Abstract
Background
High homocysteine levels may be neurotoxic and contribute to cognitive decline in older persons.
Objective
Examine the effect of supplementation with folic acid, vitamin B12 and vitamin B6 on cognitive change among women with cardiovascular disease (CVD) or CVD risk factors.
Design
The Women's Antioxidant and Folic Acid Cardiovascular Study is a randomized, placebo-controlled trial to test a combination of B vitamins (folic acid 2.5 mg, vitamin B6 50 mg, and vitamin B12 1 mg, daily) for secondary prevention of CVD. Randomization took place among 5,442 female health professionals, 40+ years, with CVD or at least three coronary risk factors in 1998 (after folic acid fortification began in the US). Shortly after randomization (mean=1.2 years), a cognitive function substudy was initiated among 2009 participants aged 65+ years. Telephone cognitive function testing was administered up to four times over 5.4 years with 5 tests of general cognition, verbal memory and category fluency. Repeated measures analyses were conducted. The primary outcome was a global composite score averaging all tests.
Results
Mean cognitive change from baseline did not differ between the B vitamin and placebo groups (difference in change in global score= 0.03, 95% CI −0.03, 0.08; p=0.30). However, supplementation appeared to confer benefits in preserving cognition among women with low baseline dietary intake of B vitamins.
Conclusions
Combined B vitamin supplementation did not delay cognitive decline among women with CVD or CVD risk factors. Possible cognitive benefits of supplementation among women with low dietary intake of B vitamins warrant further study.
INTRODUCTION
Substantial research implicates vascular factors in both cognitive decline and dementia – including cognitive outcomes not traditionally associated with vascular health, such as general cognition, episodic memory and Alzheimer's dementia (AD).(1) Thus, identifying methods to prevent cognitive decline and dementia in this high-risk group with vascular disease is important.
Addressing homocysteine levels may be promising. High levels of homocysteine have been shown to be neurotoxic: homocysteine raises intracellular amyloid beta levels (believed to be one of the earliest pathologic features in AD) (2, 3), increases plaque deposition,(3) sensitizes hippocampal neurons to amyloid beta's toxic effects(4, 5) and lowered spatial learning in animal models.(3, 6)
Thus, we hypothesize that homocysteine lowering via supplementation with folic acid, vitamin B6 and vitamin B12 may be an effective intervention against cognitive impairment in a high risk group with vascular disease or major risk factors. Two previous studies reported no cognitive benefits of supplementation with B vitamins among this high risk population, yet they have been of relatively short duration (<3 years).(7, 8)
Therefore, in a randomized placebo-controlled trial of 2009 women, with 6.6 years of treatment and 5.4 years of follow-up for cognitive function, we examined the effect of supplementation with a combination of B vitamins on cognitive function among older women with cardiovascular disease or vascular risk factors.
METHODS
In 1995 – 1996, the Women's Antioxidant Cardiovascular Study (WACS) began as a 2×2×2 randomized placebo-controlled trial of 3 antioxidants (vitamin E, vitamin C, and β-carotene) for the secondary prevention of cardiovascular disease (CVD) among 8171 women. (9) In April 1998, a fourth arm of folic acid/vitamin B6/vitamin B12 (folic acid 2.5 mg, vitamin B6 50 mg, vitamin B12 1 mg, taken daily) was added and the Women's Antioxidant and Folic Acid Cardiovascular Study (WAFACS) was initiated among 5,442 women (thus, the 5,442 women were assigned to 16 treatment assignments (2×2×2×2) that were combinations of the 3 antioxidants and 1 B vitamin supplement.).(10) Folic acid/vitamin B6/vitamin B12 and placebos were provided by BASF Corporation (Mount Olive, NJ). Eligible women were female health professionals, 40+ years, with cardiovascular disease (CVD) or at least three coronary risk factors. CVD included myocardial infarction, stroke, revascularization procedures (percutaneous transluminal angioplasty, coronary artery bypass graft, carotid endarterectomy, and peripheral artery surgery), and symptomatic angina pectoris or transient cerebral ischemia. Risk factors included current tobacco use, hypertension, high cholesterol, diabetes, parental history of premature MI, or obesity (BMI ≥ 30 kg/m2). For assessment of baseline diet, including intake of B vitamins, a semiquantitative food-frequency questionnaire (FFQ) was administered; this FFQ was developed and validated in a similar cohort of women.(11, 12) In a three-month run-in phase to assess compliance, women received placebo caplets; women who reported good compliance, had no history of cancer, active liver disease, chronic kidney failure, or use of anticoagulants, and who expressed willingness to forego the use of out-of-study vitamin supplements were randomized (an exception was made for vitamin supplements, including multivitamins that provided only up to the recommended daily allowances; any supplements that exceeded RDA levels were not permitted).
Every 12 months, the women were sent a year's supply of monthly calendar packs containing active agents or placebo. Women completed mailed questionnaires annually to update information on compliance, side effects, health and lifestyle characteristics and the occurrence of clinical endpoints. They were followed through the scheduled end (July 31, 2005); the overall follow-up of the cohort for mortality and morbidity exceeded 98% of total potential person-years of follow-up.(13) The average compliance during follow-up was 83% and did not differ significantly between the two groups.(13) All trial participants provided written informed consent; the trial was approved by the institutional review board of Brigham and Women's Hospital, Boston, and was monitored by an external data and safety monitoring board.
