Abstract
Myeloid differentiation factor 88 (MyD88) is a universal and essential signaling protein in Toll-like receptor/interleukin-1 receptor-induced activation of nuclear factor-kappa B. In this study, two MyD88 protein variants (LvMyD88 and LvMyD88-1) were identified in Litopenaeus vannamei. The LvMyD88 cDNA is 1,848 bp in length and contains an open reading frame (ORF) of 1,428 bp, whereas the LvMyD88-1 cDNA is 1,719 bp in length and has an ORF of 1,299 bp. Both variants encode proteins with death and Toll/interleukin-1 receptor domains and share 91% sequence identity. In healthy L. vannamei, the LvMyD88 genes were highly expressed in hemocytes but at a low level in the hepatopancreas. The LvMyD88s expression was induced in hemocytes after challenge with lipopolysaccharide, CpG-ODN2006, Vibrio parahaemolyticus, Staphyloccocus aureus, and white spot syndrome virus, but not by poly I∶C. Overexpression of LvMyD88 and LvMyD88-1 in Drosophila Schneider 2 cells led to activation of antimicrobial peptide genes and wsv069 (ie1), wsv303, and wsv371. These results suggested that LvMyD88 may play a role in antibacterial and antiviral response in L. vannamei. To our knowledge, this is the first report on MyD88 in shrimp and a variant of MyD88 gene in invertebrates.
Introduction
Innate immunity is the first-line of host defense in multicellular organisms against infectious oppathogens [1] and is activated upon recognition of microbial derived pathogen-associated molecular patterns (PAMPs) by host pattern recognition receptors (PRRs) [2]. Toll-like receptors (TLRs), an important family of the PRRs which are well characterized in vertebrates, initiate a signaling cascade which leads to activation of the Myeloid differentiation factor 88 (MyD88) and the transcription factor nuclear factor-kappaB (NF-κB) [3]–[5]. MyD88 consists of a Toll/interleukin-1 receptor (TIR) domain and a death domain and is the common signaling adaptor protein shared by all TLRs except TLR3 [6]–[8]. The TIR domain is essential to the interactions between TLRs and MyD88. The death domain, in turn, associates with the death domain of interleukin-1 receptor associated kinase (IRAK), to trigger downstream signaling cascades that lead to the activation of the NF-κB [9]–[11].
MyD88 was first identified in 1990 [12] and has been extensively studied in many species, including human [13], porcine [14], mouse [15], chicken [16], reptiles [17], [18], fish [19]–[26], scallop [27] and flies [28]. To date, variants of MyD88 have been reported only in several vertebrates such as humans [29], mice [30] and chicken [18]. Recent studies have shown that MyD88 genes are duplicated in common carp [25]. Although the MyD88 gene has been sequenced in diplostraca and anostraca [31] little is known about its extence in in penaeidae.
The Pacific white shrimp, Litopenaeus vannamei, is distributed along the Pacific coast ranging from the Gulf of California to the northern Peru and has become one of the most important economic penaeid shrimps worldwide, particularly in the Eastern Pacific region and Asia [32]. With rapid expansion of farming, bacterial and viral diseases have become a major concern, causing substantial economic losses in many countries since the 1990s [33]–[35]. For example, the white spot syndrome virus (WSSV) is one of the most common and destructive pathogen and is able to cause 100% mortality within 3 days to 10 days after infection [36]. Study on the L. vannamei immune system is much needed in order to design better strategies for disease prevention and control.
Our previous studies suggest that a signaling cascade similar to the TLR/MyD88/Tube/Pelle/TRAF6/NF-κB pathway may exist in L. vannamei, and could be activated by wsv449, to upregulate the expression of wsv069 (ie1), wsv303, and wsv371 [37]. Several key genes in this pathway, including the Toll genes, the Pelle gene, the TRAF6 gene, and the Rel/NF-κB homologous genes (Dorsal and Relish), have been characterized [37]–[42]. In this study, two variants of the MyD88 gene were identified in L. vannamei and their function was studied in signal transduction in response to different stimuli.
Materials and Methods
Microorganisms
Gram-negative Vibrio parahaemolyticus were cultured in a thiosulfate-citrate-bile salts-sucrose (TCBS) agar culture medium at 30°C for 18 h. Gram-positive Staphyloccocus. aureus were cultured in a nutrient broth agar at 37°C for 24 h. The V. parahaemolyticus and S. aureus cells were centrifuged at 5000 g for 10 min at 4°C, washed with 1×PBS (8 g NaCl, 0.2 g KCl, 1.44 g Na2HPO4, and 0.24 g K2HPO4, diluted with dH2O to 1 litre and with the pH adjusted to 7.3), and then resuspended in 1×PBS. The bacterial concentration was quantified as the microbial colony-forming units per milliliter (CFU/ml) and the bacterial solution adjusted to 106 CFU/ml.
The WSSV-infected L. vannamei were collected from the Hengxing shrimp farm in Zhanjiang, Guangdong Province, China, and stored at −80°C. Muscle samples (0.1 g) from the WSSV-infected L. vannamei were homogenized in 1 ml of 1×PBS and centrifuged at 5000 g for 15 min at 4°C. The supernatant was filtered through a 0.45 µm membrane, and used as the WSSV inocula.
Total RNA isolation, cDNA synthesis, and genomic DNA preparation
L. vannamei (∼8 g to 10 g each) was purchased from a shrimp market in Guangzhou, Guangdong Province, China. The RNeasy Mini Kit (Qiagen, Germany) was used to extract the total RNA from tissue samples. Residual genomic DNA was removed by RNase-free DNase I (Qiagen, Germany). The total RNA was then reverse-transcribed into first strand cDNA using a PrimeScript™ 1st Strand cDNA Synthesis Kit (TaKaRa, China) for gene cloning. For real-time quantitative polymerase chain reaction (qPCR) analysis, the cDNA samples were prepared using the PrimeScript™ RT reagent kit (TaKaRa, China). The cDNA template for the rapid amplification of the cDNA ends (RACE) PCR was prepared using the SMARTer™ RACE cDNA amplification kit (Clontech, USA). Genomic DNA was extracted from muscle samples using the Universal Genomic DNA Extraction Kit (TaKaRa, China).
