Abstract
Severe combined immunodeficiency (SCID) is potentially correctable by bone marrow transplantation if a patient has a suitable histocompatible donor. In the absence of an HLA-matched donor, lethal graft-versus-host disease (GVHD), which is mediated by alloreactive donor T cells, may occur. In an attempt to prevent GVHD in one SCID patient lacking a matched donor, we treated maternal haplomismatched bone marrow with a unique nonmitogenic T-cell-specific monoclonal antibody (anti-T12) and complement to remove mature T cells. Despite the removal of greater than 99% mature T cells, the child developed significant life-threatening GVHD, which was terminated by a 5-day course of intravenous anti-T12. Subsequently, immune reconstitution occurred by 6 wk: the mature circulating T cells proliferated in response to soluble and allo-antigens in vitro and provided help for B-cell immunoglobulin synthesis. The patient was removed from a protective environment and discharged without evidence of further infection. Both HLA and chromosomal analyses showed that the circulating cells in the patient were of maternal origin. More importantly, the maternal T cells were no longer reactive with recipient cells. Mixing experiments indicated that the state of tolerance that resulted in this chimera was not due to active suppression. We conclude that HLA-mismatched transplantation for SCID can be undertaken if mature alloreactive donor T lymphocytes are depleted before and after bone marrow grafting.
Full text
PDF

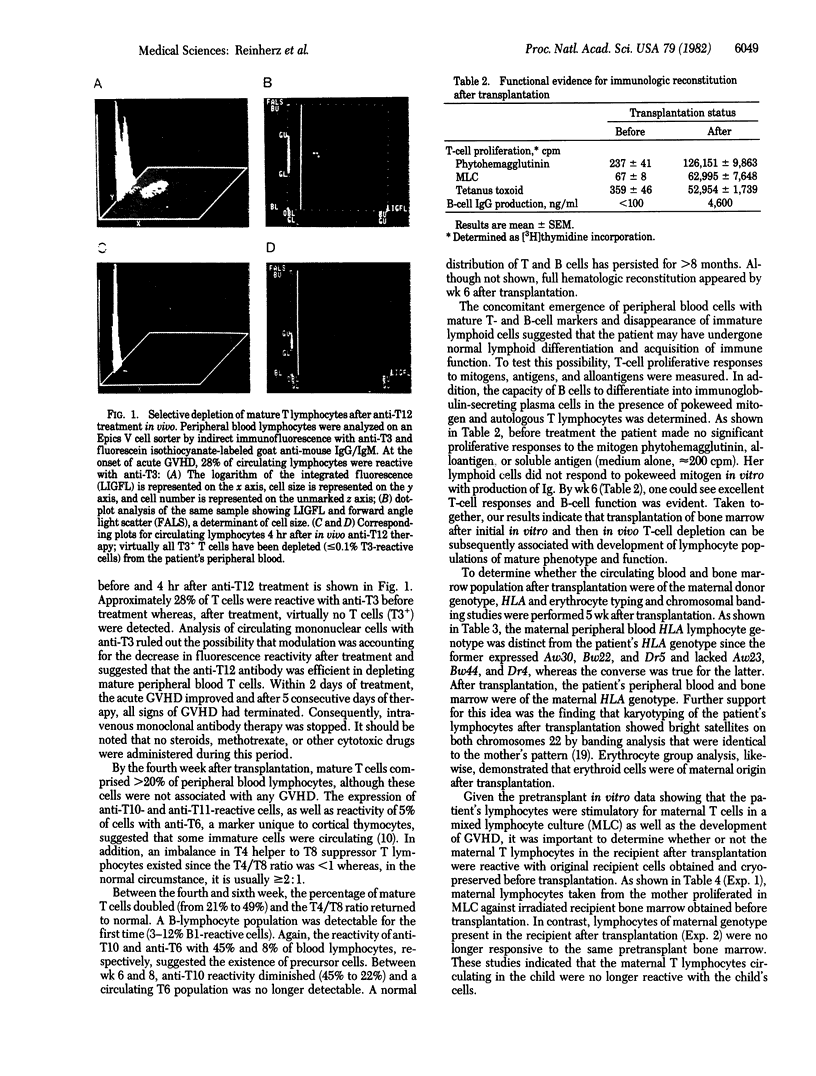
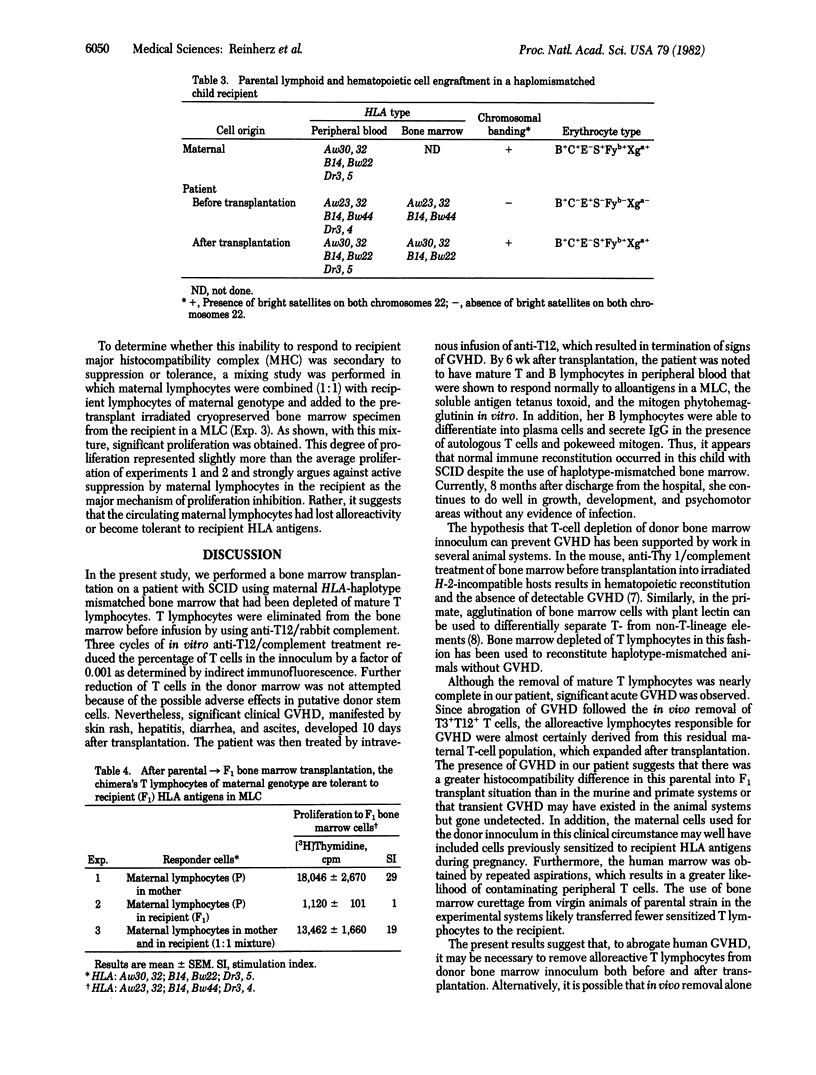

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan M. J. In a radiation chimaera, host H-2 antigens determine immune responsiveness of donor cytotoxic cells. Nature. 1977 Sep 29;269(5627):417–418. doi: 10.1038/269417a0. [DOI] [PubMed] [Google Scholar]
