Abstract
Genetic variations in toll-like receptors and cytokine genes of the innate immune pathways have been implicated in controlling parasite growth and the pathogenesis of Plasmodium falciparum mediated malaria. We previously published genetic association of TLR4 non-synonymous and TNF-α promoter polymorphisms with P.falciparum blood infection level and here we extend the study considerably by (i) investigating genetic dependence of parasite-load on interleukin-12B polymorphisms, (ii) reconstructing gene-gene interactions among candidate TLRs and cytokine loci, (iii) exploring genetic and functional impact of epistatic models and (iv) providing mechanistic insights into functionality of disease-associated regulatory polymorphisms. Our data revealed that carriage of AA (P = 0.0001) and AC (P = 0.01) genotypes of IL12B 3′UTR polymorphism was associated with a significant increase of mean log-parasitemia relative to rare homozygous genotype CC. Presence of IL12B+1188 polymorphism in five of six multifactor models reinforced its strong genetic impact on malaria phenotype. Elevation of genetic risk in two-component models compared to the corresponding single locus and reduction of IL12B (2.2 fold) and lymphotoxin-α (1.7 fold) expressions in patients'peripheral-blood-mononuclear-cells under TLR4Thr399Ile risk genotype background substantiated the role of Multifactor Dimensionality Reduction derived models. Marked reduction of promoter activity of TNF-α risk haplotype (C-C-G-G) compared to wild-type haplotype (T-C-G-G) with (84%) and without (78%) LPS stimulation and the loss of binding of transcription factors detected in-silico supported a causal role of TNF-1031. Significantly lower expression of IL12B+1188 AA (5 fold) and AC (9 fold) genotypes compared to CC and under-representation (P = 0.0048) of allele A in transcripts of patients' PBMCs suggested an Allele-Expression-Imbalance. Allele (A+1188C) dependent differential stability (2 fold) of IL12B-transcripts upon actinomycin-D treatment and observed structural modulation (P = 0.013) of RNA-ensemble were the plausible explanations for AEI. In conclusion, our data provides functional support to the hypothesis that de-regulated receptor-cytokine axis of innate immune pathway influences blood infection level in P. falciparum malaria.
Introduction
Infection with Plasmodium falciparum is still a major health problem worldwide, causing about 225 million new malaria cases each year [1]. Majority of the patients with parasite infection present febrile symptoms while a subgroup develops life-threatening complications such as severe malarial anemia (SMA) or cerebral malaria (CM). Malaria parasitemia which affects disease severity and transmission is controlled by a balance between depletion of red blood cells and immune clearance by T-helper cells, B lymphocytes, and cytokines as shown in murine model of Plasmodium chabaudi infection [2]–[4]. The role of innate immunity to restrict parasite growth in rodent model is further demonstrated by a recent study which shows when parasite dose saturates the capacity of innate response; experimentally enhanced innate immunity can control parasite density indirectly by depletion of RBCs [5]. In humans, hemoglobin degradation and heme detoxification by the obligate intracellular malaria parasite results in the formation of hemozoin (HZ) which along with malarial glycosylphosphatidylinositol (GPI) prime innate immune response by the production of pro-inflammatory cytokines through toll-like receptor mediated signaling [6], [7]. Two important functions of innate immunity in the defense against the parasite are (i) it triggers a battery of pro-inflammatory cytokines inhibiting rapid parasite growth and thereby limiting the onset of malaria pathology and (ii) it determines the type and efficiency of subsequent parasite specific adaptive immune responses through the cytokine mediators at later stages of infection [8]–[10]. An account of the etiological components of innate immunity and the mechanistic framework of their interactions will offer the much needed therapeutic alternatives for control of the global burden of malaria.
Genetic studies correlating malaria susceptibility with host immunity have a long history. Majority of the studies on the role of host genetics in determining malaria susceptibility have utilized population based case-control design and candidate or genome wide markers [11]–[20]. A general concern about the association study is that the alliance between disease phenotype and genetic loci remains tentative unless a functional causality is established. The major objective of this study is to understand the interactions and functional contribution of host genetic factors associated with a measurable phenotype, namely parasitemia, to extract an improved insight of host regulatory mechanisms controlling the blood infection level in P. falciparum mediated malaria. We have published a genetic association study of this phenotype analyzing fourteen single nucleotide polymorphisms (SNPs) located on genes encoding toll-like-receptor (TLR)-2, 4 and 9 and tumor necrosis factor-α (TNF-α) and lymphotoxin-α (LTA) and reported significant correlation of blood parasitemia with TLR4 non-synonymous and TNF-α promoter polymorphisms in Indian patients with mild malaria [21]. Here we extend this analysis by investigating genetic association between polymorphic variability of IL12B, which encodes the IL-12p40 subunit of IL12, with blood parasitemia. IL12 is a heterodimeric pro-inflammatory cytokine with pleiotropic effects, acting as a potent immune-regulatory molecule and hematopoietic growth factor in infections caused by Plasmodium parasites [22]. It is produced by phagocytes and dendritic cells in response to pathogens through toll-like and other extracellular receptors. Physiologically the most important target cells of IL12 are hematopoietic progenitors, NK cells and T cells for which it induces proliferation and production of type-1 cytokines (e.g. IFN-γ). IL12 and IFN-γ together enhance activation and production of Th1 associated classes of immunoglobulin from B cells [23]. IL12 is composed of a 35 kD subunit encoded by IL12A and 40 kD subunit encoded by IL12B. Polymorphisms in genes encoding both the subunits have been reported in a wide range of immune and inflammatory diseases including malaria [24]–[34].
Given the complexity of parasite biology and host immune system, it is unlikely that genetic variation of a single locus would provide an adequate explanation of inter-individual differences of host immune response which results in diverse clinical manifestations. To this end, identification of gene-gene interactions could enhance the power and accuracy of predicting disease outcome of a complex disorder. We have collated the genetic data on IL12B from the present report with that on toll-like receptors and cytokine loci from our previous study [21] to model the possible genetic interactions that may account for the differences in blood parasitemia in malaria patients. The functional relevance of receptor-cytokine epistatic models has been captured by analysis of receptor-genotype dependence of cytokine expression in vivo.
For a better description of genetic architecture of disease susceptibility and unambiguous identification of factors responsible for both causality and predisposition to a disease, functional appraisal of disease-associated polymorphisms is essential. There is widespread recognition that differences in gene expression may be an important source of phenotypic diversity in complex diseases [35]–[37] and that non-coding polymorphisms contribute to the variance and etiology of a trait by regulating the expression of nearby genes [38], [39]. To explore the plausible regulatory mechanisms exerted by cytokine SNPs we have characterized the allele specific events by studying their transcriptional differences in terms of reporter gene activities and allelic-expression-imbalance (AEI). Our study provides detailed insights into molecular effects of cis-regulatory variants in controlling cytokine gene expression in P. falciparum mediated malaria. However it underscores the possibility that this complex trait involves even more complex regulatory intricacies than previously anticipated.
Materials and Methods
Patient recruitment and Laboratory measures
A total of 293 mild malaria patients (age = 16–37 years) with Plasmodium falciparum infection were recruited from The Calcutta School of Tropical Medicine between September 2008 and January 2009 following WHO guidelines after obtaining the written informed consent from each study participant [40]. Patients with bacteremia, measles, acute lower respiratory tract infection, severe diarrhea with dehydration and other chronic or severe diseases such as cardiac, renal or hepatic diseases, HIV/AIDS were excluded from the study [21], [40]. Appropriate approvals have been obtained from Institutional ethical committees of University of Calcutta and The Calcutta School of Tropical Medicine, India. Parasitemia status of each patient was determined during their first visit to the clinic using Giemsa-stained blood smears and oil immersion microscopy. Detailed description of study samples and procedure for parasite enumeration can be found in our earlier report [21]. For genetic epidemiology, the patient pool from our previous study was used while for functional analyses case samples were enrolled (N = 64, age = 4–16 years) and registered in the year 2010 during July to September, from Calcutta National Medical College & Hospital, Kolkata, India as per WHO 2006 guidelines [40]. The blood samples for genetic and functional analyses were collected before any medical interventions.
Genotyping IL12B polymorphisms
Genomic DNA was extracted from peripheral blood leukocytes using a QIAamp DNA Blood Kit (Qiagen, Hilden, Germany). IL12B promoter (rs17860508) and 3′UTR (rs3212227) polymorphisms were assayed through PCR followed by an allele specific restriction enzyme digestion. The primer pairs for genotyping were listed in Table S1. PCR amplification was carried out in 10 µl reaction mixtures containing 1 U AmpliTaq Gold™ DNA polymerase, 1.5 mM MgCl2, 250 µM of each dNTP, 5 pmoles of each primer (Sigma Aldrich, St. Louis, MO). The cycling parameters consisted of an initial denaturation at 96°C for 5 mins, followed by 40 cycles of denaturation at 96°C for 30 sec, annealing at 60°C for 30 sec, extension at 72°C for 30 sec, and then completed with a final extension at 72°C for 5 mins using a thermal cycler (Applied Biosystems® GeneAmp® PCR System 9700). PCR amplicons were digested with AluI (rs17860508) and TaqI (rs3212227) (Fermentas International Inc.) at 37°C and 65°C respectively according to manufacturer's protocol. Digestion patterns were analyzed by electrophoresis in agarose gel. The accuracy of the PCR-RFLP assay was confirmed for each locus by randomly selecting 10% of total samples and sequencing the PCR products on ABI Prism 3100 Genetic Analyzer using Big-Dye Terminator v3.1 (Applied Biosystems, Foster City, CA).
Statistical analysis
All the statistical tests were performed using SPSS version 10.0 and R-program (version 2.0 package). Associations between alleles/genotypes and parasite estimate were considered “strong” and “statistically significant” in cases where the P-value<0.05.
(a) Single Gene Analysis: Distribution of allele and genotype frequencies and genetic risk assessment of IL12B polymorphisms
The allele and genotype frequencies for IL12B SNPs were estimated by gene counting and Hardy–Weinberg equilibrium was evaluated by χ2 test using Haploview (http://www.broad.mit.edu/mpg/haploview/). Pairwise comparisons between log-transformed parasitemia and genotypic classes for IL12Bpro and +1188 loci were analyzed by model based independent t test and ANOVA F-ratio. The results were further validated using non-parametric Wilcoxon Rank sum and Kruskal Wallis H tests. Bonferroni correction was done for multiple testing. For allele based risk assessment, the unprocessed parasitemia data was previously dichotomized into high (N = 88) and low (N = 205) parasitemic groups employing the two component mixture model and expectation-maximization algorithm and a threshold value of 7520 parasites/µl or 3.88 log-parasitemia was derived as the cutoff. Detailed procedure of this strategy was described earlier [21]. The proportion of the risk allele for each locus was compared between high and low parasitemic groups using a two-way contingency table.
(b) Multi-loci Analyses: Multifactor Dimensionality Reduction analysis
We adopted the multifactor dimensionality reduction (MDR) method upon merging the IL12B genotype data with those from our previous genetic epidemiologic study for identification of non-additive epistatic interactions [21], [41]–[46]. MDR is a non-parametric approach which converts multiple variables into a single attribute, thereby changing the representation space of the data. To run MDR, eight out of sixteen loci from the assembled dataset were selected as they showed an increasing/decreasing trend of log-parasitemia distribution across three genotypes which allowed us to code the genotypes into distinct risk groups. The “low”, “moderate” and “high” risk genotypic classes for each locus were designated as “0”, “1” and “2” while the “low” and “high” parasitemic groups were assigned as “0” and “1” respectively to depict the disease status. Since an equal number of sample size in high and low risk groups was a prerequisite for execution of MDR, the sample size of our analysis was kept to eighty eight, the number that represented in the high parasitemic group. Of the 205 individuals belonging to low parasitemic group, 100 distinct files each comprising of 88 individuals were generated using random numbers as seeds using R version 2.0 package to minimize the chance of detecting spurious results due to any bias. This was run on MDR software to obtain 100 independent outputs (version 2.0 beta 8.2). From eight genetic attributes (SNPs) namely TLR4Thr399Ile (rs4986791), IL12B+1188 (rs3212227), TNF-1031 (rs1799964), TNF-857 (rs1799724), TNF489 (rs1800610), LTA80 (rs2239704), LTA252 (rs909253), and TLR9P545P (rs352140), the possible multilocus genotypes were represented in a multidimensional contingency tables and classified as “high risk” if the ratio between two groups exceeded a threshold tested over the entire training data set. After reducing the dimensionality, all factor combinations were evaluated for their ability to classify the disease status in the training dataset and the best combination of factors with the minimum prediction error (1-testing balance accuracy) was calculated from the test dataset. Then the entire data was partitioned into 100 different subsets for cross-validation, from which ninety nine out of 100 subsets were assigned as a training balanced set (99/100) while the remaining one hundredth dataset (1/100) was termed as testing balanced set. To assign statistically meaningful gene-gene combinations, 100 MDR files were permutated 10,000 times using MDR permutation testing software (version 1.0 beta 2.0). The models with P value<0.05 and >95% average cross-validation consistency (CVC) were regarded as the best predictive models. To infer the genetic risk of the multifactorial models, we used odds ratio (OR) as a measure of association between genetic polymorphisms and blood infection intensity. The value of ORs was logarithmically transformed and standard errors were derived from the confidence intervals (CI). Genotype counts of the constituent loci between high and low parasitemic groups were used to calculate ORs and their corresponding 95% CIs for each gene-gene interaction model. A forest plot (blobbogram) was used to present the ORs and their 95% CIs estimated from the two-way interaction models where the odds ratio was denoted by a dot and the width of the horizontal line represented the 95% CI for the estimated OR.