Cloning the cDNA and genome of LvMyD88
Degenerate primers for cloning of LvMyD88, DPMyD88F and DPMyD88R (Table 1), were designed from conserved regions of the published MyD88 nucleotide sequences of Tribolium castaneum (EFA01304), Drosophila melanogaster (NP_610479), Mus musculus (NP_034981) and Homo sapiens (AAB49967). A cDNA fragment of LvMyD88 was initially amplified by PCR with degenerate primers using hemocytes derived cDNA. Based on the cDNA fragment, the full-length MyD88 cDNA was obtained via the 5′ and 3′RACE PCR as described previously [42]. Briefly, 5′ RACE1 and 3′ RACE1 primers (Table 1) were used for the first round 5′-end and 3′-end RACE-PCR,respectively, using the following program: 94°C for 3 min, 10 cycles of 94°C for 20 s, 62°C for 30 s (a decrease of 0.5°C per cycle), 72°C for 2 min, 30 cycles of 94°C for 20 s, 57°C for 30 s, 72°C for 2 min, and a final extension at 72°C for 10 min. These PCR conditions were also applied to the second-round 5′-end and 3′-end RACE PCR where 5′ RACE2 and 3′ RACE2 primers were used respectively. The genomic DNA sequences of LvMyD88 were obtained by PCR using the genomic DNA (the primers are listed in Table 1) using the following program: 94°C for 3 min, 34 cycles of 94°C for 30 s, 57°C for 30 s, 72°C for 3 min, followed by a final extension at 72°C for 10 min. The PCR products were cloned into the pMD-20 vector (Takara, Japan) and sequenced. The gene sequences obtained in this study have been deposited in the NCBI GenBank (http://www.ncbi.nlm.nih.gov/genbank/).
Table 1. PCR primers.
| Primers | Primer sequences (5′-3′) |
| For cDNA cloning | |
| DPMyD88Fa | CTGTACGCCCACGMNGAYA |
| DPMyD88Ra | ADTADGGGACGTAGATGTTCTTG |
| 5′ RACE1 | CACCGCTCCATGATGAGTTTAAC |
| 5′ RACE2 | CACCAATAAGGTCTCTGTTCTTGTG |
| 3′ RACE1 | CTGCTGTGATAAGTTTCTGCCA |
| 3′ RACE2 | TAGGGCAAAGGGCTATTGGAAC |
| For Genomic DNA cloning | |
| GLvMyD88-F1 | TCGGGAAGAAGTGGCAGAG |
| GLvMyD88-R1 | CTACAGTAAGAATTTGGCTATCTT |
| GLvMyD88-F2 | TTGACCAGAGGGTGCCACAGGTAG |
| GLvMyD88-R2 | CCGCTCCATGATGAGTTTAACA |
| GLvMyD88-F3 | GGCAACCACAAAATACTC |
| GLvMyD88-R3 | CAACCTTAGTATATAGATGCTCCAGTC |
| GLvMyD88-F4 | ATGAATTGTGATAAATTTGC |
| GLvMyD88-R4 | GGAAAACCCTGCATTGCC |
| For Protein expression | |
| pAcLvMyD88-Fb | CCGCTCGAGATGTCATTTCGTCGGGAAGA |
| pAcLvMyD88-Rb | CGGGGGCCCCCCAGGAATTTTGATTTTTTTC |
| For qPCR | |
| LvMyD88-F1 | GCTGTTCCACCGCCATTT |
| LvMyD88-R1 | GCATCATAGTGCTGTAGTCCAAGA |
| LvMyD88-F2 | GGCAAAGGGCTATTGGAACTAT |
| LvMyD88-R2 | ATGATCCAGACACCTCTCGTATTC |
| LvEF1α-F1 | AAGGCCCTCAAGAAGAAGTAAAT |
| LvEF1α-R1 | TTGACAACCATACCTGGCTTC |
| LvEF1α-F2 | TGCACCACGAAGCCCTTAC |
| LvEF1α-R2 | CAGGGTGGTTGAGGACGATC |
M = A or C; N = A, C, G or T/U; Y = C or T; D = not C.
Nucleotides in bold represent the restriction sites introduced for cloning.
Bioinformatics analysis
The BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/) was used to analyze the nucleotide sequences and to search for protein sequences in the databases. Multiple sequence alignment was generated using the ClustalX 2.0 program (http://www.ebi.ac.uk/tools/clustalw2). The simple modular architecture research tool (SMART, http://smart.embl-heidelberg.de) was used to analyze the protein domain topology. The neighbor-joining phylogenic trees were constructed based on the amino acid sequences using the MEGA 4.0 software (http://www.megasoftware.net/index.html) and bootstrapped for 1000 times.
Immune challenge and gene expression analysis
Twelve tissues, including the hemocytes, hepatopancreas, gill, heart, stomach, pyloric caecum, nerve, epithelium, eyestalk, intestine, seminal vesicle and muscle, were obtained from healthy L. vannamei for RNA extraction. For the reason that there were no specific primers to detect the expression level of LvMyD88-1, the expression level of LvMyD88 and LvMyD88s (the amount of LvMyD88 and LvMyD88-1) were investigated using primers LvMyD88-F1/LvMyD88-R1 and LvMyD88-F2/LvMyD88-R2, respectively. On the basis, the expression level of LvMyD88-1 was calculated using the method put forward by Pfaffl [43]. The PCR was performed in a LightCycler (Roche) with the following program: one cycle at 95°C for 30 s, 40 cycles of 95°C for 5 s, 57°C for 30 s, and 78°C for 5 s. Three replicate qPCRs were performed per sample. Elongation factor 1α (EF1α) was used as the internal control.
For the challenge experiments, healthy L. vannamei was intramuscularly injected with LPS (2 µg/g), poly I∶C (2 µg/g), CpG-ODN2006 (2 µg/g), V. parahaemolyticus (5.5×106 CFU/g), S. aureus (2.5×106 CFU/g), or WSSV (106 copies/g) at the third abdominal segment. L. vannamei injected with PBS were used as controls. At 0, 4, 8, 12, 24, 36, 48, and 72 h post-injection, three animals from each group were randomly sampled for hemocyte collection. The relative mRNA expression of the LvMyD88 genes was detected by qPCR use the same program described above.
Plasmid construction
For protein expression in Drosophila Schneider 2 (S2) cells, pAc5.1/V5-His A (Invitrogen, USA) and the PCR products amplified with pAcLvMyD88F and pAcLvMyD88R were digested with resitriction enzymes Kpn I and Apa I (Takara, Japan) and purified. The mixture was ligated at 4°C overnight and then transformed into the DH5α competent cells. Positive clones were confirmed by colony PCR and sequenced. In our previous studies [37]–[42], several luciferase reporter vectors were constructed using the promoter sequences of following genes: the Drosophila AMPs, Attacin A (AttA), Drosomycin (Drs), and Metchnikowin (Mtk); the L. vannamei AMP Penaeidin4 (PEN4); the Penaeus monodon AMP Penaeidin (PEN309, PEN453, and PEN536); and wsv069 (ie1), wsv303, and wsv371. Luciferase reporter genes, including pGL3-AttA, pGL3-Drs, pGL3-Mtk, pGL3-PEN4, pGL3-PEN309, pGL3-PEN453, pGL3-PEN536, pGL3-wsv069, pGL3-wsv303, and pGL3-wsv371, have been shown to be regulated through NF-κB activation [37], [39], [40], [44]–[46].