- Caspersson T., Zech L., Johansson C. Analysis of human metaphase chromosome set by aid of DNA-binding fluorescent agents. Exp Cell Res. 1970 Oct;62(2):490–492. doi: 10.1016/0014-4827(70)90586-0. [DOI] [PubMed] [Google Scholar]
- Diamond B. A., Yelton D. E., Scharff M. D. Monoclonal antibodies. A new technique for producing serologic reagents. N Engl J Med. 1981 May 28;304(22):1344–1349. doi: 10.1056/NEJM198105283042208. [DOI] [PubMed] [Google Scholar]
- Levey R. H., Gelfand E. W., Klemperer M. R., Sanderson A. R., Bachelor J. R., Berkel A. I., Rosen F. S. Bone-marrow transplantation in severe combined immunodeficiency syndrome. Lancet. 1971 Sep 11;2(7724):571–575. doi: 10.1016/s0140-6736(71)92151-9. [DOI] [PubMed] [Google Scholar]
- Nadler L. M., Stashenko P., Hardy R., Pesando J. M., Yunis E. J., Schlossman S. F. Monoclonal antibodies defining serologically distinct HLA-D/DR related Ia-like antigens in man. Hum Immunol. 1981 Feb;2(1):77–90. doi: 10.1016/0198-8859(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Pahwa R. N., Pahwa S. G., Good R. A. T-lymphocyte differentiation in vitro in severe combined immunodeficiency. Defects of stem cells. J Clin Invest. 1979 Dec;64(6):1632–1641. doi: 10.1172/JCI109625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Cooper M. D., Schlossman S. F., Rosen F. S. Abnormalities of T cell maturation and regulation in human beings with immunodeficiency disorders. J Clin Invest. 1981 Sep;68(3):699–705. doi: 10.1172/JCI110305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Hussey R. E., Fitzgerald K., Snow P., Terhorst C., Schlossman S. F. Antibody directed at a surface structure inhibits cytolytic but not suppressor function of human T lymphocytes. Nature. 1981 Nov 12;294(5837):168–170. doi: 10.1038/294168a0. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Morimoto C., Penta A. C., Schlossman S. F. Regulation of B cell immunoglobulin secretion by functional subsets of T lymphocytes in man. Eur J Immunol. 