenza HA transcript quantity, suggesting that caspase-1 may be a determinate of viral replication. Previous studies have shown that Casp1−/− mice infected in vivo with IAV had reduced quantities of innate immune cytokines and chemokines in the bronchoalveolar lavage, which correlated with increased severity of pneumonia and mortality (44). In contrast to our study, this study did not detect a difference in lung viral titers measured at 3 or 6 days post-IAV infection. Thus, though caspase-1 appears important for IAV-induced expression and secretion of cytokines and chemokines, caspase-1 involvement in viral replication remains unclear. Notably, the Casp-1 −/− mouse model also harbors a mutation in the Casp11 gene, rendering the mice Casp1/Casp11 double knockouts (48). Our findings in HBEC show that expression of caspase-4 (the human homolog of caspase-11) is up-regulated following IAV infection. Future studies are necessary to delineate the functions of caspase-1 and caspase-4 in antiviral response.
Though our results suggest that caspase-1 is important for the innate immune response to IAV, whether caspase-1 activity is related to inflammasome complex formation remains unclear. We were unable to detect expression of many NOD-like receptors by the qRT-PCR array, including NLRP3, which was previously shown to be expressed in AECs following IAV infection (21, 44). While protein expression cannot be excluded, these results indicate that, in AECs, the NLRP3 inflammasome may play a less prominent role than caspase-1 and other complexes in the response to IAV.
Our findings suggest that HBEC from asthmatics have an enhanced innate immune response to IAV infection. We found that HBEC from asthmatics had baseline and virus-induced differences in gene expression, and that enhanced innate immune gene expression correlated with viral replication. However, we found no overall differences in viral replication. These findings are consistent with a recent genome-wide expression study demonstrating that HBEC from asthmatics have many baseline differences in gene expression also present in cells with RV infection (16). Yet, this study and others using HBEC have shown either enhanced or no difference in RV replication in asthmatics compared to non-asthmatics (16, 17, 54). Therefore, HBEC from asthmatics may not necessarily have a more severe viral load, but rather a more sensitive and severe innate immune response, contributing to airway inflammation and pathogenesis.
Collectively, our results demonstrate that caspase-1 is important for the AEC innate immune response to IAV infection, which is enhanced in HBEC from asthmatics. Similar to other studies, our findings suggest that caspase-1 regulates the expression and secretion of cytokines besides IL-1β and IL-18, and thus may have other non-inflammasome related functions contributing to innate immunity (44). Since viral infections are the leading cause of asthma exacerbations, understanding the contribution of modified innate immune mechanisms to asthma pathogenesis is essential for the development of relevant therapies.
Supplementary Material
Key Messages.
The innate immune response to influenza virus infection is enhanced in human bronchial epithelial cells from asthmatics, which may contribute to the heightened influenza-related pathogenesis observed in asthmatics in vivo.
Caspase-1 plays an important role in the innate immune response to influenza virus infection in airway epithelial cells, and is enhanced in airway epithelial cells from asthmatics, which may contribute to virus-induced asthma pathogenesis.
Acknowledgments
Funding Sources:
This project was supported in part by the following grants: U19A1O77347 from the National Institute for Allergy and Infectious Diseases and ES013611 from the National Institute of Environmental Health Sciences. This article has been funded in part by the U.S. Environmental Protection Agency through cooperative agreement CR83346301 with the Center for Environmental Medicine, Asthma, and Lung Biology at The University of North Carolina at Chapel Hill, but does not reflect the official views of the agency and has not been subjected to the agency’s required peer and policy review. No official endorsement should be inferred.
The authors acknowledge Dr. Beverly Koller, Department of Genetics, University of North Carolina at Chapel Hill for providing the Casp1 −/− and wildtype mice and scientific input. We thank Dr. Jenny Ting, Department of Microbiology and Immunology, University of North Carolina at Chapel Hill for her insightful review and discussion of the manuscript. We acknowledge the assistance of Lisa Dailey and Maryann Bassett, and the Environmental Public Health Division of the US EPA for providing the human bronchial biopsy samples and cultures and subject information.