Dual-luciferase reporter assay
Given the unavailability of a permanent shrimp cell line, Drosophila S2 cells (Invitrogen, USA) were used to perform the functional studies of LvMyD88. The S2 cells were maintained at 28°C overnight in a Drosophila serum-free medium (SDM; Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS) prior to DNA transfection. The plasmids were then transfected with the Cellfectin II reagent (Invitrogen, USA) according to the manufacturer's instructions. For the dual-luciferase reporter assays, the S2 cells cultured in 96-well plates (TPP, Switzerland) were transfected using 0.3 µg expression plasmids, 0.2 µg reporter gene plasmids, and 0.02 µg pRL-TK renilla luciferase plasmid (Promega, USA). The pRL-TK renilla luciferase plasmid was transfected alone as an internal control. Firefly and renilla luciferase activities were measured using a Dual-Luciferase Reporter Assay System (Promega, USA) according to the manufacturer's instructions. All assays were performed in three independent transfections.
Statistical analysis
The student's t-test was used to compare the means of two samples using Microsoft Excel wherever applicable. In all cases, differences were considered significant at p<0.05. All experiments were repeated at least three times. The data are presented as the mean ± standard error (standard error of the mean, SEM).
Results
cDNA cloning and bioinformatics analysis of LvMyD88
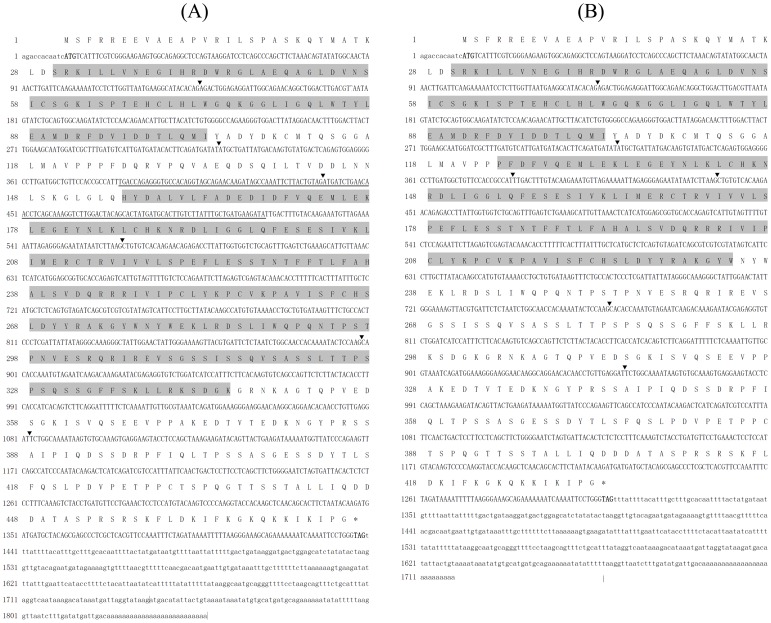
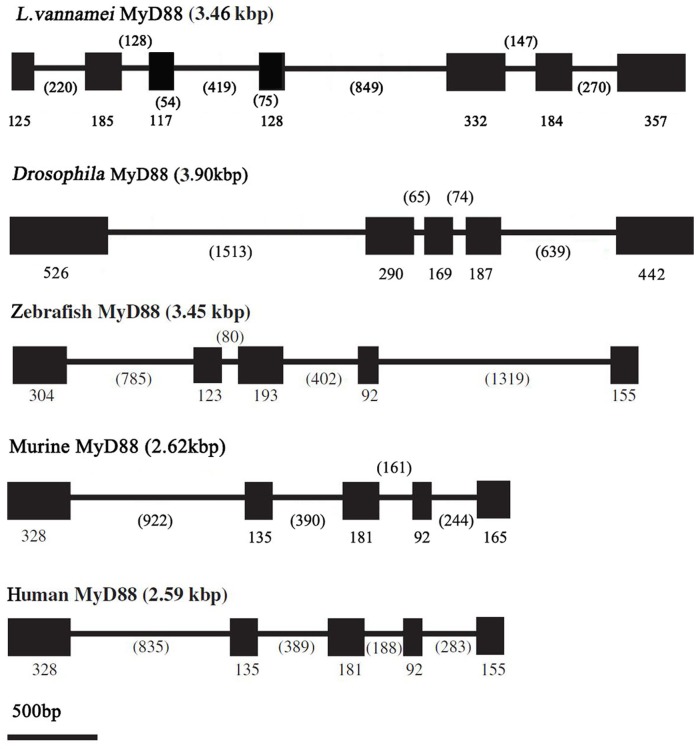
Two LvMyD88 variants, namely, LvMyD88 and LvMyD88-1, were found in L. vannamei. The full length cDNA of LvMyD88 comprises 1,848 bp with an ORF of 1,428 bp, an 11 bp 5′ untranslated region, and a 409 bp 3′ untranslated region (Fig. 1A). The full length cDNA of LvMyD88-1 contains 1,719 bp with an ORF of 1,299 bp, an 11 bp 5′ untranslated region, and a 409 bp untranslated region (Fig. 1B). The deduced amino acid sequence of LvMyD88-1 displays 91% identity with LvMyD88. The genomic sequence of LvMyD88 was also obtained, exhibiting a genomic organization which is different to that of the MyD88 genes from Drosophila, zebrafish, chicken, mouse, and human. The LvMyD88 genes consist of 7 exons and 6 introns whilst other known MyD88 genes have 5 exons and 4 introns (Fig. 2). Analysis of genome sequences showed that all the exon-intron boundaries in LvMyD88 conform to the consensus GT/AG rule for splicing [47]. However, neither splice donor consensus (GT) nor splice acceptor consensus (AG) was found in the sequences missing in LvMyD88-1. The sequences were deposited in the NCBI GenBank under Accession No. JX073566, No. JX073567 and No. JX073568.
Figure 1. Nucleotide and deduced amino acid sequences of (A) LvMyD88 and (B) LvMyD88-1 from Litopenaeus vannamei.
The nucleotide (lower row) and deduced amino acid (upper row) sequences are shown and numbered on the left. The translation initiation codon (ATG) and stop codon (TAA or TGA) are in bold. The N-terminal death domain (DD, residues 30–103) and C-terminal Toll/interleukin-1 receptor (TIR) domains (residues 156–345 and 124–234 in LvMyD88 and LvMyD88-1, respectively) are shaded. The nucleotides that are absent in LvMyD88-1 were underlined. ▾ denote the exon-exon boundaries.
Figure 2. Comparison of the MyD88 gene structures in L. vannamei, Drosophila, zebrafish, chicken, mouse, and human.
Boxes in represent exons, and the numbers indicate the length (bp) of the exons and introns.