The results of the primary trial of B vitamin supplementation have been reported previously;(13) briefly, supplementation with B vitamins was not found to protect against recurrent cardiovascular disease. There were no major adverse effects of the treatment.(13)
Cognitive Cohort
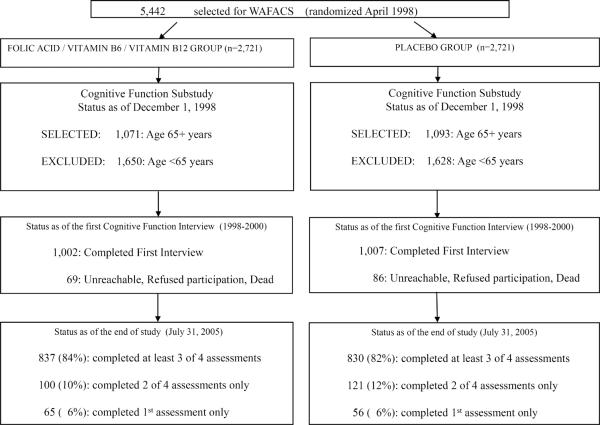
From December 1998-July 2000, mean 1.2 years after B vitamin randomization, a substudy of cognitive function was initiated among active WAFACS participants aged 65 years or older (n=2,164). Of these, 155 women (7%) were unreachable, declined participation or had died; thus 2009 (93%) women completed the initial telephone cognitive assessment (Figure 1). Participation in the initial cognitive interview was virtually identical by treatment group.
Figure 1.
Flow chart of Participation in the Cognitive Cohort of Women's Antioxidant and Folic Acid Cardiovascular Study (WAFACS)
Participants received three follow-up cognitive assessments approximately every two years. High follow-up was maintained in both the treatment and placebo groups (Figure 1): 94% completed at least one follow-up assessment, and 83% completed at least 3 of 4 assessments. In the fourth assessment, 24% of participants were not contacted for their assessment since only a short interval had passed between their third interview and the end of the trial in July 2005. Follow-up rates were nearly identical across treatment groups at each assessment.
Cognitive Function Assessment
We assessed cognitive function using a telephone cognitive battery (Table 1) with 5 tests measuring general cognition, verbal memory and category fluency. For general cognition, we used the Telephone Interview of Cognitive Status (TICS)(14) a telephone adaptation of the Mini-Mental State Examination (MMSE). For verbal memory, we administered the delayed recall of the TICS 10-word list, and the immediate and delayed recalls of the East Boston Memory Test,(15) in which a short paragraph is read and 12 key elements must be repeated immediately and 15 minutes later. Finally, for category fluency (used to measure executive retrieval functions),(16) women were asked to name as many animals as possible in one minute.
Table 1.
Baseline Characteristics of Participants in the WACS Cognitive Cohort *
| Characteristics | Folic acid / B6 / B12 (n=1002) Mean (SD; range) | Placebo (n=1007) Mean (SD; range) | p-value † |
|---|---|---|---|
| Age at randomization,years | 71.3 (4.2; 65.3–89.9) | 71.3 (4.2; 65.3–90.0) | 0.96 |
| Age at initial cognitive assessment,years | 72.5 (4.2; 66.1–90.9) | 72.5 (4.2; 66.1–91.2) | 0.99 |
| Total Physical Activity (kcal/week) | 905 (1112; 0 – 7825) | 920 (1185; 0– 19017) | 0.36 |
| Body mass index (kg/m2) | 29.0 (5.9; 15.4 – 55.6) | 28.6 (5.5; 16.3 – 54.7) | 0.37 |
| Median (25th–75th percentile) | Median (25th–75th percentile) | ||
| Total Folate intake from diet and supplements (μg/day)‡ | 455 (323 – 700) | 455 (319–685) | 0.84 |
| Total Vitamin B6 intake from diet and supplements (mg/day) | 2.6 (1.9 – 3.9) | 2.7 (1.9 – 4.01) | 0.34 |
| Total Vitamin B12 intake from diet and supplements (μg/day) | 7.3 (4.8 – 11.1) | 7.4 (4.7 – 11.1) | 0.76 |
| Total Alcohol intake (g/day) | 0 (0 – 2.9) | 0 (0 –3.5) | 0.59 |
| Percent | Percent | ||
| Highest attained education | |||
| LPVN/AD/RN § | 70.4 | 70.3 | |
| BA/MA/DR § | 29.6 | 29.7 | 0.97 |
| Cigarette smoking | |||
| Never smoker (%) | 46.5 | 47.7 | |
| Past smoker (%) | 46.6 | 44.6 | |
| Current smoker (%) | 6.9 | 7.8 | 0.58 |
| Postmenopausal hormone therapy | |||
| Never user (%) | 39.9 | 40.3 | |
| Past user (%) | 24.8 | 24.0 | |
| Current user (%) | 35.3 | 35.7 | 0.93 |
| History of MI | 21.7 | 22.1 | 0.79 |
| History of Stroke | 9.7 | 8.4 | 0.33 |
| History of Revascularization Surgery | 23.4 | 22.5 | 0.67 |
| History of Angina | 50.5 | 47.8 | 0.22 |
| History of Transient Ischemic Attack | 15.4 | 16.9 | 0.36 |
| History of Diabetes | 18.9 | 20.1 | 0.50 |
| History of Hypertension | 88.6 | 89.2 | 0.69 |
| History of Hyperlipidemia | 79.2 | 80.6 | 0.44 |
Characteristics as of randomization
For tests of differences between the two groups for all continuous variables, Wilcoxon rank-sum tests were used, and for categorical variables, chi-square tests were used
Folate intake has been calculated as folate equivalents
LPVN: Licensed practical or vocational nurse; AD: Associate's degree; RN: Registered nurse; BA: Bachelor's degree; MA: Master's degree; DR: Doctoral degree
The primary, pre-specified outcome was a global composite score averaging all five cognitive tests, using z-scores. Because verbal memory is strongly associated with risk of Alzheimer disease,(17) our key secondary outcome was a verbal memory composite score, calculated by averaging four measures of verbal memory (the immediate and delayed recalls of both the East Boston Memory Test and 10 word list). To calculate the composite scores for participants who did not complete all tests (only 0.5% of participants for the global composite score and 0.5% for the verbal memory score), we used the mean of the z-scores of the tests that were completed.
The telephone cognitive interviews were administered by trained interviewers. There was high reliability and validity of our telephone cognitive test battery. In a test-retest reliability study of the TICS, administered twice 31 days apart, we found a correlation of 0.7 (p<0.001) among 35 high-functioning, educated women. In a validation study of our telephone instrument, 61 women who had completed an extensive in-person interview were administered our brief telephone-administered assessment; we found a correlation of 0.81 comparing the overall performance on those two measures, demonstrating high validity of our telephone method. In addition, among 88 older female health professionals, cognitive impairment as determined by our telephone assessment was strongly associated with dementia diagnosis after three years; poor performance in the TICS and in verbal memory were both associated with significant 8 and 12 fold increases, respectively, of dementia.