Abbreviations
- Casp1 −/− mice
caspase-1 deficient mice
- CASP1
caspase-1
- Casp11
caspase-11
- CASP4
caspase-4
- CCL5
Chemokine (C-C motif) ligand 5
- ELISA
Enzyme-linked immunosorbent assay
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- HA
hemagglutinin
- HAU
hemagglutination units
- HBEC
human bronchial epithelial cell
- HBSS
Hank’s buffered saline solution
- IAV
influenza A virus
- IFNα
interferon alpha
- IFNβ
interferon beta
- IL
interleukin
- Influenza HxNx
Influenza Hemagglutinin x, Neuraminidase x
- IP-10
Interferon gamma-induced protein 10
- KC
keratinocyte chemoattractant
- M1
influenza matrix 1 protein
- MDCK
Madin Darby canine kidney
- MIP-2
Macrophage inflammatory protein 2
- MTEC
mouse tracheal epithelial cells
- NLR
NOD-like receptor
- NLRC5
NLR family, CARD domain containing 5
- NLRP3
NLR family, pyrin domain containing 3
- NOD
Nucleotide-binding oligomerization domain
- NOD2
Nucleotide-binding oligomerization domain protein 2
- PRR
pattern recognition receptor
- qRT-PCR
quantitative real-time polymerase chain reaction
- RIG-I
retinoic acid-inducible gene 1 protein
- RIPK2
Receptor-interacting serine/threonine-protein kinase 2
- RLR
RIG-I- like receptor
- RSV
respiratory syncytial virus
- RV
rhinovirus
- SNR
signal-to-noise
- TCID50
50% tissue culture infectious dose
- TLR
Toll-like receptor
- TNFα
tumor necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murray CS, Simpson A, Custovic A. Allergens, viruses, and asthma exacerbations. Proc Am Thorac Soc. 2004;1(2):99–104. doi: 10.1513/pats.2306027. [DOI] [PubMed] [Google Scholar]
- 2.Gern JE. Mechanisms of virus-induced asthma. J Pediatr. 2003 Feb;142(2 Suppl):S9–13. doi: 10.1067/mpd.2003.20. discussion S13–4. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993 Oct 16;307(6910):982–6. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995 May 13;310(6989):1225–9. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: Origin, effect, and prevention. J Allergy Clin Immunol. 2011 Dec;128(6):1165–74. doi: 10.1016/j.jaci.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dulek DE, Peebles RS. Viruses and asthma. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbagen.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleege L, Hallberg E, Morin C, Danila R, Lynfield R. Novel H1N1 influenza hospitalizations: Minneapolis-st. paul metropolitan area, 2008–2009. Minn Med. 2009 Nov;92(11):38–42. [PubMed] [Google Scholar]
- 8.O’Riordan S, Barton M, Yau Y, Read SE, Allen U, Tran D. Risk factors and outcomes among children admitted to hospital with pandemic H1N1 influenza. CMAJ. 2010 Jan 12;182(1):39–44. doi: 10.1503/cmaj.091724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plessa E, Diakakis P, Gardelis J, Thirios A, Koletsi P, Falagas ME. Clinical features, risk factors, and complications among pediatric patients with pandemic influenza A (H1N1) Clin Pediatr (Phila) 2010 Aug;49(8):777–81. doi: 10.1177/0009922810368558. [DOI] [PubMed] [Google Scholar]
- 10.Libster R, Bugna J, Coviello S, Hijano DR, Dunaiewsky M, Reynoso N, et al. Pediatric hospitalizations associated with 2009 pandemic influenza A (H1N1) in argentina. N Engl J Med. 2010 Jan 7;362(1):45–55. doi: 10.1056/NEJMoa0907673. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010 Mar 19;140(6):805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Kanneganti TD. Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol. 2010 Oct;10(10):688–98. doi: 10.1038/nri2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poeck H, Bscheider M, Gross O, Finger K, Roth S, Rebsamen M, et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat Immunol. 2010 Jan;11(1):63–9. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- 14.Lamkanfi M. Emerging inflammasome effector mechanisms. Nat Rev Immunol. 2011 Mar;11(3):213–20. doi: 10.1038/nri2936. [DOI] [PubMed] [Google Scholar]
- 15.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011 Apr 23;29:707–35. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bochkov YA, Hanson KM, Keles S, Brockman-Schneider RA, Jarjour NN, Gern JE. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2010 Jan;3(1):69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005 Mar 21;201(6):937–47. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006 Sep;12(9):1023–6. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 19.Hackett TL, Singhera GK, Shaheen F, Hayden P, Jackson GR, Hegele RG, et al. Intrinsic phenotypic differences of asthmatic epithelium and its inflammatory responses to RSV and air pollution. Am J Respir Cell Mol Biol. 2011 Jun 3; doi: 10.1165/rcmb.2011-0031OC. [DOI] [PubMed] [Google Scholar]