1980 Jul;10(7):570–572. doi: 10.1002/eji.1830100715. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Reisner Y., Kapoor N., Kirkpatrick D., Pollack M. S., Dupont B., Good R. A., O'Reilly R. J. Transplantation for acute leukaemia with HLA-A and B nonidentical parental marrow cells fractionated with soybean agglutinin and sheep red blood cells. Lancet. 1981 Aug 15;2(8242):327–331. doi: 10.1016/s0140-6736(81)90647-4. [DOI] [PubMed] [Google Scholar]
- Reisner Y., Kapoor N., O'Reilly R. J., Good R. A. Allogeneic bone marrow transplantation using stem cells fractionated by lectins: VI, in vitro analysis of human and monkey bone marrow cells fractionated by sheep red blood cells and soybean agglutinin. Lancet. 1980 Dec 20;2(8208-8209):1320–1324. doi: 10.1016/s0140-6736(80)92394-6. [DOI] [PubMed] [Google Scholar]
- Rosen F. S., Janeway C. A. The gamma globulins. 3. The antibody deficiency syndromes. N Engl J Med. 1966 Oct 6;275(14):769–concl. doi: 10.1056/NEJM196610062751407. [DOI] [PubMed] [Google Scholar]
- Sprent J., Boehmer H. V., Nabholz M. Association of immunity and tolerance to host H-2 determinants in irradiated F1 hybrid mice reconstituted with bone marrow cells from one parental strain. J Exp Med. 1975 Aug 1;142(2):321–331. doi: 10.1084/jem.142.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stashenko P., Nadler L. M., Hardy R., Schlossman S. F. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980 Oct;125(4):1678–1685. [PubMed] [Google Scholar]
- Zinkernagel R. M., Callahan G. N., Althage A., Cooper S., Klein P. A., Klein J. On the thymus in the differentiation of "H-2 self-recognition" by T cells: evidence for dual recognition? J Exp Med. 1978 Mar 1;147(3):882–896. doi: 10.1084/jem.147.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]