Statistical Analysis
Characteristics at baseline between randomized groups were compared using Wilcoxon rank sum tests for continuous variables and chi-square tests for proportions.
To analyze differences in patterns of change in cognition between the active and placebo groups, we selected an approach that addressed the non-linearity of the data. Because of a “learning effect,” test scores generally improved with time, particularly at the second assessment; however, this common, well-recognized phenomenon in cognitive function studies(18) does not prohibit detection of overall differences between treatment groups. To address the non-linearity of scores over time, general linear models of response profiles were fitted, with time modeled with indicator variables for assessment, rather than with a linear variables;(19) this approach imposes minimal structure on outcome trends over time, yet still permits valid estimation of effects in non-linear data. First, we evaluated mean performance at each cognitive assessment by treatment assignment using repeated measures analysis of means, accounting for correlations between assessments: E(Yij) = β0 + β1 timej + β2 groupi + β3 groupi * timei, where Yij is one of the four cognitive outcomes for group i (active or placebo) at time j (1,2,3,4). Second, mean cognitive change from baseline over the second through fourth assessments by treatment assignment was our main analytic measure and it was modeled as: E(Yij - Yi1) = β0 + β1 timej + β2 groupi, where Yij - Yi1 is the change from baseline for one of the four cognitive outcomes for group i (active or placebo) at time j (2,3,4). Because the risk factors for cognitive decline were distributed similarly between the active and placebo groups, the main analyses did not adjust for other factors. All models were fitted by maximum likelihood, incorporating the longitudinal correlation within study subjects using unstructured covariance structures and treatment assignment was modeled as fixed effects; for statistical testing, we used Wald tests.(19) For all statistical analyses, Proc Mixed in SAS (SAS release 9.1, SAS Institute Inc., Cary, NC) was used.
We examined effect modification by key risk factors (all assessed prior to randomization) for cognitive decline such as age, baseline cognitive performance, education, the presence of either prevalent CVD events versus prevalent CVD risk factors, and incident cardiovascular disease during the trial and by factors that may influence B vitamin metabolism such as alcohol drinking, cigarette smoking, multivitamin use, and dietary intake of B vitamins. Tests of effect modification were performed by evaluating interaction terms in the models of mean change in cognition from baseline. We examined the influence of non-compliance by repeating the main analyses after excluding women who were taking less than one-third of their assigned study medications.
To consider the antioxidant arms, in models adjusting for antioxidant assignments, results did not change materially from the main analysis; thus we did not include them as covariates in any models for the main analyses. Effect modification by assignment to antioxidant agents was also not observed.
Finally, to assess the impact of B vitamin supplementation on the risk of “cognitive impairment”, we fitted logistic regression models, defining the outcome as the worst 10% of the distribution of change from the initial to the final cognitive assessment. In these models, we adjusted for the follow-up time between the first and last assessments.
RESULTS
Primary analyses
The average time from randomization to the initial cognitive assessment was 1.2 years (range 0.7 – 1.7), and from randomization to the last assessment was 6.6 years (range 6.3 – 6.9). Compliance was comparable between the two treatment groups (83%).(13) Demographic and health characteristics at randomization were similar between treatment and placebo groups (Table 1).
At the first cognitive assessment, cognitive performance did not differ by treatment (Table 2); the mean difference in the global composite score between the vitamin B and placebo groups was 0.01 (95% CI −0.05, 0.07). At the final cognitive assessment (after a mean 6.6 years of treatment), there was no difference observed between the treatment and placebo groups (mean difference=0.05, 95% CI, −0.04, 0.14). Similarly, for our secondary endpoint of verbal memory, the groups did not differ at any of the cognitive assessments: for example, at the final assessment, the mean difference in performance was 0.07 (95% CI, −0.03, 0.16). The treatment group also did not show better performance in either the TICS or category fluency.
Table 2.
Cognitive function at each cognitive assessment, by B vitamin treatment
| Treatment Assignment | |||||
|---|---|---|---|---|---|
| Cognitive Test | Folic acid / B6 / B12 | Placebo | |||
| Cognitive Assessment | No. of subjects | Mean (SE) * | No. of subjects | Mean (SE) * | Mean difference [B vitamin Group - Placebo Group] (95% CI) † |
| PRIMARY ENDPOINT: Global score ‡ | |||||
| 1 | 1002 | 0.00 (0.02) | 1007 | −0.01 (0.02) | 0.01 (−0.05, 0.07) |
| 2 | 911 | 0.06 (0.03) | 923 | 0.01 (0.02) | 0.05 (−0.01, 0.12) |
| 3 | 850 | −0.04 (0.03) | 845 | −0.07 (0.03) | 0.02 (−0.06, 0.10) |
| 4 | 521 | −0.02 (0.03) | 532 | −0.07 (0.03) | 0.05 (−0.04,0.14) |
| KEY SECONDARY ENDPOINT: Verbal memory score ‡ | |||||
| 1 | 1002 | 0.00 (0.02) | 1007 | 0.00 (0.02) | 0.01 (−0.06, 0.07) |
| 2 | 911 | 0.07 (0.03) | 923 | 0.01 (0.03) | 0.06 (−0.02, 0.13) |
| 3 | 850 | 0.03 (0.03) | 845 | 0.03 (0.03) | 0.00 (−0.07, 0.08) |
| 4 | 521 | 0.03 (0.03) | 532 | −0.04 (0.03) | 0.06 (−0.03, 0.16) |
| TICS score ‡ | |||||
| 1 | 1002 | 34.35 (0.10) | 1007 | 34.38 (0.10) | −0.04 (−0.31, 0.24) |
| 2 | 911 | 34.39 (0.11) | 923 | 34.30 (0.10) | 0.09 (−0.21, 0.39) |
| 3 | 850 | 34.05 (0.11) | 845 | 33.94 (0.13) | 0.12 (−0.22, 0.45) |
| 4 | 521 | 33.94 (0.13) | 532 | 33.64 (0.15) | 0.30 (−0.09, 0.69) |
| Category fluency score | |||||
| 1 | 1000 | 16.65 (0.16) | 1007 | 16.32 (0.15) | 0.34 (−0.09, 0.76) |
| 2 | 909 | 16.82 (0.17) | 921 | 16.49 (0.16) | 0.33 (−0.12, 0.79) |
| 3 | 846 | 15.65 (0.17) | 840 | 15.33 (0.18) | 0.32 (−0.16, 0.80) |
| 4 | 519 | 16.46 (0.20) | 530 | 16.57 (0.19) | −0.11 (−0.65,0.43) |
Least squares means and standard errors
From longitudinal linear models of least square means of cognitive performance at each assessment
TICS = Telephone Interview of Cognitive Status;
Verbal score is a composite score of the z-scores of the immediate and delayed recalls of both the TICS 10-word and the East Boston Memory Test;
Global score is a composite score of the z-scores of TICS, immediate and delayed recalls of the East Boston Memory Test, category fluency, delayed recall of the TICS 10-word list
When we evaluated the mean change in cognitive performance from the initial through the final assessments, we observed similar results (Table 3), with no significant differences by treatment assignment. The mean difference in cognitive decline over time between the treatment and placebo groups was 0.03 standard units per year (95% CI,−0.03, 0.08) for the global score; 0.03 (95% CI,−0.03, 0.09) for the verbal memory score; 0.16 points (95% CI, −0.11, 0.44) for the TICS; and −0.13 points (95% CI, −0.51, 0.25) for category fluency.