- 20.Contoli M, Caramori G, Mallia P, Johnston S, Papi A. Mechanisms of respiratory virus-induced asthma exacerbations. Clin Exp Allergy. 2005 Feb;35(2):137–45. doi: 10.1111/j.1365-2222.2005.02163.x. [DOI] [PubMed] [Google Scholar]
- 21.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009 Apr 17;30(4):556–65. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciencewicki J, Brighton L, Wu WD, Madden M, Jaspers I. Diesel exhaust enhances virus- and poly(I:C)-induced toll-like receptor 3 expression and signaling in respiratory epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006 Jun;290(6):L1154–63. doi: 10.1152/ajplung.00318.2005. [DOI] [PubMed] [Google Scholar]
- 23.You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: Selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol. 2002 Dec;283(6):L1315–21. doi: 10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]
- 24.Kesic MJ, Simmons SO, Bauer R, Jaspers I. Nrf2 expression modifies influenza A entry and replication in nasal epithelial cells. Free Radic Biol Med. 2011 Apr 19; doi: 10.1016/j.freeradbiomed.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horvath KM, Brighton LE, Zhang W, Carson JL, Jaspers I. Epithelial cells from smokers modify dendritic cell responses in the context of influenza infection. Am J Respir Cell Mol Biol. 2011 Aug;45(2):237–45. doi: 10.1165/rcmb.2010-0190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W, Beck MA. Selenium deficiency induced an altered immune response and increased survival following influenza A/Puerto Rico/8/34 infection. Exp Biol Med (Maywood) 2007 Mar;232(3):412–9. [PubMed] [Google Scholar]
- 27.Jaspers I, Sheridan PA, Zhang W, Brighton LE, Chason KD, Hua X, et al. Exacerbation of allergic inflammation in mice exposed to diesel exhaust particles prior to viral infection. Part Fibre Toxicol. 2009 Aug 14;6:22. doi: 10.1186/1743-8977-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaspers I, Ciencewicki JM, Zhang W, Brighton LE, Carson JL, Beck MA, et al. Diesel exhaust enhances influenza virus infections in respiratory epithelial cells. Toxicol Sci. 2005 Jun;85(2):990–1002. doi: 10.1093/toxsci/kfi141. [DOI] [PubMed] [Google Scholar]
- 29.Jaspers I, Zhang W, Fraser A, Samet JM, Reed W. Hydrogen peroxide has opposing effects on IKK activity and IkappaBalpha breakdown in airway epithelial cells. Am J Respir Cell Mol Biol. 2001 Jun;24(6):769–77. doi: 10.1165/ajrcmb.24.6.4344. [DOI] [PubMed] [Google Scholar]
- 30.Jaspers I, Ciencewicki JM, Zhang W, Brighton LE, Carson JL, Beck MA, et al. Diesel exhaust enhances influenza virus infections in respiratory epithelial cells. Toxicol Sci. 2005 Jun;85(2):990–1002. doi: 10.1093/toxsci/kfi141. [DOI] [PubMed] [Google Scholar]
- 31.Beck MA, Nelson HK, Shi Q, Van Dael P, Schiffrin EJ, Blum S, et al. Selenium deficiency increases the pathology of an influenza virus infection. FASEB J. 2001 Jun;15(8):1481–3. [PubMed] [Google Scholar]
- 32.Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP. GenePattern 2.0. Nat Genet. 2006 May;38(5):500–1. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- 33.Lee CW, Suarez DL. Application of real-time RT-PCR for the quantitation and competitive replication study of H5 and H7 subtype avian influenza virus. J Virol Methods. 2004 Aug;119(2):151–8. doi: 10.1016/j.jviromet.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Brightling C, Berry M, Amrani Y. Targeting TNF-alpha: A novel therapeutic approach for asthma. J Allergy Clin Immunol. 2008 Jan;121(1):5,10. doi: 10.1016/j.jaci.2007.10.028. quiz 11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neveu WA, Allard JL, Raymond DM, Bourassa LM, Burns SM, Bunn JY, et al. Elevation of IL-6 in the allergic asthmatic airway is independent of inflammation but associates with loss of central airway function. Respir Res. 2010 Mar 8;11:28. doi: 10.1186/1465-9921-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tillie-Leblond I, Pugin J, Marquette CH, Lamblin C, Saulnier F, Brichet A, et al. Balance between proinflammatory cytokines and their inhibitors in bronchial lavage from patients with status asthmaticus. Am J Respir Crit Care Med. 1999 Feb;159(2):487–94. doi: 10.1164/ajrccm.159.2.9805115. [DOI] [PubMed] [Google Scholar]