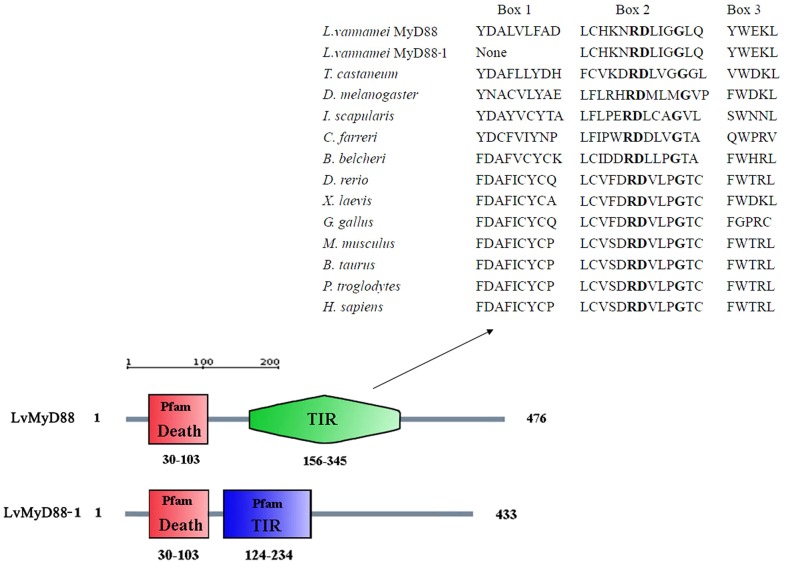
To determine the structural domains of LvMyD88, the amino acid sequence was analyzed using the SMART program.LvMyd88 and LvMyD88-1 possess an identical death domain at the N terminus but different C-terminal TIR domains. Three highly conserved regions (Box1, Box2, and Box3) present in most TIR domains were found in LvMyD88. However, the Box1 region was absent in LvMyD88-1. The RDXΦ1Φ2G motif where X represents any amino acid and Φ represents a hydrophobic residue is known to be essential for the interaction of TLRs with MyD88 [48]. This motif can be found in the Box2 of LvMyD88 and LvMyD88-1 (Fig. 3).
Figure 3. Schematic description of the domain topologies of LvMyD88 and LvMyD88-1.
LvMyD88 and LvMyD88-1 contain the same N-terminal Death domains (residues 30–103) and different C-terminal TIR domains (residues 156–345 and 124–234, respectively). The TIR domains from L. vannamei with other species were shown.
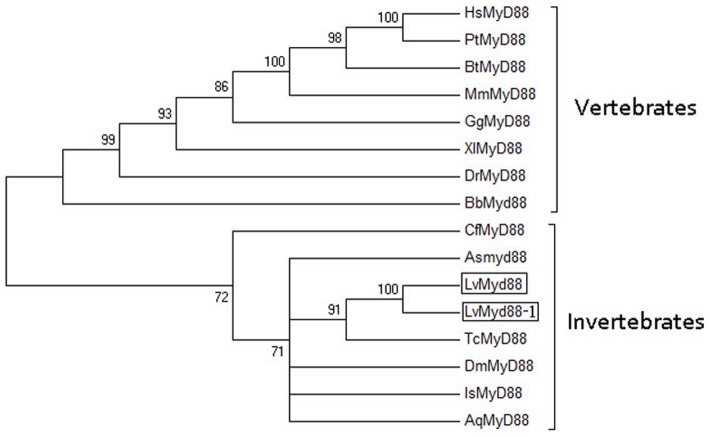
The NCBI BLAST search tool and pairwise ClustalW2 program were used to calculate the percentage of amino acid identity among MyD88s. The LvMyD88s had highest sequence identity (80%) with Xenopus laevis MyD88 (Table 2), and 50%, 31% and 43% sequence identity with the MyD88s from three invertebrate species, T. castaneum, D. melanogaster and Ixodes scapularis, respectively. In addition, a phylogenetic tree was constructed to determine the evolutionary relationship of LvMyD88 with other known MyD88 family members, showing that LvMyD88 belonged to the invertebrate group and was closely related to T. castaneum MyD88 (Fig. 4).
Table 2. Full-length amino acid sequence.
| Species | Accession number | Amina acids | Identity % | ||
| Full-length | DD | TIR | |||
| Tribolium castaneum | EFA01304 | 400 | 50% | 41% | 42% |
| Drosophila melanogaster | AAF58953 | 537 | 31% | 43% | 35% |
| Ixodes scapularis | XP_002407372 | 364 | 43% | 39% | 28% |
| Chlamys farreri | ABB76627 | 367 | 40% | 36% | 39% |
| Branchiostoma belcheri | ABQ32299 | 295 | 42% | 34% | 38% |
| Xenopus laevis | NP_001081001 | 283 | 80% | 32% | 36% |
| Danio rerio | NP_997979.2 | 284 | 30% | 33% | 35% |
| Gallus gallus | NP_001026133 | 376 | 33% | 34% | 38% |
| Mus musculus | AAC53013 | 296 | 37% | 29% | 36% |
| Bos taurus | DAA17130 | 296 | 30% | 28% | 37% |
| Pan troglodytes | NP_001123935 | 296 | 31% | 30% | 36% |
| Homo sapiens | AAC50954 | 296 | 31% | 30% | 36% |
Death domain (DD) and Toll/interleukin-1 receptor (TIR) domain identities of LvMyD88 with other species.
Figure 4. Phylogenetic tree analysis.
A rooted tree was constructed via the neighbor-joining method and bootstrapped 1000 times using the MEGA 4.0 software (http://www.megasoftware.net/index.html). LvMyD88 is boxed. LvMyD88, L. vannamei MyD88 (Accession No. JX073566); LvMyD88-1, L. vannamei MyD88-1 (Accession No. JX073567); AqMyD88, Amphimedon queenslandica MyD88 (Accession No. ADR78337); AsMyD88, Artemia sinica MyD88 (Accession No. AEJ08192); BbMyD88, Branchiostoma belcheri MyD88 (Accession No. ABQ32299); BtMyD88, Bos taurus MyD88 (Accession No. DAA17130); CfMyD88, Chlamys farreri MyD88 (Accession No. ABB76627); DmMyD88, Drosophila melanogaster MyD88 (Accession No. AAF58953); DrMyD88, Danio rerio MyD88 (Accession No. NP_997979.2); GgMyD88, Gallus gallus MyD88 (Accession No. NP_001026133); HsMyD88, Homo sapiens MyD88 (Accession No. AAC50954); IsMyD88, Ixodes scapularis MyD88 (Accession No. XP_002407372); MmMyD88, Mus musculus MyD88 (Accession No. AAC53013); PtMyD88, Pan troglodytes MyD88 (Accession No. NP_001123935); TcMyD88, Tribolium castaneum MyD88 (Accession No. EFA01304); and XlMyD88, Xenopus laevis MyD88 (Accession No. NP_001081001).
