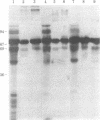
Abstract
We have compared the amino acid and carbohydrate compositions, partial amino acid sequences, immunological crossreactivity, and physical properties of secretory protein I of the parathyroid gland and chromogranin A of adrenal gland. This comparison indicates that these proteins are similar molecules. Because secretory protein I is present in secretory granules containing parathormone and is cosecreted with the hormone, and because chromogranin A is contained within chromaffin granules and, likewise, is secreted with the catecholamines, the present observations raise the possibility that this class of protein plays a general role in hormone secretion or storage mechanisms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks P., Helle K. B., Mayor D. Evidence for the presence of a chromogranin-like protein in bovine splenic nerve granules. Mol Pharmacol. 1969 Mar;5(2):210–212. [PubMed] [Google Scholar]
- Banks P., Helle K. The release of protein from the stimulated adrenal medulla. Biochem J. 1965 Dec;97(3):40C–41C. doi: 10.1042/bj0970040c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett S. F., Smith A. D. Adrenal chromaffin granules: isolation and disassembly. Methods Enzymol. 1974;31:379–389. doi: 10.1016/0076-6879(74)31042-7. [DOI] [PubMed] [Google Scholar]
- Blaschko H., Comline R. S., Schneider F. H., Silver M., Smith A. D. Secretion of a chromaffin granule protein, chromogranin, from the adrenal gland after splanchnic stimulation. Nature. 1967 Jul 1;215(5096):58–59. doi: 10.1038/215058a0. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cohn D. V., MacGregor R. R. The biosynthesis, intracellular processing, and secretion of parathormone. Endocr Rev. 1981 Winter;2(1):1–26. doi: 10.1210/edrv-2-1-1. [DOI] [PubMed] [Google Scholar]
- Cohn D. V., Morrissey J. J., Hamilton J. W., Shofstall R. E., Smardo F. L., Chu L. L. Isolation and partial characterization of secretory protein I from bovine parathyroid glands. Biochemistry. 1981 Jul 7;20(14):4135–4140. doi: 10.1021/bi00517a029. [DOI] [PubMed] [Google Scholar]
- Cohn D. V., Morrissey J. J., Shofstall R. E., Chu L. L. Cosecretion of secretory protein-I and parathormone by dispersed bovine parathyroid cells. Endocrinology. 1982 Feb;110(2):625–630. doi: 10.1210/endo-110-2-625. [DOI] [PubMed] [Google Scholar]
- Fischer-Colbrie R., Schachinger M., Zangerle R., Winkler H. Dopamine beta-hydroxylase and other glycoproteins from the soluble content and the membranes of adrenal chromaffin granules: isolation and carbohydrate analysis. J Neurochem. 1982 Mar;38(3):725–732. doi: 10.1111/j.1471-4159.1982.tb08691.x. [DOI] [PubMed] [Google Scholar]
- Geissler D., Martinek A., Margolis R. U., Margolis R. K., Skrivanek J. A., Ledeen R., König P., Winkler H. Composition and biogenesis of complex carbohydrates of ox adrenal chromaffin granules. Neuroscience. 1977;2(5):685–693. doi: 10.1016/0306-4522(77)90023-9. [DOI] [PubMed] [Google Scholar]
- Helle K. B. Biochemical studies of the chromaffin granule. II. Properties of membrane-bound and water-soluble forms of chromogranin A and dopamine- -hydroxylase activity. Biochim Biophys Acta. 1971 Aug 6;245(1):94–104. doi: 10.1016/0005-2728(71)90011-9. [DOI] [PubMed] [Google Scholar]
- Hogue-Angeletti R. A. Nonidentity of chromogranin A and dopamine beta-monooxygenase. Arch Biochem Biophys. 1977 Dec;184(2):364–372. doi: 10.1016/0003-9861(77)90363-0. [DOI] [PubMed] [Google Scholar]
- Hörtnagl H., Lochs H., Winkler H. Immunological studies on the acidic chromogranins and on dopamine beta-hydroxylase (EC 1.14.2.1) of bovine chromaffin granules. J Neurochem. 1974 Jan;22(1):197–199. doi: 10.1111/j.1471-4159.1974.tb12201.x. [DOI] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Rich A., Potts J. T., Jr Parathyroid secretion: discovery of a major calcium-dependent protein. Science. 1974 Apr 12;184(4133):167–169. doi: 10.1126/science.184.4133.167. [DOI] [PubMed] [Google Scholar]
