Abstract
Polydimethylsiloxane (DMS) is a popular material for microfluidics, but it is hydrophobic and is prone to non-specific protein adsorption. In this study, we explore methods for producing stable, protein resistant, tetraglyme plasma polymer coatings on PDMS by combining extended baking processes with multiple plasma polymer coating steps. We demonstrate that by using this approach, it is possible to produce a plasma polymer coatings that resist protein adsorption (<10 ng/cm2) and are stable to storage over at least 100 days. This methodology can translate to any plasma polymer system, enabling the introduction of a wide range of surface functionalities on PDMS surfaces.
INTRODUCTION
Microfluidic systems are becoming increasingly important for a wide range of bioengineering applications including structural biology,1, 2 bioseparations and micro-total analysis systems (μTAS),3, 4 drug discovery and development, 5, 6 and proteomics.7, 8 Materials selection for microfluidic devices is often a compromise between the manufacturability of the material and its mechanical, optical, chemical, and electrical properties. The first microfluidic devices were produced in silicon and glass since the fabrication techniques for these materials was already well-developed through work in the semi-conductor industry.9 Despite glass still being a popular choice for microfluidics, there has been a push towards polymeric alternatives, which require less fabrication time and have lower costs. Many polymer alternatives have been tried, including polycarbonate (PC),10 polymethylmethacrylate (PMMA),11 polystyrene copolymer,12 and polydimethylsiloxane (PDMS).9 Out of these materials, PDMS has become the most popular material for rapid prototyping and laboratory based microfluidics since it was originally championed by the Whitesides group.9, 13, 14, 15, 16
There are many reasons for the popularity of PDMS including the low cost of material when compared to silicon or glass, the ability of the material to conform to shapes down in to the nanometer scale,17 as well as the ease with which devices can be sealed either covalently or conformally.9 Another advantage is the simple, rapid, and safe micro-molding processes and bonding techniques used in device fabrication compared to traditional chemical etching methods.18 However, along with all the advantages of PDMS there are some fundamental drawbacks for its applications in many bioassay microfluidic applications. PDMS is hydrophobic, incompatible with many organic solvents and lacks chemical functional groups to enable simple subsequent biomolecule immobilization or polymer grafting. Of these, it is the hydrophobicity of the material, which causes most problems, including difficulties introducing fluids into microchannels, inconsistencies in flow dynamics and large amounts of uncontrolled and undesired protein adsorption.19, 20, 21, 22 This uncontrollable protein adsorption within PDMS microchannels can lead to a loss of valuable sample, changes in wall wettability, disruption to surface charge properties necessary for EOF and protein separation, distortion of channel dimensions and even channel blockage in many bio-assay applications.19, 22, 23 These problems can all lead to reductions in device specificity, sensitivity, and resolution, and even lead to complete device failure in some applications. The importance of protein adsorption within microfluidic channels was summarized in a review article by Mukhopadhyay,22 where it was stated that protein fouling within microfluidic devices affects “every application except electrophoresis of DNA.”
The ability to control surface chemistry and reduce protein fouling on PDMS is of paramount importance. This has led to much work on the surface modification of PDMS microfluidic devices to obtain desirable surface properties. The most common example of this is using oxygen plasma treatment to effectively oxidase the PDMS surface, greatly increasing its hydrophilicity,24 and therefore the ease with which aqueous flow can occur within the channel, as well as facilitating covalent sealing of devices.9 Despite being useful in some applications, the resulting surface is unstable. Oxidized PDMS surfaces undergo hydrophobic recovery where the PDMS surface returns to its original hydrophobic nature within about 15-45 min of exposure to air.25, 26 Almost all theories explaining this phenomena include the importance of low molecular weight polymer chains (often oligomers) from the bulk migrating in some way to the surface and engulfing the surface modification.27, 28, 29, 30 Hence, this mechanism will affect all PDMS surface modifications in the same way unless the method counters hydrophobic recovery. Commonly surfaces are kept in an aqueous environment to eliminate the driving force for recovery.25, 31 However, this is usually an impractical means of storage, especially when the potential use of these lab-on-a-chip type devices requires “off-the shelf,” simple to use, or high-throughput applications.