RNA extraction from PBMC fraction and cDNA synthesis
Peripheral blood mononuclear cells (PBMCs) were isolated from fresh whole blood collected from malaria patients using Histopaque 1077 (Sigma Aldrich, St. Louis, MO) double-gradient density centrifugation. Total RNA was extracted with TRI® Reagent (Sigma Aldrich, St. Louis, MO) and dissolved in DEPC treated water (Bioline). RNA (1 µg) samples were treated with 2 U of RNAse free DNAse I (Fermentas Life Sciences, UK) and incubated at 37°C for 30 mins to remove DNA contamination, reverse transcribed using random hexamers and High Capacity cDNA Reverse Transcription kit (Applied Biosystems Inc.). The optimized condition for cDNA preparation was 10 mins at 25°C, 120 mins at 37°C followed by heating at 85°C for 5 mins in a thermal cycler (Applied Biosystems® GeneAmp® PCR System 9700) and stored at −20°C.
Quantitative Real Time PCR analyses
To scrutinize the genetic impact of TLR4 non-synonymous polymorphism (rs4986791) on pro-inflammatory cytokine (IL12B and LTA) production, we compared the expression of IL12B and LTA genes under different TLR4Thr399Ile genotypic background. Since TLR4Ile-Ile and TLR4 Thr-Ile genotypes together and independently showed significantly low parasitemia compared to that of TLR4Thr-Thr, we pooled individuals having genotypes Ile-Ile with Thr-Ile genotypes and examined the pattern of cytokine gene expression with respect to patients harboring TLR4Thr-Thr genotype. The relative expression of IL12B was estimated for three +1188 genotypes by qRT-PCR using 18S rRNA as an endogenous control. The sequences of oligonucleotides used in the expression analyses were listed in Table S1. A 1∶10 fold dilution of cDNA samples were used as the template and all qPCR reactions were carried out in a 10 µl reaction volume with 5 µl of SYBR Green Master Mix (Applied Biosystems) with optimized concentrations of specific primers using Applied Biosystems 7900HT Fast Real-Time PCR System. The thermal cycler was programmed for an initial denaturation step of 5 min at 95°C and followed by 40 thermal cycles of 30 sec at 95°C, 30 sec at 60°C and 30 sec at 72°C. The experiments were carried out in triplicate including the non template controls each time. Specificity of PCR amplification for each primer pair was confirmed by melting curve analysis [47] and the relative quantification (RQ) data was analyzed between groups using the ΔΔCt method. Statistical analysis on relative expression levels was estimated using Mann–Whitney U-test (with exact probabilities) for independent samples using SPSS (ver. 10.0., SPSS Inc. Chicago, IL).
Allelic-Expression-Imbalance analysis
To examine the differential expression of IL12B 3′UTR A and C alleles, the peak heights of this transcribed SNP were compared in patients heterozygous (AC) for IL12B+1188 polymorphism by resequencing both genomic DNA (gDNA) and complementary DNA (cDNA) counterpart. IL12Bexp primers (Table S1) were used to amplify and resequence the 167 bp IL12B region encompassing the polymorphic site. Sequencing was carried out on ABI Prism 3100 Genetic Analyzer using BigDye Terminator (BDT) v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA) with 0.8 µl of the PCR product, and 5 pmoles of the primer in a 10 µl reaction mixture. The raw sequence files (.ABI) were analyzed by PeakPicker Software, originally developed and kindly provided by Dr. T. Pastinen's group [48]. Genomic DNA sequence from each sample was selected as reference and the corresponding cDNA was aligned with a default cutoff of 70%. The SNP was selected manually and the peak heights of the alleles were estimated using PeakPicker. The significant difference of IL12B+1188 A/C allele ratio between the gDNA and cDNA was tested through a bivariate Sign test in SPSS version 10.0.
Mapping of microRNA binding sites
To find out the potential microRNA binding sites within IL12B mRNA, the entire IL12B 3′UTR region was scanned using miRNA-target prediction databases such as TargetScan (www.targetscan.org/), miRBase (www.mirbase.org/), microRNA.org (www.microrna.org/), RegRNA (www.regrna.mbc.nctu.edu.tw/), MicroCosm (www.ebi.ac.uk/enright-srv/microcosm/). Target microRNAs were selected and compiled on the basis of its conserved seed match or a seed match with a higher context score. The thermodynamic stability of the mRNA-miRNA duplex was calculated using RNAHybrid (www.bibiserv.techfak.uni-bielefeld.de/rnahybrid/).
Construction of reporter fusion plasmids
(a) For TNF-α promoter assay
Five TNF-α promoter haplotypes pertaining to four SNPs (TNF-1031, TNF-857, TNF-308 and TNF-238) were selected for functional evaluation. The 1.2 kb fragment was amplified using TNFα_MluI_F.P and TNFα_XhoI_R.P (Table S1) where MluI and XhoI recognition sequences were appended into the primers for efficient cloning into pGL3 vector. TNF-α haplotypes- T-C-G-G (Hap1), T-T-G-G (Hap2) and C-C-G-G (Hap3) were directly cloned into TA vector pTZ57R/T (InsTAclone™ PCR Cloning Kit, Fermentas) using the above primers by amplifying gDNA from patients harboring the homozygous genotypes for all four loci. Haplotypes: T-C-A-G (Hap4) and T-C-G-A (Hap5) were cloned by amplifying gDNA from patients harboring genotypes T/T-C/C-G/A-G/G and T/T-C/C-G/G-G/A respectively, followed by screening the clones with restriction endonucleases NcoI for TNF-308 locus and BamHI (NEB Inc., Bethesda) for TNF-238 respectively. DNA amplification protocol was conditioned with an initial denaturation at 95°C for 5 min, followed by 35 cycles of denaturation at 95°C for 1 min, annealing at 60°C for 1 min, extension at 72°C for 1 min and a final extension of 15 min at 72°C in a thermal cycler (Applied Biosystems® GeneAmp® PCR System 9700). The unique haplotypes from the TA constructs were subsequently double-digested, purified using QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany) and subcloned in the upstream of pGL3-Basic vector (kindly gifted by Dr. Susanta Roychowdhury). The integrity of each polymorphic locus and directionality of all the resulting constructs were confirmed by sequencing using both vector and insert specific internal primers. Next, the reporter gene expressions were measured in three different cell lines under the presence and absence of endotoxin lipopolysaccharide (LPS) stimulation using Luciferase Reporter Assay System (Promega, Madison, WI).
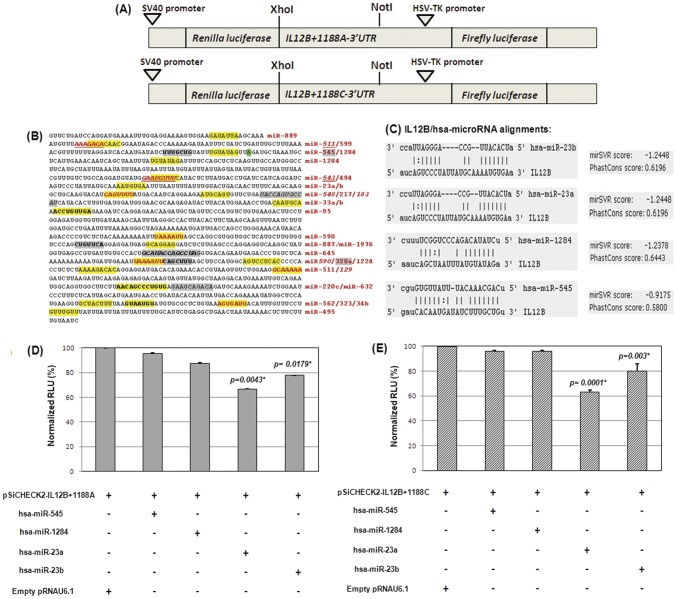
(b) For IL12B3′UTR assay
IL12B 3′UTR sequence (1047 bp) encompassing the A+1188C polymorphic site was amplified using gene specific primers (Table S1). XhoI and NotI (Fermentas International Inc.) restriction endonuclease recognition sites were incorporated in the oligos for directional cloning. Allele specific IL12B 3′UTR containing the target sites for the microRNAs was cloned into pTZ57R/T vector (InsTAclone™ PCR Cloning Kit, Fermentas) followed by subcloning into pSiCHECK2 dual luciferase reporter plasmid (kindly gifted by Dr. Soma Banerjee) in frame to the 3′ end of the cDNA encoding Renilla reniformis luciferase to produce pSiCHECK2-IL12B+1188A and pSiCHECK2-IL12+1188C constructs. Approximately 100 bp upstream and downstream sequences flanking the 70 nucleotide pre-miR sequences (www.genecards.org/) were amplified for hsa-miR-545, hsa-miR-1284, hsa-miR-23a and hsa-miR-23b using appropriate primers (Table S1) and cloned into pRNAU6.1 vector (kindly gifted by Dr. Soma Banerjee). The amplicons were inserted into pRNAU6.1 vector by double digestion with BamHI and HindIII (New England Biolabs, UK Ltd.). The orientation of the recombinant vectors was checked by sequencing. Allele specific transcript stability and miRNA-mediated IL12B 3′UTR expression were examined with SYBR Green based qRT-PCR analysis and by Dual-Luciferase Reporter (DLR) Assay System (Promega, Madison, WI) respectively.
Cell culture, Transient transfection, Luciferase and mRNA stability assay
HepG2 (hepatocellular adenocarcinoma), HEK293 (human embryonic kidney), HCT116 (colon carcinoma) and U937 (human leukemic monocyte lymphoma) cells were obtained from National Centre for Cell Science, Pune, India and maintained in DMEM medium containing 10% (v/v) fetal calf serum (Gibco BRL, Life Technologies, Grand Island, USA), 100 units/ml penicillin, 100 mg/ml streptomycin in a humidified 5% CO2 atmosphere. U937 cells were cultured in RPMI 1640 supplemented with 5 mM glutamine and 10% (v/v) heat-inactivated fetal calf serum (Gibco BRL, Life Technologies, Grand Island, USA) at 37°C in 5% CO2 atmosphere. Cells were seeded 14–16 hrs before transfection.
(a) TNF-α promoter haplotype assay
HepG2 cells (1.0×105) were plated in 12-well plates with DMEM/10% FCS medium and were transiently transfected with 0.2 µg/ml of each of the TNF-α promoter constructs (Hap1–Hap5) or empty pGL3-Basic vector cotransfected with 0.5 µg/ml pSV-β-galactosidase vector (Promega, Madison,WI) using Lipofectamine 2000 Reagent (Invitrogen, Life Technologies, Carlsbad, USA) according to the manufacturer's protocol. pGL3-Basic vector (Promega, Madison, USA) was used as control for luciferase assay and β-galactosidase vector (Promega, Madison, USA) was used as transfection control. To determine the optimum concentration of LPS (Sigma Aldrich, St. Louis, MO) for stimulation assay, a dose response experiment was performed with cells transiently transfected with TNF-Hap1 (T-C-G-G) followed by a stimulation of LPS solutions with five different concentrations such as 250 ng/ml, 500 ng/ml, 1 µg/ml, 1.5 µg/ml to 2 µg/ml and luciferase activities were measured. 0.2 µg of each of the five reporter plasmids (Hap1–Hap5) were transiently transfected. Thirty six hours post transfection, cells were stimulated with optimized dose of LPS for 4–6 hr and cellular extracts were prepared according to the manufacturer's instructions. Luminescence was measured as relative light units (RLU) in GloMax 20/20 Luminometer (Promega, Madison, USA) using 15 µl of cell supernatant. To normalize the luciferase activity, total protein concentration in each lysate was measured by the standard Bradford (BioRad) method. Transfection normalization was performed by β-galactosidase assay colorimetrically at 420 nm using O-nitrophenyl-β-D-galactopyranoside (ONPG) as substrate (Promega, Madison, WI) in JASCO V-630 spectrophotometer. Measurements of mean ± S.D were taken in triplicate. Fold change represents the difference in promoter activity for variant TNF-α haplotypes in comparison to the wild-type promoter (Hap1). All the assays were done in triplicates and repeated at least for three times. Similar experiments were also performed in HEK293 and HCT116 cell lines both under LPS stimulated and unstimulated conditions.
(b) IL12B mRNA stability assay by actinomycin-D
Actinomycin-D (2-amino-N,N′-bis[(6S,9R,10S,13R,18aS)-6,13-diisopropyl-2,5,9-trimethyl- 1,4,7,11,14-pentaoxohexadecahydro-1H-pyrrolo[2,1-i] [1], [4], [7], [10], [13] oxatetraazacyclohexadecin- 10-yl]-4,6-dimethyl-3-oxo-3H-phenoxazine-1,9-dicarboxamide), a polypeptide containing antibiotic, can inhibit transcription by binding tightly and specifically to double-helical DNA [49], [50]. It has been extensively used as a highly specific inhibitor for the formation of new de novo mRNA synthesis in both prokaryotic and eukaryotic cells [51], [52]. In this experiment, HepG2 cells were transiently transfected with plasmid DNA containing the IL12B 3′UTR fused to pSiCHECK2 vector, as described above. To determine the optimal concentration of actinomycin-D (SRL, Mumbai, India) in our experimental system, a range of concentrations of actinomycin-D (0.1–10 µg/ml) were studied. Twenty-four hours post-transfection with wild-type +1188AUTR construct, actinomycin-D was added into the media and the cells were harvested at different time points, i.e., 0, 1, 2, 4, 6, 18 and 24 hrs. Total RNA was extracted from these cells using TRIzol® Reagent (Invitrogen Life Technologies) and IL12B mRNA level was determined by RT-PCR and normalized with GAPDH and 18S rRNA genes. RNA isolated when actinomycin-D was just added was denoted as the 0-hr time point and served as negative control. It was compared with samples obtained at different time points following actinomycin-D addition to select the adequate incubation time where maximum change in expression could be found for both alleles. To compare relative mRNA stability of IL12B 3′UTR A and C containing constructs, HepG2 cells were transfected and de novo transcription was inhibited with the optimal concentration of actinomycin-D. Cells were collected after 24 hrs of actinomycin-D treatment and the mRNA levels of the transfected gene (IL12B-3′UTR/Luc) were determined by quantitative real-time PCR.