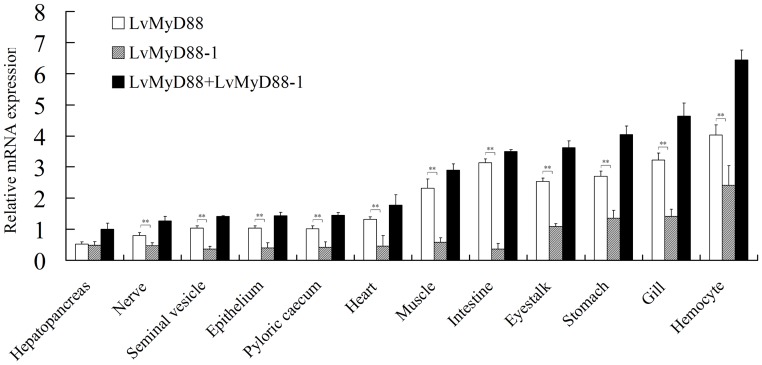
Tissue distribution of LvMyD88s in healthy L. vannamei
The constitutive expression of LvMyD88, LvMyD88-1 and LvMyD88s (the amount of LvMyD88 and LvMyD88-1) were confirmed by RT-PCR in all the examined tissues, including hepatopancreas, gill, muscle, intestine, pyloric caecum, epidermis, nerve, heart, stomach, eyestalk, seminal vesicle and hemocytes. LvMyD88s were expressed highest in hemocytes and lowest in hepatopancreas. In general, the LvMyD88 expression level was higher than that of LvMyD88-1 in all tissues examined (Fig. 5).
Figure 5. Tissue distribution of LvMyD88 expression in healthy L. vannamei.
Ten animals were used for tissue sampling. LvEF1α was used as the internal control to normalize the cDNA template used for real time PCR analysis. The data are presented as the mean ± standard error (standard error of the mean, SEM) and p<0.05 is considered significantly different.
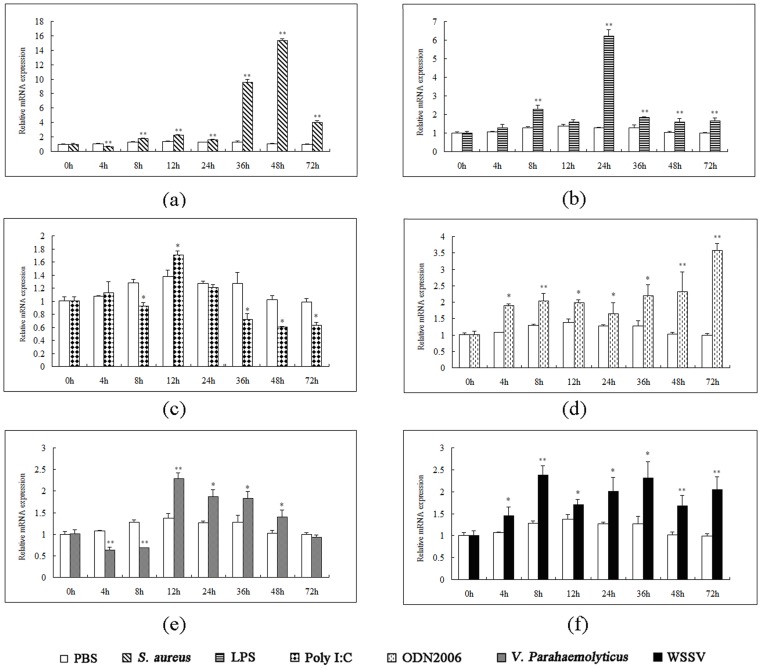
LvMyD88 expression in immune-challenged L. vannamei
Peptidoglycan (PGN) and lipoteichoic acid (LTA) in gram-positive bacteria can be recognized by TLR2 [10]. Poly I∶C, LPS and CpG-ODN2006 are the ligands for TLR3, TLR4 and TLR9, respectively [11]. In this study, gram-positive bacteria S. aureus, poly I∶C, LPS and CpG-ODN2006 were chosen to challenge the L. vannamei. In addition, gram-negative pathogenic bacterium V. parahaemolyticus and one of the most common and most destructive viral pathogens (WSSV) in shrimp aquaculture were also used to for the challenge experiments [36]. Considering that LvMyD88s (the amount of LvMyD88 and LvMyD88-1) were expressed highly in hemocytes, we selected hemocytes to study LvMyD88s expression in response to immune challenges.
S. aureus challenge increased LvMyD88s transcripts. The LvMyD88s expression level reached its peak at 48 h post-injection and sharply increase to approx 15.3 times higher than that of the control [Fig. 6(a)]. After the LPS challenge, the level of LvMyD88 transcripts started to increase at 4 h and reached the peak at 24 h, with approx 5- folds increase compared with control [Fig. 6(b)]. In contrast, injection of poly I∶C reduced gene expression at 8 h whilst induced gene expression at 12 h. Subsequently, the LvMyD88s expression recovered to the lower level than the control [Fig. 6(c)]. Injection of CpG-ODN2006 led to induced LvMyD88s expression at all time points [Figs. 6(d)]. Interestingly, LvMyD88 was downregulated at 4 and 8 h post-injection with V. parahaemolyticus but later upregulated [Fig. 6(e)]. Lastly, the WSSV infected group showed increased LvMyD88s expression when compared with the control group [Fig. 6(f)].
Figure 6. Temporal expression of LvMyD88 in immune-challenged L. vannamei.
The relative expression level of target genes was normalized to LvEF1a. The relative expression of LvMyD88 in treated groups [(a) Staphyloccocus aureus, (b) lipopolysaccharide (LPS), (c) poly I∶C, (d) CpG-ODN2006, (e) Vibrio parahaemolyticusand, (f) white spot syndrome virus (WSSV)] were compared with the control group. The results were based on three independent experiments and expressed as mean values ± SD. The statistical significance was calculated using Student's t-test (* indicates p<0.05 and ** indicates p<0.01 compared with control).
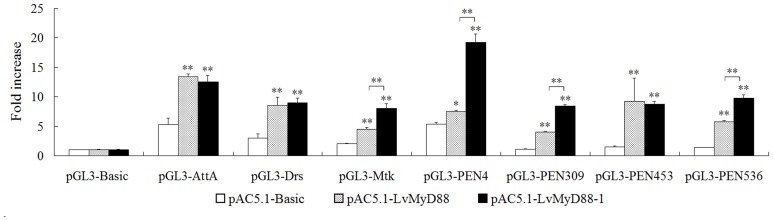
LvMyD88 activates Drosophila and shrimp AMP promoters
In Drosophila and shrimp, the AMPs are important immune factors and their expression is believed to be controlled mainly by the NF-κB signal pathway [39], [40], [45], [46]. In shrimp, it has been reported that the NF-κB signal pathway can be activated by Toll, Pelle, TRAF6, Dorsal and Relish [37]–[42]. The present study has demonstrated that both LvMyD88 and LvMyD88-1 were able to activate the promoters of Drosophila and shrimp AMP genes. LvMyD88 induced the promoter activities of the Drosophila AMPs including AttA (2.54-fold), Drs (2.83-fold), and Mtk (2.23-fold), the L. vannamei AMP PEN4 (1.40-fold) and the P. monodon AMPs such as PEN309 (3.55-fold), PEN453 (6.22-fold), and PEN536 (4.07-fold). Similar inducible effect was also detected for LvMyD88-1. It must be noted that LvMyD88-1 is more potent to drive the gene promoters of the Drosophila Mtk, L. vannamei PEN4, and P. monodon PEN309 and PEN536 than LvMyD88 (Fig. 7). All these data suggest that LvMyD88 may be involved in regulating NF-κB signaling.