Other more complex surface modification strategies on PDMS have been used for various applications including atom-transfer radical polymerization,32 surface grafting,33 chemical vapor deposition (CVD),34 UV-polymer grafting,35 gradient-induced migration of embedded ampiphilic copolymers,36 and plasma polymerization.37 Despite the relatively high number of publications looking in to surface modification of PDMS, only a few have been successfully implemented in PDMS microfluidic devices to reduce protein adsorption. These have included preferential immobilization of n-dodecyl-β-D-maltoside (DDM),21 grafting of epoxy-modified hydrophilic polymers,38 and embedding of pluronic molecules into the PDMS bulk.36 Despite these surface modification methods working well in their specific applications, researchers frequently do not discuss long term stability of the surface modifications, and it is likely that hydrophobic recovery will affect even these more complex approaches as little is done to combat this mechanism. Some work has looked at ways of reducing hydrophobic recovery through reducing the quantity of low molecular weight oligomers in the bulk of the material. This in turn, reduces the amount of motile siloxane molecules that can migrate to the surface and greatly reduces hydrophobic recovery. While solvent extraction techniques tend to be more complex to perform,39 Eddington et al. have shown that increasing the thermal ageing time (heat curing) of PDMS can significantly reduce LMW species and thus greatly reduce hydrophobic recovery.26 However, these systems only use oxygen plasma as the subsequent surface modification technique. To date, little work has focused on combining methods to reduce hydrophobic recovery with surface modification techniques that could give functional and stable PDMS surface properties.
While plasma treatments using oxygen are commonly used to oxidize PDMS surfaces, plasma polymerization actually deposits a <100 nm thick film onto materials. A wide range of monomers are been utilized to produce plasma polymer coatings with a range of specific chemical and physical properties.40 Lopez et al. first showed that it was possible to produce protein resistant, ether carbon-rich films from glyme molecules using plasma polymerization.41 The application of glyme, and a range of other plasma polymer inside glass and Teflon microfluidic devices, have been demonstrated to produce coatings with a range of specific surface charges,42 spontaneous protein immobilization properties43 and resistance to protein adsorption.44 While a limited number of groups have access to dedicated plasma polymerization systems, it is possible that existing O2 plasma units could be adapted for monomer handling or small chambers built from these systems at minimal cost. Thus, if a method for producing plasma polymers that are stable on PDMS could be established, plasma polymerization could become a very attractive, single step method for modifying open, PDMS microfluidic device.
In this study, we explore methods for the production of stable, protein resistant, plasma polymer layers from tetraglyme (ppTTg) on PDMS by utilizing the thermal curing conditions developed by Eddington et al.26 and incorporating these with a multi-layer plasma polymer deposition. Longer curing conditions aim to reduce the quantity of motile oligomers in the bulk, while single and double layer coating systems aimed to reduce migration, increase the coating stability, and enable the retention of the protein resistance over extended periods of dry shelf storage.
MATERIALS AND METHODS
PDMS preparation
PDMS (Sylgard 184, Dow Corning, UK) base and curing agent were thoroughly mixed in a 10:1 ratio in a flask on a rotating table. The mixture was then poured into a plastic Petri dish to a thickness of approximately 5 mm. This Petri dish was then placed in a vacuum desiccator where the pressure was reduced to below 1 mbar to remove any trapped air in the PDMS and avoid bubble formation in the final sample. Samples were then placed in an oven at 80 °C for 1 h. After this baking step, the samples were “cured” into a solid elastomeric material that was removed from the Petri dish and cut into small pieces (approximately 5–10 mm2).
Single coated PDMS samples were produced to test to see whether simply coating PDMS with a plasma polymer layer would produce the desired coating characteristics without the need for any further processing of the samples. These samples were simply cleaned through sonication in distilled water and then drying in a stream of nitrogen gas before being coated with the plasma polymer and tested.
Double-coated PDMS samples were produced to test whether a more advanced protocol was necessary to produce a stable plasma polymer coating on PDMS. These samples were firstly rinsed with distilled water and dried, before being placed in the plasma reactor and coated with a primary plasma polymer layer. Samples were then placed back in the oven for 72 h at 80 °C to allow hydrophobic recovery to occur. Finally, these samples were placed back in the reactor and given a second coating before surface analysis and testing.
Silicon wafer samples (〈100〉 crystal plane, single side polished, Compart Technologies, UK) were manually cut into small squares (approximately 5–10 mm2) using a fine diamond tip cutter. Samples were then blown with compressed air to remove any particulate dust particles adhering to their surfaces. Samples were then placed in a bath of isopropyl alcohol (IPA) and sonicated twice for 15 min with a solvent change between each cycle. Samples were then stored in IPA. When required, samples were removed from the solvent and blown dry with pure nitrogen before being placed in the plasma deposition chamber.