(c) IL12B 3′UTR-microRNA interaction assay
HepG2 cells (1×105) were transiently transfected with 0.1 µg/ml of the IL12B+1188 wild type (A) and variant allele (C) containing pSiCHECK2 dual reporter constructs alone and cotransfected with either 0.2 µg/ml of empty miR-vector (pRNAU6.1) and/or with the pre-miRNA (hsa-miR-545, hsa-miR-1284, hsa-miR-23a and hsa-miR-23b) cloned in pRNAU6.1. Forty eight hours later, cells were lysed; Firefly and Renilla luciferase activities were evaluated using dual luciferase reporter assay system (Promega, Madison, WI) in a GloMax 20/20 Luminometer. Renilla luciferase activity was normalized with respect to Firefly luciferase activity and total protein produced was estimated by Bradford method. All the transfection assays were done in triplicate and repeated thrice. Measurements of mean ± S.D were taken in triplicate. The pairwise comparisons between each mRNA-miRNA interactions were tested using Student-t test in GraphPad Prism (http://www.graphpad.com).
Bioinformatic analysis: SNP based DNA and RNA structural rearrangements
(a) TNF-1031 polymorphism associated DNA two dimensional configurations
DNA folding was performed with MFOLD version 3.0 (www.bioinfo.math.rpi.edu/~mfold/dna/) using the DNA energy rules in structure-melting simulations, with default parameters [53], [54], i.e., having 1 M Na+ concentration (and 0.0 M Mg2+) at 37°C. Outputs were generated in the form of structure plots based on minimum free energy (MFE) calculations, single strand (ss) frequency count and energy dot plots [55]. In addition, to identify the transcription factor binding sites (TFBS) in the genomic sequence; TFBind (http://tfbind.hgc.jp/) [56] was used to scan the 101 bp long sequence flanking the promoter SNP (rs1799964) located at 51st position. This positional pattern detection tool is able to attain high sensitivity and specificity of detection by capturing the dependencies between nucleotide positions within the TFBS.
(b) IL12B+1188 derived RNA structural ensemble prediction
In an attempt to improve our understanding of the structure and function relationship of the IL12B 3′UTR polymorphism on RNA turnover and stability, we performed an in silico structural analysis including the wild type and variant form of the locus on IL12B mRNA. An mRNA is unlikely to adopt a single and stable conformation, but it exists in a population of structures [57]; therefore instead of evaluating the minimum free energy (MFE) values of biological sequences, those of randomized sequences are now taken into account. As RNA secondary structure might affect the functionality of component regulatory elements, RNAfold program (http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi) was employed to predict secondary structures under default parameters and the folding temperature was fixed at 37°C [55], [58], [59]. In addition to the minimum free energy approach using RNA MFOLD and RNAfold, we adopted a partition function calculation method which evaluates the entire ensemble of possible RNA conformations for a given sequence and computes the effect of single nucleotide polymorphisms on RNA structural ensemble using SNPfold (http://ribosnitch.bio.unc.edu/snpfold/SNPfold.html). The structures were also generated with Sfold (http://sfold.wadsworth.org/) algorithm that computes equilibrium partition functions for all substrings of an RNA sequence based on the Turner thermodynamic parameters [60], [61]. Base-pair distances were used [62], [63] as the basis for evaluating dissimilarity between structures where the “centroid” in the entire structure ensemble space has the shortest total base-pair distance [62].
Results
The patients participating in this genetic association study were recruited according to WHO 2006 guidelines [40]. All of them displayed mild febrile symptoms, the summary of demographic, clinical and laboratory characteristics of the study participants were presented earlier [21]. In short, the crude parasitemia data derived from thick and thin blood smears deviated from normal behavior (skewness = 3.671), it was processed with log-transformation (skewness = 0.433) and regressed with respect to the covariate, age (P value of correlation between age and parasite load was 0.074). For allele based genetic analyses, cases were stratified into high and low parasitemic groups on the basis of a statistically derived threshold value of 7520 or 3.88 in terms of crude and log-parasite counts per µl respectively. The above threshold value was obtained employing a two component mixture model and Expectation Maximization algorithm [21]. No significant differences were observed in the distribution of age (P = 0.2632), gender (P = 0.3728), Hemoglobin count (P = 0.2971) and in average body weight (P = 0.3901) between the high and low parasitemic groups. Only peripheral parasitemia was found to be significantly different (high = 15227±11014; low = 2715±1705, P<0.0001) between the groups.
Genetic analysis with IL12B polymorphisms
We studied the genetic variants located in the promoter and 3′UTR region of the gene encoding IL12B by PCR based RFLP method. Two biallelic markers; rs17860508 (designated as IL12Bpro): a complex promoter insertion/deletion polymorphism -/G/GC/TTAGA/TTAGAG situated at −2703 bp upstream of the transcription initiation site and rs3212227 (designated as IL12B+1188): an A to C substitution in the 3′UTR region at +1188 position of IL12B gene were selected to examine their probable association with the blood infection intensity in two hundred and ninety three patients exhibiting Plasmodium falciparum mild malaria from Eastern India.
Genetic association between IL12B SNPs and peripheral parasite load: Genotype & allelic
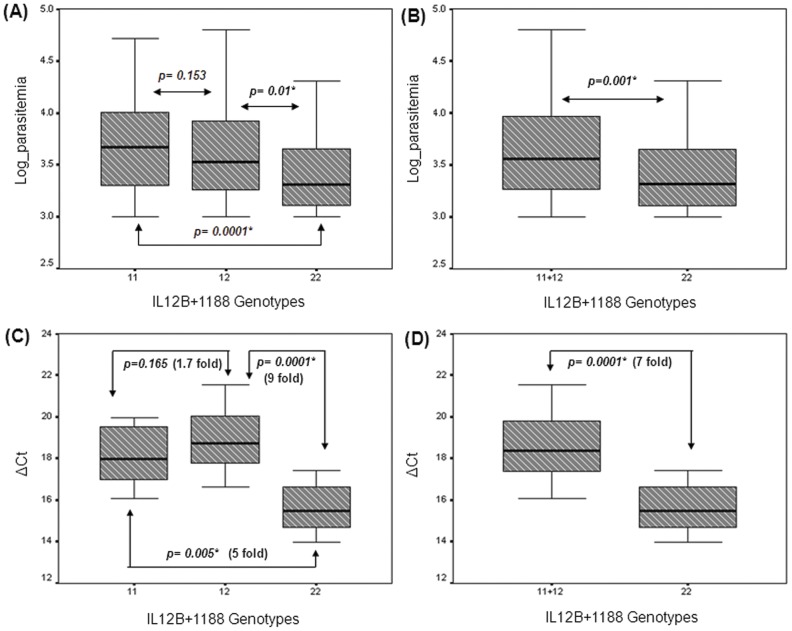
IL12Bpro (MAF: 0.357±0.002) and IL12B+1188 (MAF: 0.404±0.002) were found to be highly polymorphic in the study population and there was no deviation from Hardy-Weinberg equilibrium (P values = 0.3934 and 0.6799 for IL12Bpro and IL12B+1188 respectively). The distribution of genotypes for IL12Bpro and IL12B+1188 loci were summarized in Table 1. For model dependent association analysis, the crude parasitemia data collected from malaria patients was log transformed and subjected to linear regression with respect to age in order to obtain the standardized residuals which were then used to examine the influence of genotypes at each locus on the parasite load under different genetic models. To find out the genetic risk conferred by a SNP, IL12Bpro and IL12B+1188 were independently evaluated as covariate using three genetic models viz., dominant ((11+12) vs 22), recessive (11 vs (12+22)), and additive (11 vs 12 vs 22), where 1 represents as the risk allele using independent t-test and ANOVA (SPSS v10.0). The results of parametric analyses were substantiated by Wilcoxon Rank Sum and Kruskal Wallis H tests for two and three way comparisons respectively using the crude parasitemia data. Box-plot diagrams (Figure 1A & B) displayed a gradual decline in parasitemia across three genotypes of IL12B+1188 locus in which AA (11), AC (12) and CC (22) had the highest, intermediate and lowest median values respectively. Significant differences were obtained for the comparison 11 vs 12 vs 22 using ANOVA test (P value = 0.001) and for the following pairwise genotype comparisons namely (11+12) vs 22 (P value = 0.001); 12 vs 22 (P value = 0.014) and 11 vs 22 (P value = 0.0001) using independent t-test presented in Table 1. The P values in the parentheses were obtained after Bonferroni correction. Non-parametric statistical tests yielded similar trends in the results (Table 1). The mean log parasitemia of 11 (AA = 3.68±0.45) genotype and the heterozygote genotype (AC = 3.58±0.41), separately and jointly, differed significantly (P value = 0.001) from that of 22 (CC = 3.40±0.35), while the difference between mean parasite loads representing genotypes 11 and 12 was not statistically significant (P value = 0.153) suggesting a dominant effect of 1 (A) allele over allele 2 (C) for the IL12B+1188 locus to be the most likely explanation. Similar statistical tests were conducted on IL12Bpro (rs17860508), however none of the comparisons were significant for this polymorphism. The linkage disequilibrium estimate (r2) between these two loci that were 17,249 bp apart was 0.06 (P = 0.3052) suggesting an independent segregation of the promoter and UTR polymorphisms in our population.
Table 1. Genetic association of IL12B promoter and 3′UTR polymorphisms with parasitemia at genotypic level.
| Locus ID (MAFa± SD) | Genotype | Cases (N = 293) (Mean log parasite ± SD) | Pairwise Combination | ANOVA F-ratio | t-test (P-value) | K-W Test χ2 (P-value) | Wilcoxon Rank Sum test (P-value) |
| IL12Bpro (0.357±.002) | 11 | 125 (3.64±0.43) | 11 vs 12 | 1.761 (0.174) | 0.118 | 3.430 (0.180) | 0.091 |
| 12 | 127 (3.55±0.44) | 12 vs 22 | 0.672 | 0.965 | |||
| 22 | 41 (3.52±0.35) | 11 vs 22 | 0.113 | 0.189 | |||
| 11+12 | 252 (3.59±0.43) | 11+12 vs 22 | 0.298 | 0.298 | |||
| 12+22 | 168 (3.53±0.39) | 11 vs 12+22 | 0.062 | 0.062 | |||
| IL12B+1188 (0.404±.002) | 11 | 106 (3.68±0.45) | 11 vs 12 | 7.732 (0.001*) | 0.153 | 13.164 (0.001*) | 0.097 |
| 12 | 137 (3.58±0.41) | 12 vs 22 | 0.014* | 0.01* | |||
| 22 | 50 (3.40±0.35) | 11 vs 22 | 0.0001* | 0.0001* | |||
| 11+12 | 243 (3.62±0.43) | 11+12 vs 22 | 0.001* | 0.001* | |||
| 12+22 | 187 (3.53±0.45) | 11 vs 12+22 | 0.01* | 0.01* |
MAF denotes minor allele frequency and SD denotes standard deviation.
P-Value<0.05.
Figure 1. Association between IL12B+1188 genotypes with blood infection level and IL12B gene-expression represented in Box plots.
(A) Diagram represented the distribution of log-parasitemia across three genotypes: 11 (AA), 12 (AC) & 22 (CC) and (B) Diagram represented the comparison of log-parasitemia of minor homozygous genotype (CC) with AA and AC genotypic groups pooled. Statistical significance between pairwise comparisons was mentioned. (C) The ΔCt distribution of IL12B gene expression across AA (N = 24), AC (N = 28) and CC (N = 12) genotypes and (D) comparison of gene expression between IL12B+1188CC genotype and with that of AA and AC individuals pooled together determined by quantitative real time PCR. Statistical significance was determined by the Mann Whitney U test. P values and fold changes obtained for each pairwise combination were appended in each plot. (*) indicates these differences to be statistically significant. The bottom, middle line, and top of each box correspond to the 25th percentile, median, and the 75th percentile, respectively. Bars extend to the lowest value and to the highest value of each group.
The allele based risk assessment for IL12B+1188 was performed by stratifying the patients into “low” (N = 205) and “high” (N = 88) parasitemic groups. A detail of this procedure was described elsewhere [21]. The proportions of two alleles A and C of IL12B+1188 compared using a two way contingency table were significantly different between the groups (P<0.001). The odds ratio (OR) of comparison (1.887, 95% CI = 1.3–2.78) was due to an excess of allele A in the “high” parasitemic group (Table 2). Taken together, the results of the genetic association study supported a strong influence of IL12B+1188 polymorphism on P. falciparum infection load in our samples at both genotype and allelic levels.