Figure 7. Effects of LvMyD88 and LvMyD88-1 on the promoter activities of Drosophila and shrimp antimicrobial peptide genes (AMPs) in Drosophila S2 cells.
Drosophila S2 cells were transfected with the protein expression vector (pAC5.1 empty vector, LvMyD88, or LvMyD88-1), the reporter gene plasmid (pGL3-Basic, pGL3-AttA, pGL3-Drs, pGL3-Mtk, pGL3-PEN4, pGL3-PEN309, pGL3-PEN453, or pGL3-PEN536), and the pRL-TK Renilla luciferase plasmid (as an internal control: Promega, USA). After 48 h, the cells were harvested for measurement of luciferase activity using the dual-luciferase reporter assay system (Promega, USA). All data are representative of three independent experiments. The bars indicate the mean ± SD of the luciferase activity (n = 3). The statistical significance was calculated using Student's t-test (* indicates p<0.05 and ** indicates p<0.01compared with control).
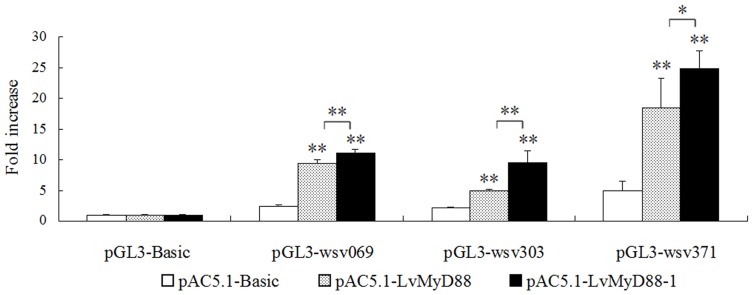
LvMyD88 activates WSSV gene promoters
A previous study suggested that the wsv069 (ie1), wsv303, and wsv371 genes are upregulated upon activation of LvPelle and LvDorsal [28]. This study showed both LvMyD88 and LvMyD88-1 upregulate viral gene expression, suggesting potential interaction between viral proteins and the host MyD88/NF-κB pathway. Expression of both LvMyD88s resulted in elevated luciferase activities for all the viral gene promoters (Fig. 8), with LvMyD88-1 gave rise to higher promoter activities than LvMyD88.
Figure 8. Overexpression of LvMyD88 and LvMyD88-1 activate viral gene expression.
The S2 cells were transfected with 0.3 µg of the protein expression vector (pAC5.1 empty vector, LvMyD88, or LvMyD88-1), the reporter gene plasmid (pGL3-Bsic, pGL3-wsv069, pGL3-wsv303, or pGL3-wsv371) and the pRL-TK Renilla luciferase plasmid (as an internal control; Promega, USA). At 48 h after transfection, the cells were harvested and analyzed using the dual-luciferase reporter assay system (Promega, USA). All data are representative of three independent experiments. The bars indicate the mean±SD of the luciferase activity (n = 3). The statistical significance was calculated using Student's t-test (* indicates p<0.05 and ** indicates p<0.01compared with the control).
Discussion
In panaeid shrimp, most of the components involved in the Toll pathway of Drosophila were identified [37]–[42]. However, the key adaptor protein co-ordinating the Toll pathway, MyD88, remains elusive. In this study, we cloned the MyD88 gene for the first time in panaeid shrimp, and discovered two variants of LvMyD88 (LvMyD88 and LvMyD88-1). Our data showed that both LvMyD88s were capable of inducing the expression of AMP genes in Drosophila and shrimp. Furthermore, overexpression of LvMyD88s may also promote viral gene expression.
MyD88 has been identified in both vertebrates and invertebrates. However, MyD88 variants have been found only in humans, mice and chicken [13], [15], [16]. Human and murine MyD88 gene contains five exons and four introns [21]. Alternative splicing of exon 2 leads to a protein lacking the inhibitor of DNA (ID) domain usually found between DD and TIR domains [29], [30]. Additional splicing variant of human MyD88 is EXC09 which lacks 19 bp in nucleotides 659–677 [13]. In chicken, MyD88 variants were not generated from alternative splicing of MyD88 pre-mRNA [16]. Chicken MyD88-2 had a single nucleotide deletion at position 859, which resulted in a protein with 77 aa residues shorter than MyD88-1, the wild type of MyD88 with 376 amino acids. Chicken MyD88-3 had a 8 aa deletion at the N terminus [18]. In this study, two MyD88 variants have been found for the first time in an invertebrate species L. vannamei. Sequences analysis showed that the LvMyD88 conformed to the consensus GT/AG rule for splicing but LvMyD88-1 did not [47]. It is likely that the difference between LvMyD88 and LvMyD88-1 is due to alternative splicing utilizing a non-canonical splice site. However, we do not know how LvMyD88-1 generated according to the present results. Future studies are need.
The two LvMyD88 variants were constitutively expressed in L. vannamei. Previous studies showed that MyD88 expression was changed after activation of TLR signaling both in vertebrate and invertebrate. Rock bream MyD88 has been shown to become up-regulated in blood, spleen and head kidney in response to experimental challenge with LPS and Edwardsiella tarda [26]. In Japanese flounder peripheral blood leukocytes, MyD88 was also found to be strongly up-regulated in response to experimental exposure to LPS, PGN, and poly I∶C [21]. In scallop, MyD88 was up-regulated in primary cultured hemocytes after LPS and PGN treatment [27]. To better understand the roles of LvMyD88 in response to exposure to various potential pathogens in L. vannamei, the expression of LvMyD88 was investigated in hemocytes after stimulation with the ligands of different TLRs, gram-negative bacterium V. parahaemolyticus, gram-positive bacterium S. aureus and viral pathogen WSSV. After poly I∶C stimulation, the transcriptional level of the LvMyD88 was lower than that of the control group at all time points except 4 h and 12 h. LvMyD88 was up-regulated after stimulation with LPS and CpG-ODN2006, V. parahaemolyticus, S. aureus and WSSV. Particularly, LvMyD88 were strongly upregulated after LPS and S. aureus challenged. It is plausible that the obvious differences in the responses in terms of MyD88 protein levels were probably due to the differences in the TLRs involved in recognizing the different PAMPs involved in each stimulation experiment. All the results suggested that LvMyD88 may play a role in innate immune in L. vannamei.
In Drosophila, it is known that the Toll pathway is central to host anti-bacterial and anti-viral response by regulating the expression of the immune related genes, including AMPs [48]–[50]. Similarly, in shrimp, AMP genes including Penaeidin, Crustin and antilipopolysaccharide factors (ALFs), are induced after bacterial and viral infection [51], [52]. Like their Drosophila counterparts, expression of shrimp AMPs is controlled by the Toll pathway through the conserved transcription factors such as NF-κB, GATA and AP-1 [52], [53]. In this study, LvMyD88 increased the promoter activities of several Drosophila and shrimp AMP genes in Drosophila S2 cells. Interestingly, LvMyD88 also enhanced the expression of wsv069 (ie1), wsv303, and wsv371, functioning as LvPelle and wsv449 (Fig. 8) [37]. All the results suggested that LvMyD88 may play the role in anti-bacterial and anti-viral response through the Toll pathway.
