Abstract
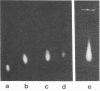
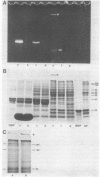
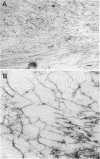
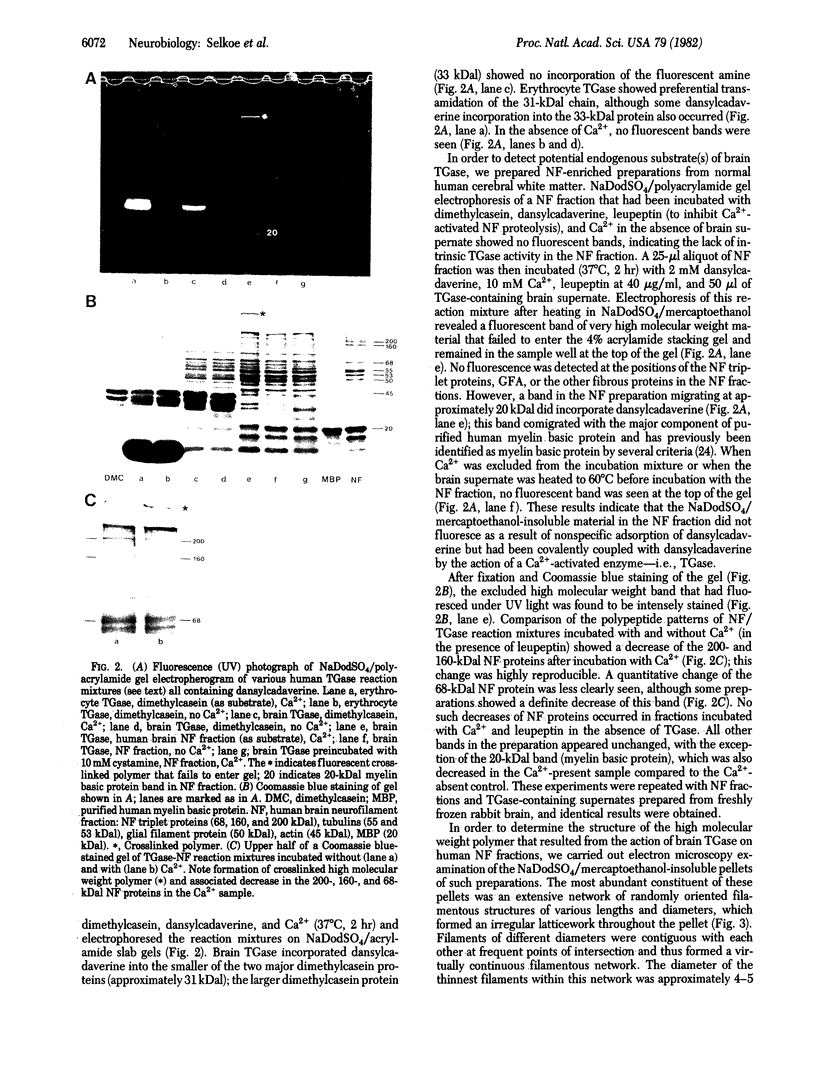
The accumulation in aged human neurons of insoluble, high molecular weight filamentous polymers apparently linked by nondisulfide covalent bonds led us to examine human brain for the presence of transglutaminase (EC 2.3.2.13) and endogenous protein substrates for this crosslinking enzyme. We demonstrate the presence in brain of a transamidating enzyme that can covalently crosslink brain proteins into insoluble polymers in vitro by forming gamma-glutamyl-epsilon-lysine intermolecular bridges. Brain transglutaminase is Ca2+ dependent, has an electrophoretic mobility similar to that of erythrocyte transglutaminase, and is active in human postmortem brain from aged normal individuals and patients with Alzheimer disease (senile dementia). Brain neurofilament fractions incubated in the presence of transglutaminase, Ca2+, and the fluorescent amine dansylcadaverine form a fluorescent, nondisulfide-bonded insoluble polymer; this process is associated with a decrease in the amount of soluble neurofilament polypeptides in the preparation. Electron microscopy of the polymeric material reveals an extensive network of connecting filaments, which can be immunostained with various neurofilament antisera. Cystamine, an inhibitor of transglutaminase, prevents the neurofilament crosslinking. Glial filaments and myelin basic protein can also serve as substrates of brain transglutaminase in vitro. Although Alzheimer disease-type paired helical filaments are not formed under the specific in vitro conditions employed, the data suggest one possible mechanism for the covalent crosslinking of filaments into insoluble polymers during human neuronal aging.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asquith R. S., Otterburn M. S., Buchanan J. H., Cole M., Fletcher J. C., Gardner K. L. The identification of epsilon-N-(gamma-L-glutamyl)-L-lysine cross-links in native wool keratins. Biochim Biophys Acta. 1970 Nov 17;221(2):342–348. doi: 10.1016/0005-2795(70)90274-6. [DOI] [PubMed] [Google Scholar]
- Birckbichler P. J., Orr G. R., Patterson M. K., Jr, Conway E., Carter H. A. Increase in proliferative markers after inhibition of transglutaminase. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5005–5008. doi: 10.1073/pnas.78.8.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birckbichler P. J., Patterson M. K., Jr Cellular transglutaminase, growth, and transformation. Ann N Y Acad Sci. 1978 Jun 20;312:354–365. doi: 10.1111/j.1749-6632.1978.tb16813.x. [DOI] [PubMed] [Google Scholar]
- Czosnek H., Soifer D., Mack K., Wisniewski H. M. Similarity of neurofilament proteins from different parts of the rabbit nervous system. Brain Res. 1981 Jul 20;216(2):387–398. doi: 10.1016/0006-8993(81)90140-2. [DOI] [PubMed] [Google Scholar]