Table 2. Allele based risk assessment in patients with high and low parasite load.
| Locus ID | Allele | High Parasitemic group (N = 88) Frequency | Low Parasitemic group (N = 205) Frequency | ORb (95% cCI) | χ2 (P-value) |
| IL12Bpro | 1 | 0.68 | 0.63 | 1.28(0.88–1.85) | 1.39 (0.238) |
| IL12B+1188 | 1 | 0.70 | 0.55 | 1.89(1.30–2.78) | (0.001*) |
Risk allele was boldfaced. N = total number of sample. χ2-test was used to estimate the differences between the allele frequencies.
OR = odds ratio.
CI = confidence interval.
P-Value<0.05.
Evaluation of gene-gene interactions: Multilocus analyses and MDR
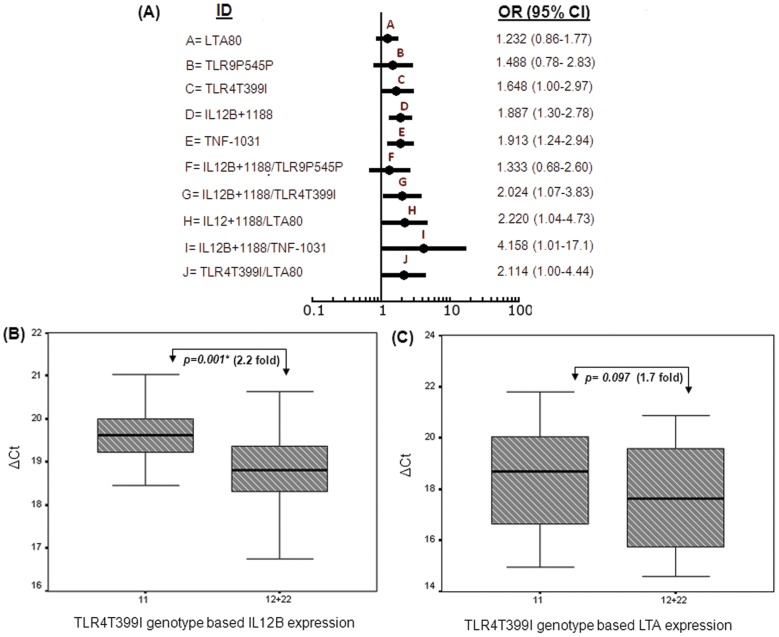
The genetic etiology of complex diseases is considered to involve interactions among multiple genetic variants and environmental conditions. Multifactorial Dimensionality Reduction [41] was performed to explore potential gene-gene interactions by combining the IL12B genotype data of this study with our previous one [21]. We identified five two-factors (Model I–V) and one three-factor (Model VI) gene-gene interaction models with an average cross-validation consistency (CVC) >95% and a P value<0.05 using MDR permutation testing program. The gene-gene partners of MDR models were summarized in Table 3. The interacting models that satisfied the above criteria, included TLR4Thr399Ile (rs4986391) and/or IL12B+1188 (rs3212227) in combination with either TNF-1031 (rs1799964) or LTA80 (rs2239704) or TLR9P545P (rs352140). The average prediction errors (PE) for these models ranged from 0.305 to 0.366. To estimate the genetic risk under a two-factor model, the proportions of individuals harboring risk genotypes for partner loci, were compared between the low and high parasitemic groups. Since IL12B+1188 was present in five out of six genetic models the odds ratio of the second locus was estimated under IL12B+1188 risk (AA+AC) background. The comparison of odds ratio (OR) and 95% confidence intervals (CI) of two-way and the constituent interacting partners were shown in the Forest plot (Figure 2A). The proportion of risk genotypes of three SNPs, namely TLR4Thr399Ile (Model I: OR = 2.024, 95% CI 1.07–3.83, P value = 0.028), TNF-1031 (Model III: OR = 4.158, 95% CI 1.01–17.1, P value = 0.033) and LTA80 (Model V: OR = 2.22, 95% CI 1.04–4.73, P value = 0.034) in association with IL12B+1188 elevated remarkably in high parasitemic groups compared to the single SNP analyses (Figure 2A; ID: G, I and H). For Model II, odds ratio for LTA80 (OR = 2.113, 95% CI 1.0–4.44, P value = 0.047) under TLR4 risk background (Thr-Thr) was also increased (Figure 2A; ID: J). Notably, the difference in mean parasitemia for LTA80 between the genotype comparison (11+12) vs 22 was not significant (OR = 1.232, 95% CI 0.861–1.766, P value = 0.074) when the locus was analyzed alone (Figure 2A; ID: A). In short, except for TLR9P545P, all two-way epistatic models yielded higher risk of developing high parasitemia compared to the single marker analyses. Model VI: TLR4Thr399Ile/IL12B+1188/LTA80 did not modify the risk significantly due to reduction of sample size in the high parasite group when three loci were pooled.
Table 3. Best predictive gene-gene interaction models identified by multifactor dimensionality reduction analysis.
| Model No. | Best Predictive Interaction Modela | Prediction Error (1-TBAb) | 10,000 Permutations P-value | c CVC (in %) |
| I | TLR4Thr399Ile/IL12B+1188 | 0.318 | 0.024* | 95 |
| II | TLR4Thr399Ile/LTA80 | 0.358 | 0.033* | 100 |
| III | IL12B+1188/TNF-1031 | 0.341 | 0.013* | 100 |
| IV | IL12B+1188/TLR9P545P | 0.329 | 0.005* | 100 |
| V | IL12B+1188/LTA80 | 0.366 | 0.042* | 100 |
| VI | TLR4Thr399Ile/IL12B+1188/LTA80 | 0.305 | 0.031* | 100 |
The best model was selected as the one with the minimum prediction error and maximum CVC.
TBA corresponds to the testing balanced accuracy defined as the prediction error (PE) = 1-TBA.
CVC corresponds to cross validation consistency.
P values for gene-gene interaction models were calculated after 10,000 permutations in MDRpt software.
Figure 2. Genetic and functional association of multifactor models obtained by Multifactorial Dimensionality Reduction analysis.
(A) Forest plots presented the comparison of risk estimates in terms of odds ratio (OR) and 95% confidence interval (CI) for significant gene-gene interaction models and component single loci. The risk corresponding to each single and two factor models was denoted by a dot and the horizontal lines represented odds ratio and 95% CI respectively. The model IDs (A–J) and respective ORs (95% CI) were given at the left and right side of each dot in the forest plot. (B) IL12B and (C) LTA gene expression in patients' PBMCs classified according to TLR4Thr399Ile genotype status by real time quantitative PCR. Distribution of ΔCt was plotted and compared between the genotypic groups. Statistical significance was determined by Mann Whitney U test. P values and corresponding fold changes obtained for each pairwise comparison were shown in the box plots. (*) indicates these differences to be statistically significant.
TLR4 encodes an extra-cellular receptor present in the antigen presenting cells (APCs) and perceives the malaria antigens to trigger a complex cascade of downstream signaling events which ultimately culminates into the production of several pro-inflammatory cytokines including IL12 and LTA [64], [65]. To translate functional impact of the above MDR derived gene-gene interaction models (Model I and II) in terms of TLR4 mediated signaling efficacy, we compared the expression levels of IL12B and LTA genes under TLR4Thr399Ile risk (11; N = 16) and non-risk (12+22; N = 33) genotype backgrounds using quantitative RT-PCR analyses. Patients harboring heterozygous and the rare homozygous genotypes (Thr-Ile and Ile-Ile) showed significantly increased (2.2 fold, Mann Whitney P value = 0.001) expression of IL12B gene (median ΔCt±S.D = 18.80±0.87) compared to those with homozygous (Thr-Thr) genotype (median ΔCt±S.D = 19.63±0.74) as shown in Figure 2B. The difference in LTA expression between TLR4Thr399Ile risk and non-risk genotypic backgrounds did not cross the threshold of statistical significance by non-parametric Mann Whitney U test (1.7 fold, P value = 0.097), though the expression of LTA gene (Figure 2C) was higher in TLR4Thr-Ile and Ile-Ile genotype background (median ΔCt±S.D = 17.66±1.7) compared to that estimated in TLR4Thr-Thr risk environment (median ΔCt±S.D = 18.43±1.5). Taken together, our results provided the genetic (Figure 2A) and functional (Figure 2B & C) validations of MDR gene-gene interaction models.
Functional analysis: TNF-α promoter and IL12B 3′UTR polymorphisms
In addition to provide functional support to the statistically inferred epistatic models, we attempted to delineate the molecular role of TNF-α promoter and IL12B 3′UTR polymorphisms using a combination of methods including quantitative RT-PCR, allelic-expression-imbalance, mRNA stability assay, luciferase based promoter (TNF-1031) and 3′UTR (IL12B+1188) activity analyses, bioinformatic prediction of putative mRNA-miRNA binding interactions and SNP based DNA, RNA secondary structure assessment.
(a) Efficiency of TNF-α promoter haplotypes under LPS stimulated and unstimulated conditions: Reporter gene assay and Bioinformatic support
To explore whether TNF-1031 polymorphism influenced the basal rate of transcription of the TNF-α gene, haplotypes pertaining to a 1.2 kb fragment spanning the proximal promoter SNPs at positions −1031, −857, −308 and −238 were subjected to reporter gene based assay. Notably, haplotypes were derived using Haploview and out of the 16 possible haplotypes, only six were observed in our population with haplotype T-C-G-G (1-1-1-1) showing the highest frequency in both high and low parasitemic groups. This reference haplotype as well as four other haplotypes that differed from the former at a single nucleotide position were cloned. The frequency of the variant haplotypes were >3% in the patient samples. Figure 3A showed the map of the promoter haplotypes cloned and assayed for reporter gene activity in HepG2 cells both in presence and absence of LPS, which was known to be one of the parasite-associated-molecular-patterns (PAMPs) recognized by TLR molecules [66]. Fold changes in variant promoter (Hap2-5) activities with respect to reference haplotype Hap1 were indicated in Figure 3B. All four variant haplotypes (Hap2-5) showed decrease in luciferase activity both in presence (250 ng/ml) of LPS (27–84%) and in absence (12–78%) of the antigen (Figure 3B). The maximum reduction in luciferase activity [78% (−LPS) and 84% (+LPS), P<0.001] was observed for Hap3 construct (C-C-G-G) which harbored the risk allele C for TNF-1031, the locus that showed significant genotypic and allelic association with blood parasite infection [21]. Our comparative promoter assay identified Hap1 and Hap3 as the strongest and weakest promoter among the five different TNF-α haplotypes in HepG2 cell line in three independent experiments. Similar experiments were performed in HEK293 and HCT116 in triplicate to compare the promoter activities of Hap1 and Hap3 (Figure 3C). A consistent reduction of the reporter gene expression under stimulated and unstimulated conditions (80%) for Hap3 was noted in both the cell lines. The control pGL3-Basic vector showed very low levels of relative luciferase activity in both the unstimulated and LPS-stimulated cells.
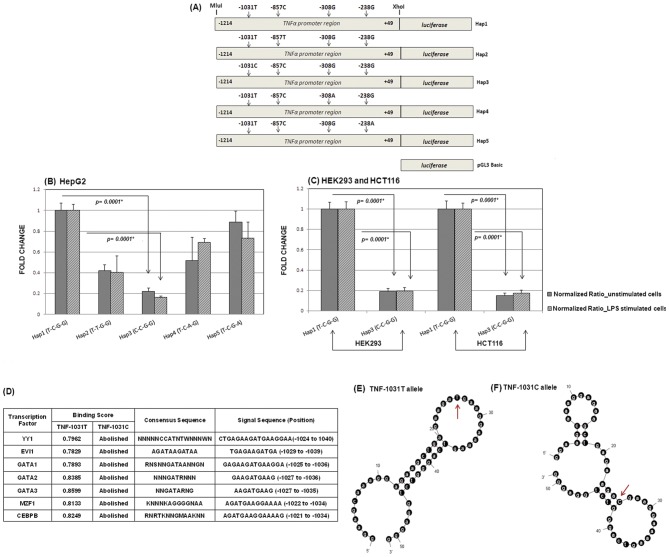
Figure 3. Results of TNF-α promoter assay.
(A) Schematic representation of reporter gene constructs for five TNF-α promoter haplotypes (Hap1–Hap5) used for transfection assays. (B) HepG2 cells (1×105 cells/ml) were transiently transfected with all promoter constructs and relative luciferase activities in supernatants were measured alone (light grey) or with cells stimulated with 250 ng/ml of LPS (dark grey) after 48 hours. Activity of wild-type promoter haplotype TNF-α-Hap1 served as reference and set at one, and the variant constructs were expressed as fold changes in relation to this. Statistical significance for all pairwise comparisons was done by t-test. P values of significant differences between haplotype expressions were marked with asterisks (*). (C) Similar experiments were performed with wild-type Hap1 and variant Hap3 constructs in HEK293 and HCT116 cells and corresponding differences in promoter activities were measured. (*) indicates the significant comparisons given as P values. (D) Putative transcription factor binding profiles for TNF-1031 T allele summarizing the TFs with their binding scores, consensus and signal sequences which were completely abolished in presence of the variant (C) allele. (E & F) MFOLD derived representative DNA secondary structures encompassing 50 bp TNF-α promoter sequence. The numbers indicate the base position while the arrowhead marks the −1031 site.