Plasma polymerization
Tetraglyme (tetra ethylene glycol dimethyl ether, TTg, >98%) was all purchased from Aldrich U.K. Prior to plasma polymerization, the monomer was degassed several times using freeze thaw cycles. All polymers were fabricated in a stainless steel T-piece reactor with an internal aluminum disc electrode.44 The experimental setup has been explained in detail previously.42 Briefly, the radio frequency power source (13.56 MHz, Coaxial Power Systems Ltd, UK) was coupled to the reactor via an impedance matching network. The substrates were placed in the reactor and the vessel was pumped down to a base pressure of 1 × 10−3 mbar. Deposition power, time and monomer flow rate were fixed initially at 50 W, 2 min and 2.5 standard cubic centimeters per minute (sccm), respectively, and after the deposition of this adhesion layer, the power was lowered to 10 W for a further 25 min. Due to the low volatility of the tetraglyme monomer, both the reactor chamber and the monomer were heated to 55 °C and 90 °C, respectively.
Protein adsorption studies
Fibrinogen from human plasma (65% purity, containing 15% sodium citrate and 20% sodium chloride, Sigma Aldrich, UK) was prepared at a concentration of 0.5 or 1 mg/ml in phosphate buffered saline (PBS) at pH 7.4 and 150 mM. Samples were placed in 2 ml of protein solution and incubated for 1 h at 37 °C before being removed and thoroughly rinsed with excess MilliQ water and dried under a stream of nitrogen prior to x-ray photoelectron spectroscopy (XPS) analysis.
Enzyme-linked immunosorbent assays (ELISAs) were used to examine the levels of protein adsorbed to the different surfaces. All solutions were freshly prepared before each experiment, and controls (no protein and no antibody samples) were run for each experiment to establish the experimental background signals. Samples were initially placed in 3 ml of 1 mg/ml human fibrinogen in PBS (pH 7.4, 150 mM) for 2 h at 37 °C in a 6 well tissue culture dish. Samples were then rinsed by exchanging the protein solution with aliquots of PBS to ensure that only protein molecules bound to the solid substrate remained. To minimize non-specific adsorption of the antibody, samples were immersed for 2 h, at 37 °C, in a blocking solution consisting of 3 ml of PBSTM (skimmed milk (5%) and Tween20 (0.05%) in PBS). Samples were then washed in PBS before the addition of anti-human fibrinogen (AbCam Ltd, Cambridge, UK) conjugated to the enzyme, horseradish peroxidase (HRP). The antibody-HRP complex concentration used was a 1:10 000 solution in PBSTM. The samples were incubated for 2 h at 37 °C and then rinsed with PBST before being transferred to a clean 24-well tissue culture plate. Then 2 ml of the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) substrate solution (8 mg ABTS and 20 μl of 30% H2O2 in 24 ml of 0.1 M Citrate Buffer at pH 4) was then placed into each of the sample wells. When a green colorimetric endpoint was reached, typically after around 2 min, the substrate solution was stopped using 1 M hydrochloric acid to ensure no further color change would occur. After the color change was stopped, 50 μl of the solution was taken out of the well and placed into a clean 96-well plate before being placed in a standard plate reader. The colorimetric optical density was then measured at 405 nm and the results were recorded. Negative control experiments without the addition of fibrinogen were also carried out. All the experiments were performed at least in triplicate.
Surface analysis
XPS
XPS spectra were acquired using an Axis Ultra DLD spectrometer (Kratos Analytical, Manchester, UK). In the spectroscopy mode, the samples were irradiated with monochromatic Al Kα source (hν = 1486.6 eV, spot size 300 μm × 700 μm). The sample was isolated electrically in order to eliminate vertical differential charging, and a low-energy electron flood source was used for charge compensation. The pressure in the analysis chamber was always maintained below 2 × 10−8 mbar for data acquisition. Survey spectra were obtained from the surface at 160 eV pass energy, 1 eV step size, from 1200 eV to −5 eV. The data were converted to VAMAS format, processed using CasaXPS (v 2.2.37). Data were quantified using empirically derived sensitivity factors. High-resolution C1s spectra were collected at pass energy of 20 eV and step size of 0.1 eV. High resolution C1s spectra were fitted with Gaussian- broadened Lorentzian functions (70% Gaussian) after linear background subtraction. Peaks were charge corrected relative to the CHx component at 285 eV.