- Dahl D., Bignami A. Immunogenic properties of the glial fibrillary acidic protein. Brain Res. 1976 Oct 29;116(1):150–157. doi: 10.1016/0006-8993(76)90257-2. [DOI] [PubMed] [Google Scholar]
- Dahl D., Bignami A. Preparation of antisera to neurofilament protein from chicken brain and human sciatic nerve. J Comp Neurol. 1977 Dec 15;176(4):645–657. doi: 10.1002/cne.901760412. [DOI] [PubMed] [Google Scholar]
- Dahl D. Isolation of neurofilament proteins and of immunologically active neurofilament degradation products from extracts of brain, spinal cord and sciatic nerve. Biochim Biophys Acta. 1981 Apr 28;668(2):299–306. doi: 10.1016/0005-2795(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Dahl D., Selkoe D. J., Pero R. T., Bignami A. Immunostaining of neurofibrillary tangles in Alzheimer's senile dementia with a neurofilament antiserum. J Neurosci. 1982 Jan;2(1):113–119. doi: 10.1523/JNEUROSCI.02-01-00113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton A., Singer S. J. Crosslinking and labeling of membrane proteins by transglutaminase-catalyzed reactions. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2568–2571. doi: 10.1073/pnas.72.7.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk J. E., Finlayson J. S. The epsilon-(gamma-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Adv Protein Chem. 1977;31:1–133. doi: 10.1016/s0065-3233(08)60217-x. [DOI] [PubMed] [Google Scholar]
- Folk J. E. Structure and catalytic properties of hepatic transglutaminase. Ann N Y Acad Sci. 1972 Dec 8;202:59–76. doi: 10.1111/j.1749-6632.1972.tb16322.x. [DOI] [PubMed] [Google Scholar]
- Harding H. W., Rogers G. E. The occurrence of the -( -glutamyl)lysine cross-link in the medulla of hair and quill. Biochim Biophys Acta. 1972 Jan 26;257(1):37–39. doi: 10.1016/0005-2795(72)90251-6. [DOI] [PubMed] [Google Scholar]
- KIDD M. Paired helical filaments in electron microscopy of Alzheimer's disease. Nature. 1963 Jan 12;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liem R. K., Yen S. H., Salomon G. D., Shelanski M. L. Intermediate filaments in nervous tissues. J Cell Biol. 1978 Dec;79(3):637–645. doi: 10.1083/jcb.79.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L., Hsu L. K., Siefring G. E., Jr, Rafferty N. S. Lens transglutaminase and cataract formation. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1356–1360. doi: 10.1073/pnas.78.3.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L., Rule N. G., Ong H. H., Furlanetto R., Jacobsen A., Downey J., Oner N., Bruner-Lorand J. Amine specificity in transpeptidation. Inhibition of fibrin cross-linking. Biochemistry. 1968 Mar;7(3):1214–1223. doi: 10.1021/bi00843a043. [DOI] [PubMed] [Google Scholar]
- Lorand L., Siefring G. E., Jr, Tong Y. S., Bruner-Lorand J., Gray A. J., Jr Dansylcadaverine specific staining for transamidating enzymes. Anal Biochem. 1979 Mar;93(2):453–458. doi: 10.1016/s0003-2697(79)80178-5. [DOI] [PubMed] [Google Scholar]