Scanning of a 101 bp region flanking 50 bp in the either direction of TNF-1031 locus by TFBind program revealed that T to C transition caused loss of binding of several transcription factors. A list of the transcription factors that putatively showed higher binding affinity for the T allele given by binding scores, corresponding consensus and signal sequences with relative nucleotide positions upstream of TNF-α coding sequence were presented in Figure 3D. The possibility of altered DNA-protein interactions in terms of changes in local secondary structure due to the sequence variation at −1031 was examined using MFOLD web server (Figure 3E and F). Out of the seventeen possible computed foldings, thirteen (76.5%) with T at −1031 position were found to be base paired while twenty (83.3%) out of twenty four possible conformations had variant C allele locked in stems. The minimum free energy (MFE) conformations constructed using 50 bp local DNA sequence encompassing TNF-1031 were shown for wild type and variant alleles (Figure 3E & F). Results of these in-silico comparisons indicated a relative reduction of accessibility of a transcription factor when the risk allele was present in the sequence. Taken together, the polymorphism at −1031 of TNF-α caused a loss of promoter activity as well as transcription factor binding as per our reporter gene assay and bioinformatics analyses respectively.
(b) Impact of IL12B+1188 (rs3212227) genotypes and alleles on gene expression: a quantitative evaluation
IL12B+1188 polymorphism showed strong genetic association with the blood parasite level in the malaria patients both at genotype and allele levels. To test the hypothesis that this 3′UTR polymorphism may modulate IL12B transcript turnover, relative expression of IL12B-mRNA was quantified using the cDNA synthesized from mRNA extracted from peripheral blood mononuclear cell (PBMC) fraction of whole blood collected from individuals harboring three different genotypes namely AA (N = 24), AC (N = 28) and CC (N = 12) using real-time quantitative PCR. Expression of IL12B was normalized for each individual separately using 18S rRNA as an endogenous control. The median and distribution of IL12B relative expression in terms of ΔCt for IL12B+1188 AA, AC and CC genotypes was presented in Figure 1C. No significant difference (P value = 0.165) in transcript levels of IL12B gene was observed between genotype groups representing AA (median ΔCt±S.D = 17.98±1.40) and AC (median ΔCt±S.D = 18.74±1.67). On the other hand, the median level of IL12B expression of AA (5 fold; P value = 0.005) and AC (9 fold; P value = 0.0001) genotypic categories were significantly lower both separately as well as collectively (7 fold, P value = 0.0001) compared to that (median ΔCt±S.D = 15.49±1.25) of non-risk CC genotype group (Figure 1C & D).
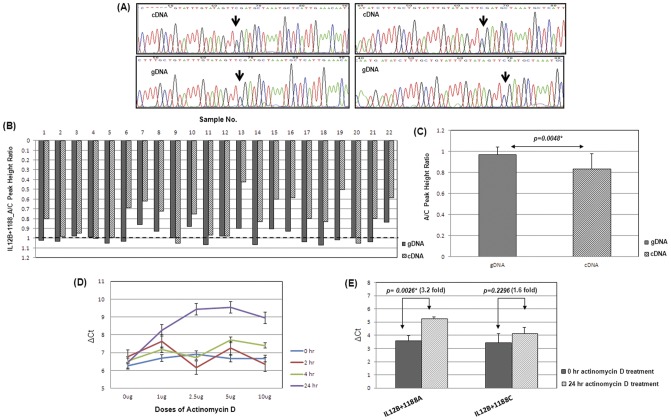
To reinforce the hypothesis that A+1188C change affects the transcription levels of IL12B gene in vivo, we assayed if there was any quantitative difference of transcripts represented by A and C alleles in patients harboring heterozygote genotype. The test for allelic-expression-imbalance (AEI) was carried out by amplification and resequencing of 167 bp fragment that covered IL12+1188 from cDNAs and gDNAs of twenty eight individuals with AC genotype. The peak heights representing A (green peak) and C (blue peak) alleles from individual electropherograms of paired samples were determined using PeakPicker software (Figure 4A). The average A to C peak height ratio ranged from 0.42–1.05 and 0.82–1.07 (Figure 4B) in cDNA and gDNA respectively and the comparison of these ratios was statistically significant (P value = 0.0048) estimated by nonparametric Sign test (Figure 4C). The allele specific expression of IL12B in the peripheral blood mononuclear cells (PBMCs) of malaria patients by 3′UTR polymorphism may be attributed to (i) differential transcript stability, (ii) influence of microRNAs and (iii) conformational change in RNA secondary structure.
Figure 4. IL12B 3′UTR based AEI analysis.
(A) Representative electropherograms showing the peak heights of the sequence encompassing IL12B+1188 locus in gDNA (lower panel) and cDNA (upper panel) extracted from the heterozygous (AC) patients' PBMC samples. The arrowhead indicates the +1188 site. Here A and C alleles are represented as green and blue peaks respectively. Difference in peak heights in sequences between cDNA and gDNA was evident. (B) Bar graph displays the A/C peak height ratios in individual heterozygous samples (N = 22). The dotted line indicates the baseline where the A/C ratio is one. (C) The departure of A/C peak height ratios from baseline in gDNA and cDNA were shown in the form of bar diagram. Each bar represents the mean height and corresponding standard deviation. The statistical difference of this distribution was measured by Sign test. P value has been indicated. (D) Dose-response and time-course assay of actinomycin-D treatment by real time PCR. (E) HepG2 cells (1×105cells/ml) were treated with the optimum dose of actinomycin-D (5 µg/ml), harvested after 0 and 24 hours after treatment and IL12B mRNA levels were measured and corrected for GAPDH mRNA for both the wild-type and variant pSiCHECK2-3′UTR constructs. Statistical significance was measured by t-test. (*) indicates the P values to be statistically significant.
IL12B+1188 allele-based mRNA stability assay: Inhibition of transcription by actinomycin-D
In order to directly assay differences in mRNA stability between IL12B 3′UTR alleles, we determined mRNA levels in cells transfected with plasmids in which 1047 bp region from IL12B 3′UTR representing A or C (+1188) allele was cloned under Renilla luciferase gene with SV40 constitutive promoter in the vector pSiCHECK2 (Figure 5A). Twenty four hours post-transfection, HepG2 cells were treated with actinomycin-D (5 µg/ml) to suppress de novo transcription. Figure 4D represents the dose response and time kinetics of actinomycin-D treatment that enabled us to select for the optimum treatment conditions for the drug. mRNA levels at 0 and 24 hours after the drug treatment were quantified by real-time PCR using IL12B and GAPDH (as endogenous control) specific primers. As shown in Figure 4E, the IL12B mRNA levels had declined 3.2 and 1.6 folds respectively for A (ΔCt at 0 hr = 3.57±0.39 vs ΔCt at 24 hr = 5.23±0.17) and C (ΔCt at 0 hr = 3.44±0.68 vs ΔCt at 24 hr = 4.12±0.47) alleles compared to their respective starting levels suggesting that A+1188C polymorphism differentially influenced IL12B mRNA stability.
Figure 5. IL12B mRNA-microRNA interaction assay.
(A) Schematic representation of reporter gene constructs for IL12B 3′UTR A and C alleles used for transfection assays. (B) Entire 3′UTR region was mapped for putative microRNA binding sites. The highlighted, boldfaced and underlined segments within IL12B sequence were the seed sequences for the miRNAs. (C) Schematic representation of the score and seed position of four miRNAs on target IL12B. (D & E) Normalized luciferase relative light units (RLU) in HepG2 cells were measured for IL12B+1188 A and C allele containing pSiCHECK2 constructs with (+) and without (−) the effect of miRNAs. Co-transfection of the empty pRNAU6.1 (+) vector with pSiCHECK2-IL12B+1188 construct was set as 100% and reductions in luciferase expression in presence of four miRNAs were measured in relation to this. hsa-miR-23a and hsa-miR-23b resulted in significant reduction in luciferase activities for both pSiCHECK2-IL12B+1188A and pSiCHECK2-IL12B+1188C constructs. Statistical significance was measured with t-test. (*) indicates the P values and percentage reduction to be statistically significant.
Influence of microRNA regulators targeting IL12B 3′UTR
To identify the putative microRNA binding sites in the 3′UTR of IL12B gene we employed a consensus approach by using different miRNA target prediction softwares, viz. TargetScan, MiRanda, MicroCosm, miRBase and RegRNA. A comprehensive microRNA map of IL12B 3′UTR predicted consensusly by at least three different databases was presented in the Figure 5B. Four candidate microRNAs from this list were selected for cell based reporter gene assay as they were located in the closest proximity of the polymorphic site (hsa-miR545 and hsa-miR-1284) and conserved among species (hsa-miR23a and hsa-miR-23b). Their respective seed sequences were shown in Figure 5C.
The precursor microRNA (pre-miR) sequences for each of these four regulatory RNAs were extracted from GeneCard database followed by amplification of a region encompassing 100 bp upstream and downstream of the mature miRNA and cloning in appropriate orientation into pRNAU6.1 vector. To verify whether A+1188C SNP alters the binding and regulation incurred by the above candidate microRNAs, we transfected each of these pre-miR constructs in HepG2 cells along with the 1047 bp segment of IL12B 3′UTR containing either +1188A or +1188C variant cloned into the 3′UTR of luciferase gene in pSiCHECK2 plasmid. Reporter plasmid co-transfected with empty miR-pRNAU6.1 vector served as the control and luciferase expression was measured 48 hours post-transfection. All the experiments were done in triplicate and repeated thrice and the change of luciferase gene expression was denoted as normalized RLU (in percentage) relative to the control. Co-transfection of hsa-miR-23a (33.33%±0.08, P value = 0.0043) and hsa-miR-23b (22.22%±0.04, P value = 0.0179) with pSiCHECK2-IL12B-+1188A resulted in significant reduction in Renilla luciferase expression (Figure 5D). Similar reduction of reporter gene expression was observed when hsa-miR-23a (36.6%±1.15, P value = 0.0001) and hsa-miR-23b (20%±2.06, P value = 0.003) were transfected with pSiCHECK2-IL12B-+1188C (Figure 5E). On the other hand, luciferase activity from neither pSiCHECK2-IL12B-+1188A nor pSiCHECK2-IL12B-+1188C construct was altered by hsa-miR-545 (A = 4.35%±0.4 and C = 4.08%±0.5) and hsa-miR-1284 (A = 12.61%±0.5 and C = 4.1%±0.6). Similar trend of hsa-miR-23a and hsa-miR-23b mediated reduction of luciferase activity from pSiCHECK2-IL12B-+1188 A & C constructs were observed in HCT116 and U937 cells (data not shown). Taken together, our results suggest that hsa-miR-23a and hsa-miR-23b bind to IL12B 3′UTR, however, none of them exerts any allele specific influence over the IL12B expression.
Allele dependent conformational changes in RNA secondary structure
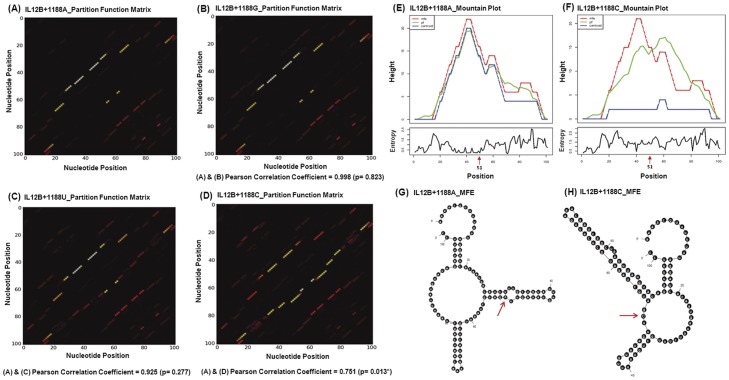
There is increasing evidence that cis-regulatory RNA structural elements within mRNAs mediate post-transcriptional gene regulation by determining several aspects of the mRNA life cycle such as stability, localization, and translational efficiency [67], [68]. However, RNA structures are dynamic and evolved to adopt multiple conformations forming an ensemble that may be best described by a partition function defined as the probabilities of all possible base pairings [69]. To predict if A+1188C polymorphism provides any localized effect on the structural ensemble of IL12B mRNA, we reconstructed foldings of 101 nucleotide RNA sequences using four different bases (A, G, U and C) in the central 51st position, keeping 50 nucleotides upstream and downstream sequences with respect to +1188 site identical. The partition function matrices computed for all four sequences using SNPfold were represented as dot-plots where probability of each base-pairing was denoted by a dot (Figure 6A to D). The pairwise Pearson correlation coefficients of A to G, A to U and A to C were 0.998, 0.925 and 0.751 respectively indicating a significant (P value = 0.013) modulation of overall structural assembly for A to C change (Figure 6A to D).
Figure 6. IL12B 3′UTR polymorphism based RNA ensemble structures.
(A–D) display the SNPfold derived partition function heat maps generated for 101 nucleotide sequences harboring A (risk), G, U and C (non-risk) alleles respectively at the 51st position. The partition function matrix illustrates the base-pairing probabilities represented by dots. We estimated the pairwise Pearson correlation coefficient with respect to wild-type A allele and P values to quantify the overall modulation in the RNA structural ensemble caused by a mutation. (*) indicates the P value to be statistically significant. (E & F) show the mountain plot diagrams for IL12+1188A and C allele for 101 bases using RNAfold. The upper panel demonstrates the height vs position graph in which the red, green and blue lines depict the minimum free energy structure, the partition function of all possible RNA secondary structures and ensemble centroid structure respectively. The lower panel represents the entropy vs position profile and arrowhead denotes the 51st position, the location of A+1188C. (G & H) shows allele specific minimum free energy (MFE) conformations generated from RNA MFOLD. Numbers indicate the base position while the arrow directs the position of the polymorphic site.