Water contact angle measurements
Water contact angles were measured to test the hydrophilicity of each plasma polymer film produced. They were also used to measure the stability and potential hydrophobic recovery of PDMS coated with plasma polymer films as has been shown elsewhere.26 Water contact angles were measured using a Rame-Hart goniometer (Rame-Hart, NJ, USA) directly onto samples on the sample stage. A 2 μl of distilled water was deposited on to the sample surface using a syringe. The contact angle between the sample surface and the water drop was then measured using the microscope to give the sessile contact angle.
RESULTS
XPS analysis of the coatings
Surface characterization of plasma polymer coated PDMS was achieved through XPS elemental quantification of survey spectra and curve fitting of C1s spectra. Control substrates of pure silicon wafers (positive control) and uncoated PDMS were used throughout the study to allow for comparison between native PDMS, coated PDMS and optimal coatings.
Table TABLE I. shows the average elemental quantification of each surface modification after curve fitting of the survey scans, as well as the oxygen to carbon ratio. The table also contains the theoretical values that are obtained from calculation of the monomer precursor's chemical structure to show the amount of monomer structure retention.
TABLE I.
Elemental composition from XPS survey spectra for ppTTg plasma polymer coatings on Si wafers and PDMS substrates. All values represent the mean elemental composition percentage + SD, calculated from 6 replicates for each sample.
| Sample | %C | %O | %Si | O/C Ratio |
|---|---|---|---|---|
| Native PDMS | 45.5 ± 0.7 | 26.6 ± 1.0 | 27.9 ± 0.7 | 0.58 ± 0.01 |
| Single ppTTg coated PDMS | 65.8 ± 3.3 | 28.2 ± 1.8 | 6.0 ± 1.7 | 0.43 ± 0.02 |
| Double ppTTg coated PDMS | 68.2 ± 1.4 | 29.9 ± 1.6 | 1.9 ± 0.2 | 0.45 ± 0.02 |
| ppTTg coated silicon wafer | 67.5 ± 1.0 | 32.5 ± 1.5 | 0 | 0.48 ± 0.02 |
| Theoretical composition of tetraglyme | 66.7 | 33.3 | 0 | 0.5 |
The elemental composition of uncoated PDMS is close to the theoretical elemental composition of the repeating unit of the polymer, and also to that achieved in a previous study.27 When the plasma polymer was deposited onto each of the substrates, there was a significant change in surface chemistry. There was an increase in carbon and oxygen, as well as decreases in silicon signal detected across all of the substrates, producing O/C ratios just under 0.5 for all plasma polymer coated surfaces. The total attenuation of silicon signal for coated silicon wafer samples suggested that plasma polymer layers were thicker than 10 μm, as this is the approximate sampling depth of XPS analysis on organic films.45 Analysis of the single layer ppTTg coatings on PDMS showed that there was approximately 6% silicon content on the surface. Double layer ppTTg coatings exhibited much lower silicon content than single layer coatings (1.9% compared to 6%), but this quantity was still significant as it was higher than the silicon substrate positive control sample.
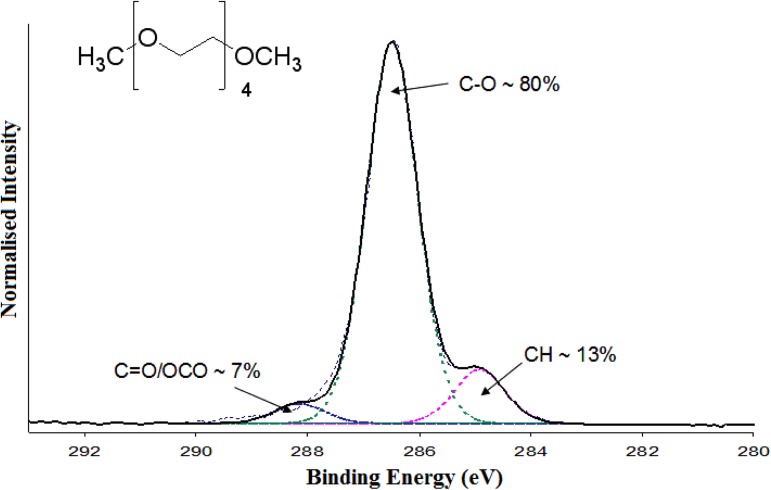
Table TABLE II. shows the results from analysis of the XPS high-resolution C1s spectra from each of the samples. The uncoated PDMS showed a broad peak between 284.6 eV and 285.0 eV which represents the carbon from PDMS in either a silicon (284.6 eV) or hydrocarbon bond (285.0 eV), which was to be expected from previous work.46 When plasma polymers were deposited onto the PDMS surface, there were significant changes in surface chemistry. Figure 1 shows a typical high-resolution C 1s spectrum of a ppTTg coating, from a double-coated PDMS sample, as an example of how peak fitting and designation was completed. The deposition of ppTTg onto PDMS introduced ether carbon groups (%C-O) on to the surface. However, the double ppTTg coating and positive control samples, where ppTTg was deposited on silicon wafer, both possessed approximately 80% ether carbon groups, whereas the single layer coating only possessed approximately 65%. Previous work has demonstrated higher ether carbon content (up to around ∼80%) is desired for a functional tetraglyme coating.44, 47, 48, 49
TABLE II.