In summary, this study of LvMyD88 in L. vannamei supported the view that an evolutionarily conserved TLR/MyD88/Tube/Pelle/TRAF6/NF-κB signaling pathway may exist in shrimp [37]. It may help better understand the innate immune pathway in shrimp, which would be beneficial to the prevention of various diseases in shrimp culture, and provide valuable information for the study of origin and evolution of innate immunity.
Acknowledgments
The authors thank Dr. Jun Zou from Scottish Fish Immunology Research Centre in University of Aberdeen for his modification of the whole text.
Funding Statement
This research was supported by the National Natural Science Foundation of China under Grant No. U1131002, the National High Technology Research and Development Program of China (973 Program) 2012CB114401, the China Agriculture Research System, the Special Fund for Agro-scientific Research in the Public Interest 201103034, the Foundation of Administration of Ocean and Fisheries of Guangdong Province A201101B02, the Open Project of the State Key Laboratory of Biocontrol (SKLBC09K04) and the Foundation of Science and Technology Bureau of Guangdong Province ( 2011A 020102002 and 2009A 020102002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124: 783–801. [DOI] [PubMed] [Google Scholar]
- 2. Medzhitov R, Janeway C Jr (2000) Innate immune recognition: mechanisms and pathways. Immunol Rev 173: 89–97. [DOI] [PubMed] [Google Scholar]
- 3. Kawai T, Akira S (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34: 637. [DOI] [PubMed] [Google Scholar]
- 4. Moresco EM, LaVine D, Beutler B (2011) Toll-like receptors. Curr Biol 21: R488–493. [DOI] [PubMed] [Google Scholar]
- 5. Silverman N, Maniatis T (2001) NF-kappaB signaling pathways in mammalian and insect innate immunity. Genes Dev 15: 2321–2342. [DOI] [PubMed] [Google Scholar]
- 6. Kenny EF, O'Neill LAJ (2008) Signalling adaptors used by Toll-like receptors: an update. Cytokine 43: 342–349. [DOI] [PubMed] [Google Scholar]
- 7. O'Neill LA, Bowie AG (2007) The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 7: 353–364. [DOI] [PubMed] [Google Scholar]
- 8. Janssens S, Beyaert R (2002) A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem Sci 27: 474–482. [DOI] [PubMed] [Google Scholar]
- 9. Kawai T, Akira S (2007) Signaling to NF-[kappa] B by Toll-like receptors. Trends in molecular medicine 13: 460–469. [DOI] [PubMed] [Google Scholar]
- 10. Akira S, Takeda K (2004) Toll-like receptor signalling. Nature Reviews Immunology 4: 499–511. [DOI] [PubMed] [Google Scholar]
- 11. West AP, Koblansky AA, Ghosh S (2006) Recognition and signaling by toll-like receptors. Annu Rev Cell Dev Biol 22: 409–437. [DOI] [PubMed] [Google Scholar]
- 12. Lord KA, Hoffman-Liebermann B, Liebermann DA (1990) Nucleotide sequence and expression of a cDNA encoding MyD88, a novel myeloid differentiation primary response gene induced by IL6. Oncogene 5: 1095–1097. [PubMed] [Google Scholar]
- 13. Bonnert TP, Garka KE, Parnet P, Sonoda G, Testa JR, et al. (1997) The cloning and characterization of human MyD88: a member of an IL-1 receptor related family. FEBS Lett 402: 81–84. [DOI] [PubMed] [Google Scholar]
- 14. Tohno M, Shimazu T, Aso H, Kawai Y, Saito T, et al. (2007) Molecular cloning and functional characterization of porcine MyD88 essential for TLR signaling. Cell Mol Immunol 4: 369–376. [PubMed] [Google Scholar]
- 15. Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, et al. (2002) Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci U S A 99: 16899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wheaton S, Lambourne MD, Sarson AJ, Brisbin JT, Mayameei A, et al. (2007) Molecular cloning and expression analysis of chicken MyD88 and TRIF genes. DNA Seq 18: 480–486. [DOI] [PubMed] [Google Scholar]
- 17. Prothmann C, Armstrong NJ, Rupp RAW (2000) The Toll/IL-1 receptor binding protein MyD88 is required for Xenopus axis formation. Mechanisms of Development 97: 85–92. [DOI] [PubMed] [Google Scholar]
- 18. Li X, Zhu B, Chen N, Hu H, Chen J, et al. (2011) Molecular characterization and functional analysis of MyD88 in Chinese soft-shelled turtle Trionyx sinensis. Fish Shellfish Immunol 30: 33–38. [DOI] [PubMed] [Google Scholar]
- 19. van der Sar AM, Stockhammer OW, van der Laan C, Spaink HP, Bitter W, et al. (2006) MyD88 innate immune function in a zebrafish embryo infection model. Infect Immun 74: 2436–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meijer AH, Gabby Krens SF, Medina Rodriguez IA, He S, Bitter W, et al. (2004) Expression analysis of the Toll-like receptor and TIR domain adaptor families of zebrafish. Mol Immunol 40: 773–783. [DOI] [PubMed] [Google Scholar]
- 21. Takano T, Kondo H, Hirono I, Saito-Taki T, Endo M, et al. (2006) Identification and characterization of a myeloid differentiation factor 88 (MyD88) cDNA and gene in Japanese flounder, Paralichthys olivaceus. Dev Comp Immunol 30: 807–816. [DOI] [PubMed] [Google Scholar]
- 22. Yao CL, Kong P, Wang ZY, Ji PF, Liu XD, et al. (2009) Molecular cloning and expression of MyD88 in large yellow croaker, Pseudosciaena crocea. Fish Shellfish Immunol 26: 249–255. [DOI] [PubMed] [Google Scholar]
- 23. Rebl A, Goldammer T, Fischer U, Kollner B, Seyfert HM (2009) Characterization of two key molecules of teleost innate immunity from rainbow trout (Oncorhynchus mykiss): MyD88 and SAA. Vet Immunol Immunopathol 131: 122–126. [DOI] [PubMed] [Google Scholar]
- 24. Skjaeveland I, Iliev DB, Strandskog G, Jorgensen JB (2009) Identification and characterization of TLR8 and MyD88 homologs in Atlantic salmon (Salmo salar). Dev Comp Immunol 33: 1011–1017. [DOI] [PubMed] [Google Scholar]
- 25. Kongchum P, Hallerman EM, Hulata G, David L, Palti Y (2011) Molecular cloning, characterization and expression analysis of TLR9, MyD88 and TRAF6 genes in common carp (Cyprinus carpio). Fish Shellfish Immunol 30: 361–371. [DOI] [PubMed] [Google Scholar]