Mountain plots were generated for 101 nucleotide sequences harboring A and C alleles (Figure 6E & F). A mountain plot represents the secondary structure in a plot of height versus position where the height m(k) is given by the number of base pairs enclosing the base at position k. The red, green and blue curves represent minimum free energy structure, thermodynamic ensemble of RNA structures and the centroid structure respectively. The overlap between the red and green lines in Figure 6E suggested that structure produced by A allele was closer to that predicted by MFE. The blue plateau in Figure 6F indicated the central tendency of the C allele to remain in open conformation. Deviation from the base line in the position versus entropy graph of C allele shown in lower panel signified a higher randomness and positional entropy in the local structures involving C allele (Figure 6F). This also gave rise to a higher Ensemble Diversity (ED) in the structural constellation generated by C at position 51 (EDA = 20.73 vs EDC = 34.06). Taken together, the in silico modeling of RNA secondary structure indicated an overall conformational dissimilarity in the base-pairing probabilities of the RNA thermodynamic ensemble marked by A and C alleles which could result in an alternative cis-regulatory functions mediated by IL12B 3′UTR polymorphism.
Discussion
Malaria epidemiology studies have shown that, within human populations, a high degree of variation exists between individuals with respect to malaria susceptibility phenotypes, including parasite load, disease incidence, severity, and the magnitude and type of immune responses to malaria antigens [70]. Immune response to malaria has been an object of extensive investigation aiming at understanding the genetic regulation since an inappropriate immune response leads to uncontrolled parasite replication and is detrimental to host fitness [71]. A large body of the literature has demonstrated that protection against severe P. falciparum malaria is provided by distinct genetic traits, while relatively less is known about the mild events which clearly constitute a substantial fraction of global socioeconomic burden of malaria [72]–[74]. To understand the role of host genetics in the susceptibility and clinical course of mild malaria, we in this study, primarily rely on reconstruction of genetic interactions among toll-like receptors (TLRs) and pro-inflammatory cytokine genes together with an indepth functional appraisal of determinants associated with variation in blood parasite level.
Over the past several years, genetic epidemiology studies technologically have progressed from investigating few candidate markers to interrogating thousands of variants in genome-wide association studies (GWAS) while the data analysis mostly focuses on detecting single SNP effects [75], [76]. Looking beyond the boundary of additive inheritance of SNPs using marker by marker approach may offer a better exercise to identify genetic determinants and biological mechanisms involved in disease etiology. Keeping this in mind, we have used MDR to reduce the dimensionality of the multilocus genetic data pertaining to immune response components to identify combinations of polymorphisms associated with the risk of high parasite load. MDR introduced by Ritchie, et al. is a computational strategy to detect and characterize gene-gene interactions that may be associated with disease susceptibility [41]. So far, MDR and its extensions such as GMDR, FAM-MDR, MDR-PDT, EMDR [77]–[80] have identified many interacting genetic variants underlying a wide variety of complex human diseases such as Alzheimer's disease, asthma, atrial fibrillation, autism, bladder cancer, hypertension, nicotine dependency, prostate cancer, schizophrenia, sporadic breast cancer and type II diabetes [81]–[89]. In our study single SNP and multifactor dimensionality reduction analyses together identified three genetic loci which served as important factors in controlling blood parasitemia in mild malaria. These loci include TLR4Thr399Ile, TNF-1031 and IL12B+1188. Two additional loci viz. LTA80 and TLR9P545P appear in combination with TLR4Thr399Ile and IL12B+1188 suggesting that the formers fail to show statistically significant association in independent analyses because of their minor effects on trait variation (Table 3) [90]. Notably, the minor allele frequencies of both LTA80 and TLR9P545P were approximately 40% in our samples [21]. The presence of IL12B+1188 in five of six models and elevation of odds ratio of TLR4Thr399Ile, TNF-1031 and LTA80 in combination with IL12B+1188 are suggestive of a pivotal genetic influence of IL12B on malaria phenotype. This is supported by the fact that different malaria phenotypes such as hyperparasitemia, severe malarial anemia or cerebral malaria are often found to be associated with IL12B gene [34], [91]–[94] while the genetic alliance between parasitemia with LTA is seldom unequivocal [95]–[97]. A study conducted among 198 individuals belonging to 34 families living in Burkina Faso using the pedigree-based generalized multifactor dimensionality reduction approach, identified statistical interactions among immune genes including IL12B 3′ untranslated region, IL12Bpro, LTA+80 for mild malaria, maximum parasitemia or asymptomatic parasitemia [98] underscoring the usefulness of modeling gene-gene interaction in genetic dissection of complex diseases (Table 3).
The association of high parasitemia with IL12B+1188 (Table 1) and significantly low expression of the cytokine in individuals harboring risk genotypes (Figure 1) indicate that the susceptible individuals are deficient in controlling parasite replication due to inadequate level of IL12B transcript in mild malaria patients. Critical role of IL12B in malaria has been demonstrated by other studies that show IL12 production is inversely associated with disease severity in human malaria [94], [99], [100] and the molecule is extremely effective in correcting malarial anemia in murine model [101], [102]. Reduced expression of IL12B and LTA under TLR4 risk genotype background may be attributed to altered TLR4 signaling (Figure 2). To the best of our knowledge, this is the first report that provides functional validation of statistical epistatic models demonstrating in vivo suppression of cytokine gene expression due to genetic deficiency of TLR4 signaling. The attenuated TLR4 signaling by the variant receptors may be ascribed to altered distribution of electrostatic surface potential as shown by homology modeling in our previous study [21]. However, it should be borne in mind, a comprehensive profiling of transcriptome and network analyses are necessary to identify additional genetic partners involved in the pathway.
Pinpointing phenotypically causal variants from a large fraction of SNPs that show statistical association to diseases remains a major challenge. Only a handful of disease-associated variants occur at non-synonymous (nsSNPs) sites while a majority are located on the non-coding genomic regions (ncSNPs) adjacent to protein coding genes suggesting that the latter may be involved in transcriptional regulation. Global expression quantitative trait loci (eQTL) analyses in yeast, mice, and humans have detected significant levels of cis and trans-eQTL that simultaneously regulate a large fraction of the transcriptome [103]–[108]. We previously reported that the polymorphism located at TNF-1031 displayed significant association with peripheral parasite load [21]. Here we showed that any variation from the TNF-α wild-type haplotype resulted in reduced promoter efficiency and the maximal reduction was observed for the haplotype that was altered at −1031 locus. The dysfunctioning of TNF-α promoter due to −1031 polymorphism may be attributed to allelic differences in binding of transcription factors (TFs). A number of transcription factor binding sites (TFBSs) were abolished in presence of the variant allele (C) which assumed a sterically inaccessible locked-in conformation as depicted in the bioinformatic outputs (Figure 3D–F). Chromatin immunoprecipitation or electrophoretic mobility shift assays (EMSA) are necessary to validate in-silico prediction of altered binding affinity between putative TFs and promoter SNP. Reduction of transcriptional activity due to single nucleotide variation in TNF-α promoter has been reported for the polymorphisms located at −863 [109], −376 [110] and −238 [111].
Allelic-expression-imbalance (AEI) phenomenon which serves as an integrative quantitative measure of any and all cis-acting regulatory variants [112] is highly context-specific [113] and widespread not only in humans and mice but in most organisms [114]–[120]. It can affect the transcriptome by altering stability [121], processing efficiency [122], isoform expression of mRNAs [123], [124] and inducing epigenetic changes [125], [126]. The unequal expression of IL12B between marker genotypes and allelic imbalance detected in malaria patients' PBMC samples strongly suggest that IL12B+1188 polymorphism executes a cis-regulatory function. To interpret the probable mechanisms of IL12B+1188 mediated AEI, we have investigated allele specific transcript stability, differential interaction of IL12B 3′UTR with candidate microRNAs and allele-dependent modulation of RNA sub-optimal structures. The remarkable modulation of local RNA secondary structures due to A to C transversion observed in this study may affect the transcript stability directly or through the association of RNA-binding proteins (RBPs) indirectly [69]. Many RNA binding proteins have both sequence-specificity and RNA-secondary structure binding preferences and are known to co-regulate functionally related transcripts [127]. From SNP-targeted studies, it has been estimated that 20% of the measured transcripts show 1.5 fold differences between alleles while 30% show 1.2 fold differences [118], [128]. However, it is still obscure how small allelic imbalance may cause a phenotype and how accurate the bioinformatic predictions are with actual experimental evidences.
Recent studies have also shown that 3′UTRs contain recognition motifs for microRNAs (miRNAs) which play important gene-regulatory roles by pairing to their target mRNAs to direct posttranscriptional repression. The most accurate predictors of miRNA target sites relies on conserved matches to the ∼7 bp seed region near the 5′end of the microRNA and also on the accessibility of target sites. The four candidate microRNAs (hsa-miR-545, hsa-miR-1284, hsa-miR-23a and hsa-miR-23b) predicted in common from multiple databases target IL12B 3′UTR sequence located proximally to A+1188C. Our data showed hsa-miR23a and hsa-miR-23b interacts with IL12B 3′UTR to repress gene expression without any allelic bias. This may be attributed to our reductionist approach to candidate microRNA prediction and limited ability of the current prediction algorithms that calculate target efficacy based on interactions between the mRNA with itself and the mRNA with a miRNA. This is of particular importance because mRNA-miRNA interaction occurs in a complex cellular environment in which mRNAs and miRNAs are likely to be bound by cellular RNA-binding proteins, which are currently impossible to account for in silico. Our findings exemplify that diversity of posttranscriptional gene regulation may extend beyond microRNAs and underscores the importance of characterization of structural cis-regulatory elements and their interaction partners in the context of mRNA stability. Recently, there has been renewed interest in identifying the effects of disease-associated noncoding SNPs on changes in RNA structure [129], [130] which may regulate gene expression at virtually every possible stage ranging from local chromatin remodeling to mRNA translation [131]–[133]. Taken together, our findings convincingly demonstrated inadequate gene expression of pro-inflammatory cytokines, namely IL12B and TNF-α attributable to cis-regulatory polymorphisms together with loss of efficacious TLR4 mediated signaling due to nsSNP contribute to uncontrolled parasite growth in P.falciparum malaria in an epistatic manner. In the study of genetics of complex diseases, to determine which of the multitude of variants carried by an individual are responsible for a given phenotype remains a massive task. Our study emphasizes the need to evaluate gene-gene interaction and biological credibility of hundreds of common non-coding variants with low effect size as powerful complementary research strategies to illuminate the genetic architecture of common diseases.
Supporting Information
Oligonucleotide sequences.
(DOC)
Acknowledgments
We are grateful to all study participants for their cooperation. We thank CAS (UGC), DST-FIST, and IPLS (DBT) for providing some of the instrument facilities at the Department of Biochemistry, University of Calcutta. We are thankful to Ms. Sanmitra Basu and Ms. Rebecca Chowdhury of Department of Biochemistry, University of Calcutta for critical review of the manuscript.
Funding Statement
This work was supported by the Department of Science and Technology, New Delhi (SERC Fast Track Scheme - SR/FTP/L-56/2005 dated 25.04.2006) and University Grants Commission (Major Research Project - F. No 33-232/2007(SR) dated 13.03.2008). MB is supported by a pre-doctoral fellowship from University Grants Commission-Special Assistance Programme-The Research Fellowship in Sciences for meritorious students (UGC-SAP-RFSMS) scheme, and Council of Scientific and Industrial Research (CSIR)-Senior Research Fellowship (Sanction no. 09/(0829)/2010-EMR-I dated 29.3.11). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization (2011) World Malaria Report. GenevaSwitzerland: World Health Organization.