- 37.Venge J, Lampinen M, Hakansson L, Rak S, Venge P. Identification of IL-5 and RANTES as the major eosinophil chemoattractants in the asthmatic lung. J Allergy Clin Immunol. 1996 May;97(5):1110–5. doi: 10.1016/s0091-6749(96)70265-8. [DOI] [PubMed] [Google Scholar]
- 38.Culley FJ, Pennycook AM, Tregoning JS, Dodd JS, Walzl G, Wells TN, et al. Role of CCL5 (RANTES) in viral lung disease. J Virol. 2006 Aug;80(16):8151–7. doi: 10.1128/JVI.00496-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wark PA, Bucchieri F, Johnston SL, Gibson PG, Hamilton L, Mimica J, et al. IFN-gamma-induced protein 10 is a novel biomarker of rhinovirus-induced asthma exacerbations. J Allergy Clin Immunol. 2007 Sep;120(3):586–93. doi: 10.1016/j.jaci.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janssen-Heininger YM, Poynter ME, Aesif SW, Pantano C, Ather JL, Reynaert NL, et al. Nuclear factor kappaB, airway epithelium, and asthma: Avenues for redox control. Proc Am Thorac Soc. 2009 May 1;6(3):249–55. doi: 10.1513/pats.200806-054RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hart LA, Krishnan VL, Adcock IM, Barnes PJ, Chung KF. Activation and localization of transcription factor, nuclear factor-kappaB, in asthma. Am J Respir Crit Care Med. 1998 Nov;158(5 Pt 1):1585–92. doi: 10.1164/ajrccm.158.5.9706116. [DOI] [PubMed] [Google Scholar]
- 42.Lamkanfi M, Kalai M, Saelens X, Declercq W, Vandenabeele P. Caspase-1 activates nuclear factor of the kappa-enhancer in B cells independently of its enzymatic activity. J Biol Chem. 2004 Jun 4;279(23):24785–93. doi: 10.1074/jbc.M400985200. [DOI] [PubMed] [Google Scholar]
- 43.Werts C, le Bourhis L, Liu J, Magalhaes JG, Carneiro LA, Fritz JH, et al. Nod1 and Nod2 induce CCL5/RANTES through the NF-kappaB pathway. Eur J Immunol. 2007 Sep;37(9):2499–508. doi: 10.1002/eji.200737069. [DOI] [PubMed] [Google Scholar]
- 44.Thomas PG, Dash P, Aldridge JR, Jr, Ellebedy AH, Reynolds C, Funk AJ, et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009 Apr 17;30(4):566–75. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ichinohe T, Pang IK, Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol. 2010 May;11(5):404–10. doi: 10.1038/ni.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bian ZM, Elner SG, Elner VM. Dual involvement of caspase-4 in inflammatory and ER stress-induced apoptotic responses in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2009 Dec;50(12):6006–14. doi: 10.1167/iovs.09-3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberson EC, Tully JE, Guala AS, Reiss JN, Godburn KE, Pociask DA, et al. Influenza induces ER stress, caspase-12-dependent apoptosis and JNK mediated TGF-{beta} release in lung epithelial cells. Am J Respir Cell Mol Biol. 2011 Jul 28; doi: 10.1165/rcmb.2010-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kayagaki N, Warming S, Lamkanfi M, Walle LV, Louie S, Dong J, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011 Oct 16; doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 49.Wang S, Miura M, Jung YK, Zhu H, Li E, Yuan J. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell. 1998 Feb 20;92(4):501–9. doi: 10.1016/s0092-8674(00)80943-5. [DOI] [PubMed] [Google Scholar]
- 50.Bryan NB, Dorfleutner A, Rojanasakul Y, Stehlik C. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J Immunol. 2009 Mar 1;182(5):3173–82. doi: 10.4049/jimmunol.0802367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masumoto J, Taniguchi S, Ayukawa K, Sarvotham H, Kishino T, Niikawa N, et al. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem. 1999 Nov 26;274(48):33835–8. doi: 10.1074/jbc.274.48.33835. [DOI] [PubMed] [Google Scholar]
- 52.Brough D, Rothwell NJ. Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J Cell Sci. 2007 Mar 1;120(Pt 5):772–81. doi: 10.1242/jcs.03377. [DOI] [PubMed] [Google Scholar]
- 53.Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, et al. The pyroptosome: A supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007 Sep;14(9):1590–604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopez-Souza N, Favoreto S, Wong H, Ward T, Yagi S, Schnurr D, et al. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J Allergy Clin Immunol. 2009 Jun;123(6):1384,90.e2. doi: 10.1016/j.jaci.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.