Table 3.
Mean cognitive decline over follow-up, by B vitamin treatment
| Cognitive Test | Mean difference in cognitive decline [Folic acid / B6 / B12 Group - Placebo Group] (95% CI) | p-value † |
|---|---|---|
| PRIMARY ENDPOINT: Global score * | ||
| From initial cognitive assessment to: | ||
| 2nd cognitive assessment | 0.04 (−0.02, 0.10) | 0.21 |
| 3rd cognitive assessment | 0.01 (−0.06, 0.08) | 0.74 |
| 4th cognitive assessment | 0.04 (−0.05, 0.13) | 0.40 |
| Average over follow-up | 0.03 (−0.03, 0.08) | 0.30 |
| KEY SECONDARY ENDPOINT: Verbal memory score * | ||
| From initial cognitive assessment to: | ||
| 2nd cognitive assessment | 0.05 (−0.02,0.12) | 0.18 |
| 3rd cognitive assessment | 0.00 (−0.08, 0.07) | 0.96 |
| 4th cognitive assessment | 0.05 (−0.04,0.15) | 0.26 |
| Average over follow-up | 0.03 (−0.03, 0.09) | 0.36 |
| TICS score * | ||
| From initial cognitive assessment to: | ||
| 2nd cognitive assessment | 0.12 (−0.18, 0.42) | 0.45 |
| 3rd cognitive assessment | 0.15 (−0.18,0.49) | 0.37 |
| 4th cognitive assessment | 0.34 (−0.05, 0.74) | 0.09 |
| Average over follow-up | 0.16 (−0.11, 0.44) | 0.25 |
| Category fluency score | ||
| From initial cognitive assessment to: | ||
| 2nd cognitive assessment | −0.06 (−0.52, 0.40) | 0.80 |
| 3rd cognitive assessment | −0.06 (−0.52, 0.41) | 0.81 |
| 4th cognitive assessment | −0.46 (−1.01, 0.09) | 0.10 |
| Average over follow-up | −0.13 (−0.51, 0.25) | 0.50 |
TICS = Telephone Interview of Cognitive Status;
Verbal score is a composite score of the z-scores of the immediate and delayed recalls of both the TICS 10-word and the East Boston Memory Test; Global score is a composite score of the z-scores of the TICS, immediate and delayed recalls of the East Boston Memory Test, category fluency, delayed recall of the TICS 10-word list
From longitudinal linear models of least square means of change in cognitive performance from baseline at each assessment and averaged over the follow-up. The number of subjects in each assessment is shown in Table 2.
The B vitamin group did not differ in their odds of substantial cognitive decline from the first through the final assessment compared with the placebo group. Compared with placebo, the relative risk (RR) for the B vitamin group was 1.04 (95% CI, 0.69, 1.56) in the global score (defined as 0.8 point decline), RR=1.08 (95% CI, 0.72, 1.63) for the verbal memory score (defined as 1.0 point decline), and RR=0.82 (95% CI, 0.55, 1.22) for the TICS. For category fluency there was some suggestion that supplementation was associated with increased risk of decline: RR=1.51 (95% CI, 0.97, 2.35; p=0.07) for the decline in category fluency.
Sub-group analyses
We investigated whether the effect of B vitamin supplementation differed by various characteristics (Table 4). For the primary outcome of the global score, we did not observe any statistically significant effect modification. However, we observed a general trend across the outcomes, where B vitamin supplementation conferred cognitive benefits among a subset of women with low levels of total intakes of B vitamins. We defined the cutpoints for “low” intake for vitamin B6 as <1.9 mg / day and for folate, as <279 μg / day; these cutpoints were based on the intake levels that were found to be significantly associated with elevated homocysteine (≥13 μmol / L) in the Framingham study.(20) For vitamin B12, there were no such low dietary intake threshold levels, thus, we used the RDI of 2.4 μg for older persons.(21) Finally, we created strata of women with either “low” intake for at least one of the three B vitamins or with adequate intakes in all three B vitamins.
Table 4.