- 2. Stevenson MM, Tam MF (1993) Differential induction of helper T cell subsets during blood-stage Plasmodium chabaudi AS infection in resistant and susceptible mice. Clin Exp Immunol 92: 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taylor-Robinson AW, Phillips RS (1994) B cells are required for the switch from Th1- to Th2-regulated immune responses to Plasmodium chabaudi chabaudi infection. Infect Immun 62: 2490–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ing R, Stevenson MM (2009) Dendritic cell and NK cell reciprocal cross talk promotes gamma interferon-dependent immunity to blood-stage Plasmodium chabaudi AS infection in mice. Infect Immun 77: 770–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Metcalf CJ, Graham AL, Huijben S, Barclay VC, Long GH, et al. (2011) Partitioning regulatory mechanisms of within-host malaria dynamics using the effective propagation number. Science 333: 984–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Francis SE, Sullivan DJ Jr, Goldberg DE (1997) Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu Rev Microbiol 51: 97–123. [DOI] [PubMed] [Google Scholar]
- 7. Franklin BS, Parroche P, Ataide MA, Lauw F, Ropert C, et al. (2009) Malaria primes the innate immune response due to interferon-gamma induced enhancement of toll-like receptor expression and function. Proc Natl Acad Sci U S A 106: 5789–5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walther M, Woodruff J, Edele F, Jeffries D, Tongren JE, et al. (2006) Innate immune responses to human malaria: heterogeneous cytokine responses to blood-stage Plasmodium falciparum correlate with parasitological and clinical outcomes. J Immunol 177: 5736–5745. [DOI] [PubMed] [Google Scholar]
- 9. Medzhitov R, Janeway C Jr (2000) Innate immunity. N Engl J Med 343: 338–344. [DOI] [PubMed] [Google Scholar]
- 10. Stevenson MM, Riley EM (2004) Innate immunity to malaria. Nat Rev Immunol 4: 169–180. [DOI] [PubMed] [Google Scholar]
- 11. Abel L (1999) Genetic epidemiology in the study of susceptibility/resistance to malaria in the human population. Bull Soc Pathol Exot 92: 256–260. [PubMed] [Google Scholar]
- 12. Hill AV (1996) Genetic susceptibility to malaria and other infectious diseases: from the MHC to the whole genome. Parasitology 112 Suppl: S75–84. [DOI] [PubMed] [Google Scholar]
- 13. Hill AV (1999) The immunogenetics of resistance to malaria. Proc Assoc Am Physicians 111: 272–277. [DOI] [PubMed] [Google Scholar]
- 14. Kwiatkowski D (1999) The molecular genetic approach to malarial pathogenesis and immunity. Parassitologia 41: 233–240. [PubMed] [Google Scholar]
- 15. Ndiaye R, Sakuntabhai A, Casademont I, Rogier C, Tall A, et al. (2005) Genetic study of ICAM1 in clinical malaria in Senegal. Tissue Antigens 65: 474–480. [DOI] [PubMed] [Google Scholar]
- 16. Diakite M, Clark TG, Auburn S, Campino S, Fry AE, et al. (2009) A genetic association study in the Gambia using tagging polymorphisms in the major histocompatibility complex class III region implicates a HLA-B associated transcript 2 polymorphism in severe malaria susceptibility. Hum Genet 125: 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sikora M, Ferrer-Admetlla A, Laayouni H, Menendez C, Mayor A, et al. (2009) A variant in the gene FUT9 is associated with susceptibility to placental malaria infection. Hum Mol Genet 18: 3136–3144. [DOI] [PubMed] [Google Scholar]
- 18. Sikora M, Laayouni H, Menendez C, Mayor A, Bardaji A, et al. (2011) A targeted association study of immunity genes and networks suggests novel associations with placental malaria infection. PLoS One 6: e24996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Driss A, Hibbert JM, Wilson NO, Iqbal SA, Adamkiewicz TV, et al. (2011) Genetic polymorphisms linked to susceptibility to malaria. Malar J 10: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Timmann C, Evans JA, Konig IR, Kleensang A, Ruschendorf F, et al. (2007) Genome-wide linkage analysis of malaria infection intensity and mild disease. PLoS Genet 3: e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Basu M, Maji AK, Chakraborty A, Banerjee R, Mullick S, et al. (2010) Genetic association of Toll-like-receptor 4 and tumor necrosis factor-alpha polymorphisms with Plasmodium falciparum blood infection levels. Infect Genet Evol 10: 686–696. [DOI] [PubMed] [Google Scholar]
- 22. Stevenson MM, Su Z, Sam H, Mohan K (2001) Modulation of host responses to blood-stage malaria by interleukin-12: from therapy to adjuvant activity. Microbes Infect 3: 49–59. [DOI] [PubMed] [Google Scholar]
- 23. Trinchieri G (2003) Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 3: 133–146. [DOI] [PubMed] [Google Scholar]
- 24. Hall MA, McGlinn E, Coakley G, Fisher SA, Boki K, et al. (2000) Genetic polymorphism of IL-12 p40 gene in immune-mediated disease. Genes Immun 1: 219–224. [DOI] [PubMed] [Google Scholar]
- 26. Morahan G, Huang D, Ymer SI, Cancilla MR, Stephen K, et al. (2001) Linkage disequilibrium of a type 1 diabetes susceptibility locus with a regulatory IL12B allele. Nat Genet 27: 218–221. [DOI] [PubMed] [Google Scholar]
- 27. Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, et al. (2006) A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314: 1461–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nair RP, Ruether A, Stuart PE, Jenisch S, Tejasvi T, et al. (2008) Polymorphisms of the IL12B and IL23R genes are associated with psoriasis. J Invest Dermatol 128: 1653–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mangino M, Braund P, Singh R, Steeds R, Stevens S, et al. (2008) Association analysis of IL-12B and IL-23R polymorphisms in myocardial infarction. J Mol Med (Berl) 86: 99–103. [DOI] [PubMed] [Google Scholar]
- 30. Miteva LD, Manolova IM, Ivanova MG, Rashkov RK, Stoilov RM, et al. (2012) Functional genetic polymorphisms in interleukin-12B gene in association with systemic lupus erythematosus. Rheumatol Int 32: 53–59. [DOI] [PubMed] [Google Scholar]
- 31. Tso HW, Lau YL, Tam CM, Wong HS, Chiang AK (2004) Associations between IL12B polymorphisms and tuberculosis in the Hong Kong Chinese population. J Infect Dis 190: 913–919. [DOI] [PubMed] [Google Scholar]
- 32. Zhang L, Prather D, Vanden Eng J, Crawford S, Kariuki S, et al. (2010) Polymorphisms in genes of interleukin 12 and its receptors and their association with protection against severe malarial anaemia in children in western Kenya. Malar J 9: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keller CC, Yamo O, Ouma C, Ong'echa JM, Ounah D, et al. (2006) Acquisition of hemozoin by monocytes down-regulates interleukin-12 p40 (IL-12p40) transcripts and circulating IL-12p70 through an IL-10-dependent mechanism: in vivo and in vitro findings in severe malarial anemia. Infect Immun 74: 5249–5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morahan G, Boutlis CS, Huang D, Pain A, Saunders JR, et al. (2002) A promoter polymorphism in the gene encoding interleukin-12 p40 (IL12B) is associated with mortality from cerebral malaria and with reduced nitric oxide production. Genes Immun 3: 414–418. [DOI] [PubMed] [Google Scholar]
- 35. Fehrmann RS, Jansen RC, Veldink JH, Westra HJ, Arends D, et al. (2011) Trans-eQTLs reveal that independent genetic variants associated with a complex phenotype converge on intermediate genes, with a major role for the HLA. PLoS Genet 7: e1002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frazer KA, Murray SS, Schork NJ, Topol EJ (2009) Human genetic variation and its contribution to complex traits. Nat Rev Genet 10: 241–251. [DOI] [PubMed] [Google Scholar]
- 37. Jais PH (2005) How frequent is altered gene expression among susceptibility genes to human complex disorders? Genet Med 7: 83–96. [DOI] [PubMed] [Google Scholar]
- 38. Cookson W, Liang L, Abecasis G, Moffatt M, Lathrop M (2009) Mapping complex disease traits with global gene expression. Nat Rev Genet 10: 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nicolae DL, Gamazon E, Zhang W, Duan S, Dolan ME, et al. (2010) Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. PLoS Genet 6: e1000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization (2006) Guidelines for the treatment of malaria. GenevaSwitzerland: World Health Organization.
- 41. Ritchie MD, Hahn LW, Roodi N, Bailey LR, Dupont WD, et al. (2001) Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am J Hum Genet 69: 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ritchie MD, Hahn LW, Moore JH (2003) Power of multifactor dimensionality reduction for detecting gene-gene interactions in the presence of genotyping error, missing data, phenocopy, and genetic heterogeneity. Genet Epidemiol 24: 150–157. [DOI] [PubMed] [Google Scholar]
- 43. Moore JH (2004) Computational analysis of gene-gene interactions using multifactor dimensionality reduction. Expert Rev Mol Diagn 4: 795–803. [DOI] [PubMed] [Google Scholar]
- 44. Moore JH, Gilbert JC, Tsai CT, Chiang FT, Holden T, et al. (2006) A flexible computational framework for detecting, characterizing, and interpreting statistical patterns of epistasis in genetic studies of human disease susceptibility. J Theor Biol 241: 252–261. [DOI] [PubMed] [Google Scholar]
- 45. Moore JH, Williams SM (2009) Epistasis and its implications for personal genetics. Am J Hum Genet 85: 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hahn LW, Ritchie MD, Moore JH (2003) Multifactor dimensionality reduction software for detecting gene-gene and gene-environment interactions. Bioinformatics 19: 376–382. [DOI] [PubMed] [Google Scholar]
- 47. Ririe KM, Rasmussen RP, Wittwer CT (1997) Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Anal Biochem 245: 154–160. [DOI] [PubMed] [Google Scholar]
- 48. Ge B, Gurd S, Gaudin T, Dore C, Lepage P, et al. (2005) Survey of allelic expression using EST mining. Genome Res 15: 1584–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Waksman SA, Woodruff HB (1940) Bacteriostatic and bacteriocidal substances produced by soil actinomycetes. Proc Soc Exper Biol 45: 609–614. [Google Scholar]
- 50. Sobell HM (1985) Actinomycin and DNA transcription. Proc Natl Acad Sci U S A 82: 5328–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berg JM, Tymoczko JL, Stryer L (2002) Biochemistry. New York: W H Freeman.
- 52.Tripathi G (2010) Cellular and Biochemical Science. New Delhi: I. K. International Pvt Ltd. 567 p.
- 53. Zuker M (1989) Computer prediction of RNA structure. Methods Enzymol 180: 262–288. [DOI] [PubMed] [Google Scholar]
- 54. SantaLucia J Jr (1998) A unified view of polymer, dumbbell, and oligonucleotide DNA nearest-neighbor thermodynamics. Proc Natl Acad Sci U S A 95: 1460–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31: 3406–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tsunoda T, Takagi T (1999) Estimating transcription factor bindability on DNA. Bioinformatics 15: 622–630. [DOI] [PubMed] [Google Scholar]
- 57. Betts L, Spremulli LL (1994) Analysis of the role of the Shine-Dalgarno sequence and mRNA secondary structure on the efficiency of translational initiation in the Euglena gracilis chloroplast atpH mRNA. J Biol Chem 269: 26456–26463. [PubMed] [Google Scholar]
- 58. Bindewald E, Shapiro BA (2006) RNA secondary structure prediction from sequence alignments using a network of k-nearest neighbor classifiers. RNA 12: 342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hofacker IL, Stadler PF (2006) Memory efficient folding algorithms for circular RNA secondary structures. Bioinformatics 22: 1172–1176. [DOI] [PubMed] [Google Scholar]
- 60. Mathews DH, Sabina J, Zuker M, Turner DH (1999) Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J Mol Biol 288: 911–940. [DOI] [PubMed] [Google Scholar]
- 61. Xia T, SantaLucia J Jr, Burkard ME, Kierzek R, Schroeder SJ, et al. (1998) Thermodynamic parameters for an expanded nearest-neighbor model for formation of RNA duplexes with Watson-Crick base pairs. Biochemistry 37: 14719–14735. [DOI] [PubMed] [Google Scholar]
- 62. Ding Y, Chan CY, Lawrence CE (2005) RNA secondary structure prediction by centroids in a Boltzmann weighted ensemble. RNA 11: 1157–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ding Y, Chan CY, Lawrence CE (2006) Clustering of RNA secondary structures with application to messenger RNAs. J Mol Biol 359: 554–571. [DOI] [PubMed] [Google Scholar]
- 64. Schuetze N, Schoeneberger S, Mueller U, Freudenberg MA, Alber G, et al. (2005) IL-12 family members: differential kinetics of their TLR4-mediated induction by Salmonella enteritidis and the impact of IL-10 in bone marrow-derived macrophages. Int Immunol 17: 649–659. [DOI] [PubMed] [Google Scholar]
- 65. May L, van Bodegom D, Frolich M, van Lieshout L, Slagboom PE, et al. (2010) Polymorphisms in TLR4 and TLR2 genes, cytokine production and survival in rural Ghana. Eur J Hum Genet 18: 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kim HM, Park BS, Kim JI, Kim SE, Lee J, et al. (2007) Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 130: 906–917. [DOI] [PubMed] [Google Scholar]
- 67. Namy O, Rousset JP, Napthine S, Brierley I (2004) Reprogrammed genetic decoding in cellular gene expression. Mol Cell 13: 157–168. [DOI] [PubMed] [Google Scholar]
- 68. Garneau NL, Wilusz J, Wilusz CJ (2007) The highways and byways of mRNA decay. Nat Rev Mol Cell Biol 8: 113–126. [DOI] [PubMed] [Google Scholar]
- 69. Halvorsen M, Martin JS, Broadaway S, Laederach A (2010) Disease-associated mutations that alter the RNA structural ensemble. PLoS Genet 6: e1001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Verra F, Mangano VD, Modiano D (2009) Genetics of susceptibility to Plasmodium falciparum: from classical malaria resistance genes towards genome-wide association studies. Parasite Immunol 31: 234–253. [DOI] [PubMed] [Google Scholar]