- Kiang W. L., Krusius T., Finne J., Margolis R. U., Margolis R. K. Glycoproteins and proteoglycans of the chromaffin granule matrix. J Biol Chem. 1982 Feb 25;257(4):1651–1659. [PubMed] [Google Scholar]
- Kirshner A. G., Kirshner N. A specific soluble protein from the catecholamine storage vesicles of bovine adrenal medulla. II. Physical characterization. Biochim Biophys Acta. 1969 May;181(1):219–225. doi: 10.1016/0005-2795(69)90244-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morrissey J. J., Cohn D. V. The effects of calcium and magnesium on the secretion of parathormone and parathyroid secretory protein by isolated porcine parathyroid cells. Endocrinology. 1978 Dec;103(6):2081–2090. doi: 10.1210/endo-103-6-2081. [DOI] [PubMed] [Google Scholar]
- Morrissey J. J., Hamilton J. W., Cohn D. V. The secretion of parathormone and glycosylated proteins by parathyroid cells in culture. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1279–1286. doi: 10.1016/0006-291x(78)90326-1. [DOI] [PubMed] [Google Scholar]
- Morrissey J. J., Shofstall R. E., Hamilton J. W., Cohn D. V. Synthesis, intracellular distribution, and secretion of multiple forms of parathyroid secretory protein-I. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6406–6410. doi: 10.1073/pnas.77.11.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravazzola M., Orci L., Habener J. F., Potts J. T., Jr Parathyroid secretory protein: Immunocytochemical localisation within cells that contain parathyroid hormone. Lancet. 1978 Aug 12;2(8085):371–372. doi: 10.1016/s0140-6736(78)92967-7. [DOI] [PubMed] [Google Scholar]
- Rush R. A., Thomas P. E., Kindler S. H., Udenfriend S. The interaction of dopamine-beta-hydroxylase with concanavalin A and its use in enzyme purification. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1301–1305. doi: 10.1016/0006-291x(74)90837-7. [DOI] [PubMed] [Google Scholar]
- Sage H. J., Smith W. J., Kirshner N. Mechanism of secretion from the adrenal medulla. I. A microquantitative immunologic assay for bovine adrenal catecholamine storage vesicle protein and its application to studies of the secretory process. Mol Pharmacol. 1967 Jan;3(1):81–89. [PubMed] [Google Scholar]
- Schneider F. H., Smith A. D., Winkler H. Secretion from the adrenal medulla: biochemical evidence for exocytosis. Br J Pharmacol Chemother. 1967 Sep;31(1):94–104. doi: 10.1111/j.1476-5381.1967.tb01980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. Purification and properties of an acidic protein from chromaffin granules of bovine adrenal medulla. Biochem J. 1967 May;103(2):483–492. doi: 10.1042/bj1030483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieder N., Ziegler E., Winkler H., Smith A. D. Some properties of soluble proteins from chromaffin granules of different species. Biochem Pharmacol. 1968 Aug;17(8):1553–1556. doi: 10.1016/0006-2952(68)90214-1. [DOI] [PubMed] [Google Scholar]
- Viveros O. H., Diliberto E. J., Jr, Hazum E., Chang K. J. Opiate-like materials in the adrenal medulla: evidence for storage and secretion with catecholamines. Mol Pharmacol. 1979 Nov;16(3):1101–1108. [PubMed] [Google Scholar]
- Winkler H., Schöpf J. A., Hörtnagl H. Bovine adrenal medulla: subcellular distribution of newly synthesised catecholamines, nucleotides and chromogranins. Naunyn Schmiedebergs Arch Pharmacol. 1972;273(1):43–61. doi: 10.1007/BF00508079. [DOI] [PubMed] [Google Scholar]
- Winkler H. The composition of adrenal chromaffin granules: an assessment of controversial results. Neuroscience. 1976;1(2):65–80. doi: 10.1016/0306-4522(76)90001-4. [DOI] [PubMed] [Google Scholar]
- Winkler H., Westhead E. The molecular organization of adrenal chromaffin granules. Neuroscience. 1980;5(11):1803–1823. doi: 10.1016/0306-4522(80)90031-7. [DOI] [PubMed] [Google Scholar]