- Lorand L., Weissmann L. B., Epel D. L., Bruner-Lorand J. Role of the intrinsic transglutaminase in the Ca2+-mediated crosslinking of erythrocyte proteins. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4479–4481. doi: 10.1073/pnas.73.12.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matacić S., Loewy A. G. The identification of isopeptide crosslinks in insoluble fibrin. Biochem Biophys Res Commun. 1968 Feb 26;30(4):356–362. doi: 10.1016/0006-291x(68)90750-x. [DOI] [PubMed] [Google Scholar]
- Pant H. C., Gainer H. Properties of a calcium-activated protease in squid axoplasm which selectively degrades neurofilament proteins. J Neurobiol. 1980;11(1):1–12. doi: 10.1002/neu.480110102. [DOI] [PubMed] [Google Scholar]
- Pisano J. J., Finlayson J. S., Peyton M. P. [Cross-link in fibrin polymerized by factor 13: epsilon-(gamma-glutamyl)lysine]. Science. 1968 May 24;160(3830):892–893. doi: 10.1126/science.160.3830.892. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell. 1979 Nov;18(3):681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Brown B. A., Salazar F. J., Marotta C. A. Myelin basic protein in Alzheimer disease neuronal fractions and mammalian neurofilament preparations. Ann Neurol. 1981 Nov;10(5):429–436. doi: 10.1002/ana.410100505. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Ihara Y., Salazar F. J. Alzheimer's disease: insolubility of partially purified paired helical filaments in sodium dodecyl sulfate and urea. Science. 1982 Mar 5;215(4537):1243–1245. doi: 10.1126/science.6120571. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Liem R. K., Yen S. H., Shelanski M. L. Biochemical and immunological characterization of neurofilaments in experimental neurofibrillary degeneration induced by aluminum. Brain Res. 1979 Mar 16;163(2):235–252. doi: 10.1016/0006-8993(79)90352-4. [DOI] [PubMed] [Google Scholar]
- Siefring G. E., Jr, Apostol A. B., Velasco P. T., Lorand L. Enzymatic basis for the Ca2+-induced cross-linking of membrane proteins in intact human erythrocytes. Biochemistry. 1978 Jun 27;17(13):2598–2604. doi: 10.1021/bi00606a022. [DOI] [PubMed] [Google Scholar]
- Stenberg P., Stenflo J. A rapid and specific fluorescent activity staining procedure for transamidating enzymes. Anal Biochem. 1979 Mar;93(2):445–452. doi: 10.1016/s0003-2697(79)80177-3. [DOI] [PubMed] [Google Scholar]
- TERRY R. D., GONATAS N. K., WEISS M. ULTRASTRUCTURAL STUDIES IN ALZHEIMER'S PRESENILE DEMENTIA. Am J Pathol. 1964 Feb;44:269–297. [PMC free article] [PubMed] [Google Scholar]
- Tomlinson B. E., Blessed G., Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970 Sep;11(3):205–242. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- Wiśniewski H. M., Narang H. K., Terry R. D. Neurofibrillary tangles of paired helical filaments. J Neurol Sci. 1976 Feb;27(2):173–181. doi: 10.1016/0022-510x(76)90059-9. [DOI] [PubMed] [Google Scholar]