- 71. Graham AL, Allen JE, Read AF (2005) Evolutionary causes and consequences of immunopathology. Ann Rev Ecol Evol Syst 36: 373–398. [Google Scholar]
- 72. May J, Evans JA, Timmann C, Ehmen C, Busch W, et al. (2007) Hemoglobin variants and disease manifestations in severe falciparum malaria. JAMA 297: 2220–2226. [DOI] [PubMed] [Google Scholar]
- 73. Hill AV (2001) The genomics and genetics of human infectious disease susceptibility. Annu Rev Genomics Hum Genet 2: 373–400. [DOI] [PubMed] [Google Scholar]
- 74. Allison AC (2009) Genetic control of resistance to human malaria. Curr Opin Immunol 21: 499–505. [DOI] [PubMed] [Google Scholar]
- 75. Mechanic LE, Chen HS, Amos CI, Chatterjee N, Cox NJ, et al. (2011) Next generation analytic tools for large scale genetic epidemiology studies of complex diseases. Genet Epidemiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Billings LK, Florez JC (2010) The genetics of type 2 diabetes: what have we learned from GWAS? Ann N Y Acad Sci 1212: 59–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lou XY, Chen GB, Yan L, Ma JZ, Mangold JE, et al. (2008) A combinatorial approach to detecting gene-gene and gene-environment interactions in family studies. Am J Hum Genet 83: 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cattaert T, Urrea V, Naj AC, De Lobel L, De Wit V, et al. (2010) FAM-MDR: a flexible family-based multifactor dimensionality reduction technique to detect epistasis using related individuals. PLoS One 5: e10304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mei H, Cuccaro ML, Martin ER (2007) Multifactor dimensionality reduction-phenomics: a novel method to capture genetic heterogeneity with use of phenotypic variables. Am J Hum Genet 81: 1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mei H, Ma D, Ashley-Koch A, Martin ER (2005) Extension of multifactor dimensionality reduction for identifying multilocus effects in the GAW14 simulated data. BMC Genet 6 Suppl 1: S145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Liang X, Slifer M, Martin ER, Schnetz-Boutaud N, Bartlett J, et al. (2009) Genomic convergence to identify candidate genes for Alzheimer disease on chromosome 10. Hum Mutat 30: 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chan IH, Tang NL, Leung TF, Huang W, Lam YY, et al. (2008) Study of gene-gene interactions for endophenotypic quantitative traits in Chinese asthmatic children. Allergy 63: 1031–1039. [DOI] [PubMed] [Google Scholar]
- 83. Asselbergs FW, Moore JH, van den Berg MP, Rimm EB, de Boer RA, et al. (2006) A role for CETP TaqIB polymorphism in determining susceptibility to atrial fibrillation: a nested case control study. BMC Med Genet 7: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ma DQ, Whitehead PL, Menold MM, Martin ER, Ashley-Koch AE, et al. (2005) Identification of significant association and gene-gene interaction of GABA receptor subunit genes in autism. Am J Hum Genet 77: 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Andrew AS, Nelson HH, Kelsey KT, Moore JH, Meng AC, et al. (2006) Concordance of multiple analytical approaches demonstrates a complex relationship between DNA repair gene SNPs, smoking and bladder cancer susceptibility. Carcinogenesis 27: 1030–1037. [DOI] [PubMed] [Google Scholar]
- 86. Li MD, Mangold JE, Seneviratne C, Chen GB, Ma JZ, et al. (2009) Association and interaction analyses of GABBR1 and GABBR2 with nicotine dependence in European- and African-American populations. PLoS One 4: e7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Beuten J, Gelfond J, Franke J, Weldon K, Crandall A, et al. (2009) Single and multigenic analysis of the association between variants in 12 steroid hormone metabolism genes and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 18: 1869–1880. [DOI] [PubMed] [Google Scholar]
- 88. Qin S, Zhao X, Pan Y, Liu J, Feng G, et al. (2005) An association study of the N-methyl-D-aspartate receptor NR1 subunit gene (GRIN1) and NR2B subunit gene (GRIN2B) in schizophrenia with universal DNA microarray. Eur J Hum Genet 13: 807–814. [DOI] [PubMed] [Google Scholar]
- 89. Neuman R, Wasson J, Atzmon G, Wainstein J, Yerushalmi Y, et al. (2010) Gene-gene interactions lead to higher risk for development of type 2 diabetes in an Ashkenazi Jewish population. PLoS One 5: e9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Park JH, Gail MH, Weinberg CR, Carroll RJ, Chung CC, et al. (2011) Distribution of allele frequencies and effect sizes and their interrelationships for common genetic susceptibility variants. Proc Natl Acad Sci U S A 108: 18026–18031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Marquet S, Doumbo O, Cabantous S, Poudiougou B, Argiro L, et al. (2008) A functional promoter variant in IL12B predisposes to cerebral malaria. Hum Mol Genet 17: 2190–2195. [DOI] [PubMed] [Google Scholar]
- 92. Ong'echa JM, Raballah EO, Kempaiah PM, Anyona SB, Were T, et al. (2011) Polymorphic variability in the 3′ untranslated region (UTR) of IL12B is associated with susceptibility to severe anaemia in Kenyan children with acute Plasmodium falciparum malaria. BMC Genet 12: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Phawong C, Ouma C, Tangteerawatana P, Thongshoob J, Were T, et al. (2010) Haplotypes of IL12B promoter polymorphisms condition susceptibility to severe malaria and functional changes in cytokine levels in Thai adults. Immunogenetics 62: 345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Boutlis CS, Lagog M, Chaisavaneeyakorn S, Misukonis MA, Bockarie MJ, et al. (2003) Plasma interleukin-12 in malaria-tolerant papua new guineans: inverse correlation with Plasmodium falciparum parasitemia and peripheral blood mononuclear cell nitric oxide synthase activity. Infect Immun 71: 6354–6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Barbier M, Delahaye NF, Fumoux F, Rihet P (2008) Family-based association of a low producing lymphotoxin-alpha allele with reduced Plasmodium falciparum parasitemia. Microbes Infect 10: 673–679. [DOI] [PubMed] [Google Scholar]
- 96. Clark TG, Diakite M, Auburn S, Campino S, Fry AE, et al. (2009) Tumor necrosis factor and lymphotoxin-alpha polymorphisms and severe malaria in African populations. J Infect Dis 199: 569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Randall LM, Kenangalem E, Lampah DA, Tjitra E, Mwaikambo ED, et al. (2010) A study of the TNF/LTA/LTB locus and susceptibility to severe malaria in highland papuan children and adults. Malar J 9: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Atkinson A, Barbier M, Afridi S, Fumoux F, Rihet P (2011) Evidence for epistasis between hemoglobin C and immune genes in human P. falciparum malaria: a family study in Burkina Faso. Genes Immun 12: 481–489. [DOI] [PubMed] [Google Scholar]
- 99. Perkins DJ, Weinberg JB, Kremsner PG (2000) Reduced interleukin-12 and transforming growth factor-beta1 in severe childhood malaria: relationship of cytokine balance with disease severity. J Infect Dis 182: 988–992. [DOI] [PubMed] [Google Scholar]
- 100. Ong'echa JM, Remo AM, Kristoff J, Hittner JB, Were T, et al. (2008) Increased circulating interleukin (IL)-23 in children with malarial anemia: in vivo and in vitro relationship with co-regulatory cytokines IL-12 and IL-10. Clin Immunol 126: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Stevenson MM, Su Z, Sam H, Mohan K (2001) Modulation of host responses to blood-stage malaria by interleukin-12: from therapy to adjuvant activity. Microbes Infect 3: 49–59. [DOI] [PubMed] [Google Scholar]
- 102. Mohan K, Stevenson MM (1998) Interleukin-12 corrects severe anemia during blood-stage Plasmodium chabaudi AS in susceptible A/J mice. Exp Hematol 26: 45–52. [PubMed] [Google Scholar]
- 103. Jansen RC, Nap JP (2001) Genetical genomics: the added value from segregation. Trends Genet 17: 388–391. [DOI] [PubMed] [Google Scholar]
- 104. Doerge RW (2002) Mapping and analysis of quantitative trait loci in experimental populations. Nat Rev Genet 3: 43–52. [DOI] [PubMed] [Google Scholar]
- 105. Schadt EE, Monks SA, Drake TA, Lusis AJ, Che N, et al. (2003) Genetics of gene expression surveyed in maize, mouse and man. Nature 422: 297–302. [DOI] [PubMed] [Google Scholar]
- 106. Brem RB, Yvert G, Clinton R, Kruglyak L (2002) Genetic dissection of transcriptional regulation in budding yeast. Science 296: 752–755. [DOI] [PubMed] [Google Scholar]
- 107. Morley M, Molony CM, Weber TM, Devlin JL, Ewens KG, et al. (2004) Genetic analysis of genome-wide variation in human gene expression. Nature 430: 743–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. West MA, Kim K, Kliebenstein DJ, van Leeuwen H, Michelmore RW, et al. (2007) Global eQTL mapping reveals the complex genetic architecture of transcript-level variation in Arabidopsis. Genetics 175: 1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Skoog T, van't Hooft FM, Kallin B, Jovinge S, Boquist S, et al. (1999) A common functional polymorphism (C→A substitution at position −863) in the promoter region of the tumour necrosis factor-alpha (TNF-alpha) gene associated with reduced circulating levels of TNF-alpha. Hum Mol Genet 8: 1443–1449. [DOI] [PubMed] [Google Scholar]
- 110. Knight JC, Udalova I, Hill AV, Greenwood BM, Peshu N, et al. (1999) A polymorphism that affects OCT-1 binding to the TNF promoter region is associated with severe malaria. Nat Genet 22: 145–150. [DOI] [PubMed] [Google Scholar]
- 111. Kaluza W, Reuss E, Grossmann S, Hug R, Schopf RE, et al. (2000) Different transcriptional activity and in vitro TNF-alpha production in psoriasis patients carrying the TNF-alpha 238A promoter polymorphism. J Invest Dermatol 114: 1180–1183. [DOI] [PubMed] [Google Scholar]
- 112. Wang D, Sadee W (2006) Searching for polymorphisms that affect gene expression and mRNA processing: example ABCB1 (MDR1). AAPS J 8: E515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Knight JC (2004) Allele-specific gene expression uncovered. Trends Genet 20: 113–116. [DOI] [PubMed] [Google Scholar]
- 114. Yan H, Yuan W, Velculescu VE, Vogelstein B, Kinzler KW (2002) Allelic variation in human gene expression. Science 297: 1143. [DOI] [PubMed] [Google Scholar]
- 115. Lo HS, Wang Z, Hu Y, Yang HH, Gere S, et al. (2003) Allelic variation in gene expression is common in the human genome. Genome Res 13: 1855–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cowles CR, Hirschhorn JN, Altshuler D, Lander ES (2002) Detection of regulatory variation in mouse genes. Nat Genet 32: 432–437. [DOI] [PubMed] [Google Scholar]
- 117. Verlaan DJ, Ge B, Grundberg E, Hoberman R, Lam KC, et al. (2009) Targeted screening of cis-regulatory variation in human haplotypes. Genome Res 19: 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ge B, Pokholok DK, Kwan T, Grundberg E, Morcos L, et al. (2009) Global patterns of cis variation in human cells revealed by high-density allelic expression analysis. Nat Genet 41: 1216–1222. [DOI] [PubMed] [Google Scholar]
- 119. Dimas AS, Deutsch S, Stranger BE, Montgomery SB, Borel C, et al. (2009) Common regulatory variation impacts gene expression in a cell type-dependent manner. Science 325: 1246–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Majewski J, Pastinen T (2011) The study of eQTL variations by RNA-seq: from SNPs to phenotypes. Trends Genet 27: 72–79. [DOI] [PubMed] [Google Scholar]
- 121. Carter AM, Sachchithananthan M, Stasinopoulos S, Maurer F, Medcalf RL (2002) Prothrombin G20210A is a bifunctional gene polymorphism. Thromb Haemost 87: 846–853. [PubMed] [Google Scholar]
- 122. Gehring NH, Frede U, Neu-Yilik G, Hundsdoerfer P, Vetter B, et al. (2001) Increased efficiency of mRNA 3′ end formation: a new genetic mechanism contributing to hereditary thrombophilia. Nat Genet 28: 389–392. [DOI] [PubMed] [Google Scholar]
- 123. Gretarsdottir S, Thorleifsson G, Reynisdottir ST, Manolescu A, Jonsdottir S, et al. (2003) The gene encoding phosphodiesterase 4D confers risk of ischemic stroke. Nat Genet 35: 131–138. [DOI] [PubMed] [Google Scholar]
- 124. Ueda H, Howson JM, Esposito L, Heward J, Snook H, et al. (2003) Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 423: 506–511. [DOI] [PubMed] [Google Scholar]
- 125. Ober C, Aldrich CL, Chervoneva I, Billstrand C, Rahimov F, et al. (2003) Variation in the HLA-G promoter region influences miscarriage rates. Am J Hum Genet 72: 1425–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Pastinen T, Hudson TJ (2004) Cis-acting regulatory variation in the human genome. Science 306: 647–650. [DOI] [PubMed] [Google Scholar]
- 127. Blencowe B, Brenner S, Hughes T, Morris Q (2009) Post-transcriptional gene regulation: RNA-protein interactions, RNA processing, mRNA stability and localization. Pac Symp Biocomput 545–548. [PubMed] [Google Scholar]
- 128. Zhang K, Li JB, Gao Y, Egli D, Xie B, et al. (2009) Digital RNA allelotyping reveals tissue-specific and allele-specific gene expression in human. Nat Methods 6: 613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Martin JS, Halvorsen M, Davis-Neulander L, Ritz J, Gopinath C, et al. (2012) Structural effects of linkage disequilibrium on the transcriptome. RNA 18: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kiryu H, Asai K (2012) Rchange: algorithms for computing energy changes of RNA secondary structures in response to base mutations. Bioinformatics 28: 1093–1101. [DOI] [PubMed] [Google Scholar]
- 131. Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, et al. (2007) Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129: 1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT (2008) Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 322: 750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Mercer TR, Dinger ME, Mattick JS (2009) Long non-coding RNAs: insights into functions. Nat Rev Genet 10: 155–159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Oligonucleotide sequences.
(DOC)