- 26. Whang I, Lee Y, Kim H, Jung SJ, Oh MJ, et al. (2011) Characterization and expression analysis of the myeloid differentiation factor 88 (MyD88) in rock bream Oplegnathus fasciatus. Mol Biol Rep 38: 3911–3920. [DOI] [PubMed] [Google Scholar]
- 27. Qiu L, Song L, Yu Y, Xu W, Ni D, et al. (2007) Identification and characterization of a myeloid differentiation factor 88 (MyD88) cDNA from Zhikong scallop Chlamys farreri. Fish Shellfish Immunol 23: 614–623. [DOI] [PubMed] [Google Scholar]
- 28. Tauszig-Delamasure S, Bilak H, Capovilla M, Hoffmann JA, Imler JL (2001) Drosophila MyD88 is required for the response to fungal and Gram-positive bacterial infections. Nature immunology 3: 91–97. [DOI] [PubMed] [Google Scholar]
- 29. Hardiman G, Rock FL, Balasubramanian S, Kastelein RA, Bazan JF (1996) Molecular characterization and modular analysis of human MyD88. Oncogene 13: 2467–2475. [PubMed] [Google Scholar]
- 30. Janssens S, Burns K, Tschopp J, Beyaert R (2002) Regulation of interleukin-1- and lipopolysaccharide-induced NF-kappaB activation by alternative splicing of MyD88. Curr Biol 12: 467–471. [DOI] [PubMed] [Google Scholar]
- 31. McTaggart SJ, Obbard DJ, Conlon C, Little TJ (2012) Immune genes undergo more adaptive evolution than non-immune system genes in Daphnia pulex. BMC Evolutionary Biology 12: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang YS, Peng SE, Yu HT, Liu FC, Wang CH, et al. (2004) Genetic and phenotypic variations of isolates of shrimp Taura syndrome virus found in Penaeus monodon and Metapenaeus ensis in Taiwan. Journal of General Virology 85: 2963–2968. [DOI] [PubMed] [Google Scholar]
- 33. Bachère E (2000) Shrimp immunity and disease control. Aquaculture 191: 3–11. [Google Scholar]
- 34. Lightner DV, Poulos BT, Tang-Nelson KF, Pantoja CR, Nunan LM, et al. (2006) Application of molecular diagnostic methods to penaeid shrimp diseases: advances of the past 10 years for control of viral diseases in farmed shrimp. Dev Biol (Basel) 126: 117–122; discussion 325–116. [PubMed] [Google Scholar]
- 35. Lightner DV, Redman RM (2007) Opportunities for training in shrimp diseases. Dev Biol (Basel) 129: 137–146. [PubMed] [Google Scholar]
- 36. Lin YR, Hung HC, Leu JH, Wang HC, Kou GH, et al. (2011) The Role of Aldehyde Dehydrogenase and Hsp70 in Suppression of White Spot Syndrome Virus Replication at High Temperature. Journal of virology 85: 3517–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang PH, Gu ZH, Wan DH, Zhang MY, Weng SP, et al. (2011) The shrimp NF-kappaB pathway is activated by white spot syndrome virus (WSSV) 449 to facilitate the expression of WSSV069 (ie1), WSSV303 and WSSV371. PLoS One 6: e24773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang LS, Yin ZX, Liao JX, Huang XD, Guo CJ, et al. (2007) A Toll receptor in shrimp. Mol Immunol 44: 1999–2008. [DOI] [PubMed] [Google Scholar]
- 39. Wang PH, Liang JP, Gu ZH, Wan DH, Weng SP, et al. (2012) Molecular cloning, characterization and expression analysis of two novel Tolls (LvToll2 and LvToll3) and three putative Spatzle-like Toll ligands (LvSpz1–3) from Litopenaeus vannamei. Dev Comp Immunol 36: 359–371. [DOI] [PubMed] [Google Scholar]
- 40. Wang PH, Wan DH, Gu ZH, Deng XX, Weng SP, et al. (2011) Litopenaeus vannamei tumor necrosis factor receptor-associated factor 6 (TRAF6) responds to Vibrio alginolyticus and white spot syndrome virus (WSSV) infection and activates antimicrobial peptide genes. Dev Comp Immunol 35: 105–114. [DOI] [PubMed] [Google Scholar]
- 41. Huang XD, Yin ZX, Jia XT, Liang JP, Ai HS, et al. (2010) Identification and functional study of a shrimp Dorsal homologue. Dev Comp Immunol 34: 107–113. [DOI] [PubMed] [Google Scholar]
- 42. Huang XD, Yin ZX, Liao JX, Wang PH, Yang LS, et al. (2009) Identification and functional study of a shrimp Relish homologue. Fish Shellfish Immunol 27: 230–238. [DOI] [PubMed] [Google Scholar]
- 43. Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic acids research 29: e45–e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ho SH, Song YL (2009) Cloning of penaeidin gene promoter in tiger shrimp (Penaeus monodon). Fish Shellfish Immunol 27: 73–77. [DOI] [PubMed] [Google Scholar]
- 45. O'Leary NA, Gross PS (2006) Genomic structure and transcriptional regulation of the penaeidin gene family from Litopenaeus vannamei. Gene 371: 75–83. [DOI] [PubMed] [Google Scholar]
- 46. Wang PH, Gu ZH, Huang XD, Liu BD, Deng XX, et al. (2009) An immune deficiency homolog from the white shrimp, Litopenaeus vannamei, activates antimicrobial peptide genes. Mol Immunol 46: 1897–1904. [DOI] [PubMed] [Google Scholar]
- 47. Sharp PA, Burge CB (1997) Classification of introns: U2-type or U12-type. Cell 91: 875–879. [DOI] [PubMed] [Google Scholar]
- 48. Xu Y, Tao X, Shen B, Horng T, Medzhitov R, et al. (2000) Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature 408: 111–115. [DOI] [PubMed] [Google Scholar]
- 49. Wang XW, Tan NS, Ho B, Ding JL (2006) Evidence for the ancient origin of the NF-κB/IκB cascade: its archaic role in pathogen infection and immunity. Proc Natl Acad Sci U S A 103: 4204–4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fan ZH, Wang XW, Lu J, Ho B, Ding JL (2008) Elucidating the function of an ancient NF-kappaB p100 homologue, CrRelish, in antibacterial defense. Infect Immun 76: 664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hoffmann JA (2003) The immune response of Drosophila. Nature 426: 33–38. [DOI] [PubMed] [Google Scholar]
- 52. Lemaitre B, Hoffmann J (2007) The host defense of Drosophila melanogaster. Annu Rev Immunol 25: 697–743. [DOI] [PubMed] [Google Scholar]
- 53. Amparyup P, Kondo H, Hirono I, Aoki T, Tassanakajon A (2008) Molecular cloning, genomic organization and recombinant expression of a crustin-like antimicrobial peptide from black tiger shrimp Penaeus monodon. Mol Immunol 45: 1085–1093. [DOI] [PubMed] [Google Scholar]