Mean decline between B vitamin and placebo groups: effect modification by major risk factors for cognitive decline*
| Characteristics | Mean difference in cognitive decline † [Folic acid / B6 / B12 Group - Placebo Group] (95% CI) | |||
|---|---|---|---|---|
|
| ||||
| Global Score | Verbal Score | TICS | Category fluency | |
| Age at 1st assessment | ||||
| ≤ 72 years (n= 987) | 0.05 (−0.02, 0.13) | 0.07 (−0.02, 0.15) | 0.11 (−0.27,0.49) | −0.10 (−0.66, 0.46) |
| > 72 years (n= 1022) | 0.01 (−0.08, 0.09) | −0.01 (−0.10, 0.08) | 0.21 (−0.19, 0.61) | −0.16 (−0.69, 0.36) |
| P† | P=0.20 | P=0.71 | P=0.87 | |
| Dietary folate intake | ||||
| <279 mg/day (n= 293) | 0.14 (−0.01, 0.29) | 0.14 (−0.04, 0.31) | 1.24 (0.47,2.00) | −0.19 (−0.61, 0.24) |
| ≥279 mg/day (n=1,637) | 0.01 (−0.05, 0.07) | 0.00 (−0.06, 0.07) | −0.04 (−0.34, 0.26) | 0.00 (−0.99, 0.99) |
| P=0.11 | P=0.16 | P=0.002 | P=0.73 | |
| Dietary vitamin B6 intake | ||||
| < 1.9 mg/day (n= 524) | 0.02 (−0.05, 0.08) | 0.07 (−0.05, 0.20) | 0.56 (0.03, 1.10) | −0.69 (−1.43, 0.05) |
| ≥1.9 mg/day (n=1,406) | 0.05 (−0.06, 0.15) | 0.01 (−0.07, 0.08) | 0.00 (−0.33, 0.33) | 0.03 (−0.43, 0.49) |
| P=0.63 | P=0.35 | P=0.08 | P=0.10 | |
| Dietary vitamin B12 intake | ||||
| <2.4 μg/day (n= 82) | 0.19 (−0.06, 0.44) | 0.10 (−0.21, 0.41) | 1.35 (0.16, 2.54) | 1.62 (0.11, 3.13) |
| ≥2.4 μg/day (n=1848) | 0.02 (−0.04, 0.08) | 0.02 (−0.04, 0.09) | 0.10 (−0.18, 0.39) | −0.24 (−0.64, 0.17) |
| P=0.19 | P=0.62 | P=0.05 | P=0.02 | |
| Overall dietary vitamin B intake | ||||
| “Low” ‡ (n= 573) | 0.09 (−0.01, 0.20) | 0.12 (0.00, 0.24) | 0.74 (0.23, 1.25) | −0.39 (−1.10, 0.32) |
| “Adequate”‡ (n=1357) | 0.00 (−0.07, 0.06) | −0.02 (−0.09, 0.06) | −0.10 (−0.43, 0.24) | −0.07 (−0.54, 0.40) |
| P=0.11 | P=0.06 | P=0.01 | P=0.46 | |
| 1st cognitive assessment score ‡ | ||||
| Low (n=201) | 0.11 (−0.14, 0.37) | 0.01 (−0.24, 0.26) | 1.00 (−0.16, 2.17) | 0.17 (−1.05, 1.40) |
| Normal (n= 1808) | 0.02 (−0.03, 0.07) | 0.04 (−0.03, 0.10) | 0.07 (−0.20, 0.33) | −0.10 (−0.49, 0.28) |
| P=0.49 | P=0.82 | P=0.12 | P=0.67 | |
| Highest attained education∥ | ||||
| LPVN/AD/RN (n=1,330) | 0.04 (−0.03, 0.10) | 0.03 (−0.04, 0.11) | 0.34 (0.00, 0.67) | −0.07 (−0.54, 0.41) |
| BA, MA, DR (n= 560) | 0.02 (−0.09, 0.13) | 0.02 (−0.10, 0.15) | −0.12 (−0.65, 0.41) | −0.38 (−1.11, 0.34) |
| P=0.77 | P=0.90 | P=0.15 | P=0.47 | |
| Cigarette smoking | ||||
| Never smoker (n= 946) | 0.02 (−0.06, 0.10) | 0.05 (−0.04, 0.14) | 0.16 (−0.25, 0.56) | 0.50 (−1.04, 0.04) |
| Ever smoker (n=1,063) | 0.04 (−0.04, 0.11) | 0.01 (−0.08, 0.09) | 0.16 (−0.21, 0.54) | 0.21 (−0.33, 0.75) |
| P=0.79 | P=0.46 | P=0.98 | P=0.07 | |
| Alcohol drinking | ||||
| Non-drinker (n= 1006) | 0.02 (−0.06, 0.10) | 0.02 (−0.07, 0.10) | 0.06 (−0.33, 0.46) | 0.02 (−0.52, 0.55) |
| Drinker (n= 924) | 0.03 (−0.05, 0.11) | 0.03 (−0.06, 0.12) | 0.25 (−0.15, 0.64) | −0.36 (−0.93, 0.21) |
| P=0.87 | P=0.79 | P=0.52 | P=0.35 | |
| Multivitamin | ||||
| Non-user (n=1546) | 0.02 (−0.04, 0.09) | 0.03 (−0.04, 0.10) | 0.19 (−0.14, 0.51) | −0.21 (−0.64, 0.23) |
| User (n= 463) | 0.05 (−0.05, 0.16) | 0.03 (−0.10, 0.15) | 0.09 (−0.44, 0.61) | 0.12 (−0.69, 0.93) |
| P=0.65 | P=0.96 | P=0.76 | P=0.49 | |
| Prevalent CVD event/Risk factors¶ | ||||
| CVD event (n=1525) | 0.01 (−0.05, 0.07) | 0.02 (−0.06, 0.09) | 0.02 (−0.31, 0.34) | −0.15 (−0.59, 0.29) |
| Risk factors (n= 484) | 0.09 (−0.01, 0.20) | 0.07 (−0.04, 0.19) | 0.63 (0.12, 1.14) | −0.06 (−0.82, 0.71) |
| P=0.19 | P=0.44 | P=0.05 | P=0.83 | |
| Incident Cardiovascular disease** | ||||
| Absent (n=1624) | 0.03 (−0.02, 0.09) | 0.03 (−0.03, 0.10) | 0.15 (−0.13, 0.43) | −0.11 (−0.50, 0.28) |
| Present (n= 385) | −0.01 (−0.14, 0.12) | 0.00 (−0.15, 0.15) | 0.31 (−0.27, 0.89) | −0.41 (−1.32, 0.50) |
| P=0.54 | P=0.72 | P=0.59 | P=0.52 | |
Characteristics as of randomization, except for incident cardiovascular disease which occurred during follow-up;
From longitudinal linear models of least square means of change in cognitive performance from baseline at each assessment and averaged over the follow-up. The number of subjects in each assessment is shown in Table 2 ; P =p value for interaction for testing effect modification in longitudinal linear models.
“Low” overall dietary vitamin B intake is defined as those with low intakes in at least one of the three B vitamins: folate (<279 mg/day) or vitamin B6 (<1.9 mg/day) or vitamin B12 (<2.4 μg/day); “Adequate” overall dietary vitamin B intake was defined as adequate intakes of all three B vitamins (folate ≥279 mg/day and vitamin B6 ≥1.9 mg/day and vitamin B12≥2.4 μg/day)
Participants with “Low” score for the global score (n=201), verbal score (n=201) and animal score (n=197) were those who were in the lowest 10% of scores; for “Low” TICS score, we used the established cutpoint of 31 (n=199).
LPVN: Licensed practical or vocational nurse; AD: Associates degree; RN: Registered nurse; BA: Bachelor's degree; MA: Masters degree; DR: Doctoral degree
“Prevalent CVD” events included myocardial infarction, stroke, revascularization procedures (percutaneous transluminal angioplasty, coronary artery bypass graft, carotid endarterectomy, and peripheral artery surgery), and symptomatic angina pectoris or transient cerebral ischemia. “Risk factors” included current tobacco use, hypertension, high cholesterol, diabetes, parental history of premature MI, or obesity (BMI ≥ 30 kg/m2)
Incident cardiovascular disease includes all non-fatal MI, non-fatal stroke, revascularization surgery, or cardiovascular death that occurred during follow-up, updated at each cognitive assessment
Although there were no significant interactions with dietary intake of individual B vitamins for the global composite score, we observed a suggestion of benefit of combined B vitamin supplementation for the TICS among those with low dietary intakes of folate (mean difference in change from baseline = 1.24 points, 95% CI, 0.47, 2.00), vitamin B6 (mean difference in change = 0.56 points, 95% CI, 0.03, 1.10) or vitamin B12 (mean difference in change = 1.35 points, 95% CI, 0.16, 2.54) but not among those with adequate intakes of these vitamins; the p for interaction was significant for folate (p = 0.002). For category fluency, we observed similar effect modifications, particularly with dietary intake of vitamin B12 (p=0.02): among those with low vitamin B12 intake, the mean difference in change was 1.62 (95% CI, 0.11, 3.13). When we separated women into those with low intake in at least one B vitamin versus women with adequate intakes in all B vitamins, we observed that B vitamin supplementation significantly slowed the rate of decline in the TICS by 0.74 units (95% CI, 0.23, 1.25), but not for those with adequate intakes (p-interaction=0.01).
When we evaluated the influence of compliance, by removing participants from observation when they reported non-compliance (taking less than one third of the assigned pills) we did not major observe differences in the effect of supplementation. For example, for the global score, the mean difference in cognitive decline over time between the treatment and placebo groups (0.03 standard units (95% CI,−0.03, 0.08)) was similar to the main analysis.
DISCUSSION
In this long-term, randomized placebo-controlled trial of 2009 older women at high risk of cognitive decline due to existing cardiovascular disease or risk factors, use of a dietary supplement of combined vitamin B6, folic acid and vitamin B12, did not confer cognitive benefits. However, a suggestive pattern of reduced risk of cognitive decline with supplementation was observed among women with low dietary intake of B vitamins at baseline.
There have been multiple randomized trials that have examined folic acid, vitamin B6, and vitamin B12, separately or in combination, in relation to cognitive function or dementia, in a variety of older populations.(7, 8, 22–30) Because of the heterogeneity in the doses (e.g. range 0.4 – 15 mg / day of folic acid), combinations, duration of study (range 5 weeks – 3 years), sample size (range 7 – 3097), proportion of the study population with elevated homocysteine, and characteristics of the study population (e.g. restricted to those with cognitive impairment / dementia or restricted to those with vascular disease) it is difficult to derive a firm conclusion;(31) however, the collective results are not convincing for beneficial effects of B vitamin supplementation on cognitive health.(32)
A post-hoc power analysis, based on the mean change in cognitive function actually observed in the placebo group, and its standard deviation, showed that there was at least 80% power to detect mean differences in cognitive decline between the treatment and placebo groups which were equivalent to the mean difference in cognitive decline observed between participants who were 3.5 years apart in age. That is, we had adequate power to detect an effect of B vitamin treatment that was equivalent to delaying cognitive aging by 3.5 years in these subjects, which is a fairly modest effect. Thus, the null results could not be largely attributed to a lack of statistical power.
This present study is unique in that it provides data from a large study sample (n=2009) of older women at elevated risk due to vascular disease or risk factors, with the longest duration of treatment (6.6 years) of B vitamins to date. There have only been two other studies(7, 8) of participants with vascular disease, and these both included a relatively short period (1 – 2 years) of treatment. Both studies administered 2.5 mg of folic acid in various combinations with vitamin B6 or B12 and like the present study, reported no effect of B vitamin supplementation on cognitive function. These null results for cognitive decline are consistent with the results from randomized trials that have also found no benefits of B vitamin supplementation in the secondary prevention of cardiovascular disease. (7, 13, 33–38)
Although additional supplementation with B vitamins might not confer any cognitive benefits for older populations that are generally well-nourished, our data is consistent with the possibility that supplementation of B vitamins may be beneficial in the small segment of the population who still have low dietary intakes of folate, vitamin B6 and vitamin B12. Durga et al (27) also found that older persons with elevated homocysteine and low folate intake (mean intake was < 200 μg / day) given folic acid supplementation (0.8 mg / day), showed slower decline over 3 years in global cognitive functioning, memory and information processing. Nevertheless, these subgroup analysis results need to be interpreted with caution and need to be further confirmed in similarly large studies of long duration.
WAFACS began in April 1998, 3 months after the mandated grain folic acid fortification, which has led to generally increased background dietary intakes of folic acid;(39) further, our cognitive assessments began 1.2 years after randomization. At the end of the study, we evaluated plasma levels of folate and homocysteine among a sample of 150 participants on B vitamins and 150 on placebo to assess whether there were still substantial differences in folate status between the two groups.(13) Those with high plasma folate levels ≥ 25 ng / mL (or 56.7 nmol / L) comprised 16.7% of the participants assigned to placebo and 85.3% of the participants assigned to B vitamins. However, the difference in plasma homocysteine levels between the groups was modest: the median was 11.8 μmol/L in the placebo group and 9.8 μmol/L in the treated group, for an average difference of 2.0 μmol/L (or 17%), which may have been insufficient for major cognitive benefits in this generally well-nourished population. It is possible, however, that among those with low dietary intakes of B vitamins at baseline, the differences in homocysteine levels between the active and placebo groups may have been larger; this could be one explanation for the apparent cognitive benefits we observed of vitamin B supplementation in this subgroup.
Other limitations of this study should be considered. Cognitive testing began a mean 1.2 years after randomization; thus we were unable to evaluate change in cognitive performance from randomization. It is possible that vitamin B supplementation may have conferred some very early cognitive changes, and if so, we would have missed such changes when we compared performance after our first cognitive assessment 1.2 years subsequent to randomization. We believe that such an early effect, though possible, is not highly biologically plausible. Moreover, since we observed no absolute differences in cognitive function 1.2 years after treatment initiation and since it is likely that cognitive function was equally distributed in the two groups at randomization (indeed, the distribution of numerous risk factors for cognitive impairment was comparable across treatment groups at randomization), then it seems unlikely that we could have missed any meaningful changes in cognitive from randomization to our initial cognitive testing. Another limitation was non-compliance which may bias associations towards the null; however, compliance rates were generally high and similar in the treatment and placebo groups.
In conclusion, in this large-scale, long-term randomized placebo-controlled trial of B vitamin supplementation and cognitive decline, no cognitive benefits were observed among women at high risk of cardiovascular disease. However, we observed a general pattern of cognitive benefits with supplementation among the subset of women with low dietary intake of the B vitamins at baseline; this finding may have been due to chance but warrants further study. Although preventing deficiency of essential vitamins such as folate, vitamin B6 and vitamin B12 remains an important health concern for older persons, additional supplementation with B vitamins is unlikely to provide cognitive benefits for those who are generally well-nourished. Alternative strategies for maintaining cognitive health in high-risk older individuals need to be investigated.
Acknowledgements
We are indebted to the 2009 participants in the Women's Antioxidant and Folic Acid Cardiovascular Study who also participated in the cognitive substudy for their dedicated and conscientious collaboration; to the entire staff of the Women's Antioxidant and Folic Acid Cardiovascular Study: including Elaine Zaharris, Jean MacFadyen, Eleanor Danielson, Marilyn Chown, Shamikhah Curry, Margarette Haubourg, Felicia Zangi, Tony Laurinaitis, Geneva McNair, Philomena Quinn, Harriet Samuelson, Ara Sarkissian, and Martin Van Denburgh; to Michelle Albert, Gavin Blake, Claudia Chae, Michael Fisher, Carlos Kase, Tobias Kurth, I-Min Lee, Aruna Pradhan, Paul Ridker, Jackie Suk, and James Taylor for their assistance in the conduct of the trial.
This work is supported by grants AG15933, HL046959 from the National Institutes of Health.
Footnotes
Contributors: All authors conceived and designed the study. Drs. Kang and Grodstein carried out the study. Drs. Kang and Cook analyzed the data. Dr. Kang drafted the manuscript and all authors critically reviewed the paper and approved the final draft for publication.
Competing interests: None declared for all authors.
REFERENCES
- 1.Launer LJ. Demonstrating the case that AD is a vascular disease: epidemiologic evidence. Ageing Res Rev. 2002;1:61–77. doi: 10.1016/s0047-6374(01)00364-5. [DOI] [PubMed] [Google Scholar]
- 2.Hasegawa T, Ukai W, Jo DG, et al. Homocysteic acid induces intraneuronal accumulation of neurotoxic Abeta42: implications for the pathogenesis of Alzheimer's disease. J Neurosci Res. 2005;80:869–76. doi: 10.1002/jnr.20514. [DOI] [PubMed] [Google Scholar]
- 3.Fuso A, Nicolia V, Cavallaro RA, et al. B-vitamin deprivation induces hyperhomocysteinemia and brain S-adenosylhomocysteine, depletes brain S-adenosylmethionine, and enhances PS1 and BACE expression and amyloid-beta deposition in mice. Mol Cell Neurosci. 2008;37:731–46. doi: 10.1016/j.mcn.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Beal MF, Swartz KJ, Finn SF, Mazurek MF, Kowall NW. Neurochemical characterization of excitotoxin lesions in the cerebral cortex. J Neurosci. 1991;11:147–158. doi: 10.1523/JNEUROSCI.11-01-00147.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kruman II, Kumaravel TS, Lohani A, et al. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer's disease. J Neurosci. 2002;22:1752–62. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Troen AM, Shukitt-Hale B, Chao WH, et al. The cognitive impact of nutritional homocysteinemia in apolipoprotein-E deficient mice. J Alzheimers Dis. 2006;9:381–92. doi: 10.3233/jad-2006-9403. [DOI] [PubMed] [Google Scholar]
- 7.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. Jama. 2004;291:565–75. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 8.Stott DJ, MacIntosh G, Lowe GD, et al. Randomized controlled trial of homocysteine-lowering vitamin treatment in elderly patients with vascular disease. Am J Clin Nutr. 2005;82:1320–6. doi: 10.1093/ajcn/82.6.1320. [DOI] [PubMed] [Google Scholar]
- 9.Bassuk SS, Albert CM, Cook NR, et al. The Women's Antioxidant Cardiovascular Study: design and baseline characteristics of participants. J Womens Health (Larchmt) 2004;13:99–117. doi: 10.1089/154099904322836519. [DOI] [PubMed] [Google Scholar]
- 10.Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease:a randomized trial. JAMA. 2008;299:2027–2036. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 12.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127:188–99. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 13.Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease. JAMA. 2008;299:2027–2036. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandt J, Folstein MF. Telephone Interview for Cognitive Status: Professional Manual. Psychological Assessment Resources, Inc; Lutz, FL: 2003. [Google Scholar]
- 15.Scherr PA, Albert MS, Funkenstein HH, et al. Correlates of cognitive function in an elderly community population. Am J Epidemiol. 1988;128:1084–1101. doi: 10.1093/oxfordjournals.aje.a115051. [DOI] [PubMed] [Google Scholar]
- 16.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 17.Small BJ, Fratiglioni L, Viitanen M, Winblad B, Backman L. The course of cognitive impairment in preclinical Alzheimer disease: three- and 6-year follow-up of a population based sample. Arch Neurol. 2000;57:839–844. doi: 10.1001/archneur.57.6.839. [DOI] [PubMed] [Google Scholar]
- 18.Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2959–68. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 19.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Chapter 5. John Wiley & Sons, Inc; Hoboken, NJ: 2004. Modelling the Mean: Analyzing Response Profiles; pp. 103–139. [Google Scholar]
- 20.Selhub J, Jacques PF, Wilson PW, Rush D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. Jama. 1993;270:2693–8. doi: 10.1001/jama.1993.03510220049033. [DOI] [PubMed] [Google Scholar]
- 21.Food and Nutrition Board IoM . Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. 1998. [PubMed] [Google Scholar]
- 22.Shaw DM, Macsweeney DA, Johnson AL, et al. Folate and amine metabolites in senile dementia: a combined trial and biochemical study. Psychol Med. 1971;1:166–71. doi: 10.1017/s003329170000009x. [DOI] [PubMed] [Google Scholar]
- 23.McMahon JA, Green TJ, Skeaff CM, Knight RG, Mann JI, Williams SM. A controlled trial of homocysteine lowering and cognitive performance. N Engl J Med. 2006;354:2764–72. doi: 10.1056/NEJMoa054025. [DOI] [PubMed] [Google Scholar]
- 24.Eussen SJ, de Groot LC, Joosten LW, et al. Effect of oral vitamin B-12 with or without folic acid on cognitive function in older people with mild vitamin B-12 deficiency: a randomized, placebo-controlled trial. Am J Clin Nutr. 2006;84:361–70. doi: 10.1093/ajcn/84.1.361. [DOI] [PubMed] [Google Scholar]
- 25.Sommer BR, Hoff AL, Costa M. Folic acid supplementation in dementia: a preliminary report. J Geriatr Psychiatry Neurol. 2003;16:156–9. doi: 10.1177/0891988703256052. [DOI] [PubMed] [Google Scholar]
- 26.Fioravanti M, Ferrario E, Massaia M, et al. Low folate levels in the cognitive decline of elderly patients and the efficacy of folate as a treatment for improving memory deficits. Arch Gerontol Geriatr. 1997;26:1–13. doi: 10.1016/s0167-4943(97)00028-9. [DOI] [PubMed] [Google Scholar]
- 27.Durga J, van Boxtel MP, Schouten EG, et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007;369:208–16. doi: 10.1016/S0140-6736(07)60109-3. [DOI] [PubMed] [Google Scholar]
- 28.Lewerin C, Matousek M, Steen G, Johansson B, Steen B, Nilsson-Ehle H. Significant correlations of plasma homocysteine and serum methylmalonic acid with movement and cognitive performance in elderly subjects but no improvement from short-term vitamin therapy: a placebo-controlled randomized study. Am J Clin Nutr. 2005;81:1155–62. doi: 10.1093/ajcn/81.5.1155. [DOI] [PubMed] [Google Scholar]
- 29.Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55:1449–55. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 30.Bryan J, Calvaresi E, Hughes D. Short-term folate, vitamin B-12 or vitamin B-6 supplementation slightly affects memory performance but not mood in women of various ages. J Nutr. 2002;132:1345–1356. doi: 10.1093/jn/132.6.1345. [DOI] [PubMed] [Google Scholar]
- 31.Balk EM, Raman G, Tatsioni A, Chung M, Lau J, Rosenberg IH. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167:21–30. doi: 10.1001/archinte.167.1.21. [DOI] [PubMed] [Google Scholar]
- 32.Gillette-Guyonnet S, Abellan Van Kan G, Andrieu S, et al. IANA task force on nutrition and cognitive decline with aging. J Nutr Health Aging. 2007;11:132–52. [PubMed] [Google Scholar]
- 33.Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–77. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 34.Lange H, Suryapranata H, De Luca G, et al. Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med. 2004;350:2673–81. doi: 10.1056/NEJMoa032845. [DOI] [PubMed] [Google Scholar]
- 35.Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ. Secondary prevention with folic acid: results of the Goes extension study. Heart. 2005;91:1213–4. doi: 10.1136/hrt.2004.035030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonaa KH, Njolstad I, Ueland PM, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–88. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 37.Baker F, Picton D, Blackwood S, Hunt J, Erskine M, Dyas M. Blinded comparison of folic acid and placebo in patients with ischaemic heart disease: an outcome trial [abstract] Circulation. 2002;106(suppl II):741S. [Google Scholar]
- 38.Schnyder G, Roffi M, Flammer Y, Pin R, Hess OM. Effect of homocysteine-lowering therapy with folic acid, vitamin B(12), and vitamin B(6) on clinical outcome after percutaneous coronary intervention: the Swiss Heart study: a randomized controlled trial. JAMA. 2002;288:973–9. doi: 10.1001/jama.288.8.973. [DOI] [PubMed] [Google Scholar]
- 39.Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340:1449–54. doi: 10.1056/NEJM199905133401901. [DOI] [PubMed] [Google Scholar]