Carbon environments calculated from peak fitting of XPS high resolution C 1s spectra for ppTTg coated on PDMS and silicon wafer substrates. All values represent the mean elemental composition percentage + SD, calculated from 6 replicates for each sample.
| Sample | % -C/H/Si (285.0 eV) | % -O (286.6 eV) | % =O (288.0 eV) |
|---|---|---|---|
| ppTTg coated Silicon Wafer | 11.8 ± 1.2 | 81.9 ± 1.5 | 6.3 ± 1.0 |
| Single ppTTg coated PDMS | 30.0 ± 2.2 | 65.1 ± 3.5 | 4.9 ± 1.9 |
| Double ppTTg coated PDMS | 13.6 ± 1.6 | 79.5 ± 2.5 | 6.9 ± 1.8 |
| Native PDMS | 100.0 ± 0 | 0 ± 0 | 0 ± 0 |
Figure 1.
XPS high resolution C 1s spectra of double ppTTg coated PDMS.
The presence of silicon on the surface was one of the major indicators of hydrophobic recovery of PDMS, and further understanding of this silicon content was essential for interpreting the results achieved. Hydrophobic recovery occurs in dry conditions. Thus, it is reasonable to assume that introducing the samples into the UHV environment of the XPS (typically in the range of 10−7–10−9 mbar) may elicit some level of PDMS migration to the surface. Analysis of regions around the silicon peaks on the XPS wide scan for all three ppTTg coated sample types showed constant background intensities around the peaks of interest.50 This suggested that the silicon content was restricted to the outermost layers of the surface, as substrate or contributions from within the film would result in increased background scattering in the data. This would indicate that siloxane molecules were migrating to the surface after plasma polymerization occurred, either in air before entrance into the XPS, or during exposure to the UHV vacuum.
To analyze this, measurements of the water contact angle on all samples were made before and after exposure to the UHV XPS environment. While there was no change detected on the coated silicon wafer samples, both of the PDMS substrates showed significant increases in contact angle after exposure to UHV.50 The difference between the contact angles suggested that the UHV was driving some migration of hydrophobic molecules from the PDMS bulk to the surface. Critically, prior to exposure to the UHV both the silicon wafer and double coated PDMS samples had the same contact angle, indicating that the coatings as viewed by contact angle measurement were the same on each substrate.
Stability of ppTTg coatings
The XPS chemical characterization results for samples aged in air are displayed in Table TABLE III.. They show that the control samples, both positive and negative, remain almost unchanged throughout the 100-day period. Previously published work has shown that plasma polymerized films can undergo chemical changes when exposed and aged in varying conditions.51, 52, 53 Plasma polymer films often contain trapped radicals which can react with oxygen that diffuses into the layer, and oxidation or hydrolysis can occur which creates irreversible changes in chemical properties.54 In the case of ppTTg films this change could lead to loss of ether carbon groups, and hence its low-fouling properties. Table TABLE III. shows that the ppTTg coated Si wafer samples do not significantly change in either elemental composition or ether carbon content over a 100-day period, showing that the coatings were stable over this time. This has been shown in a previous study and suggests that ppTTg coatings were relatively stable in air over such periods of time.44 Double ppTTg coated PDMS also remained largely unchanged in terms of both elemental composition and ether carbon content over the 100-day period. However, there were small increases in silicon content over the 100 day period (1.9% to 3.1%), which was not statistically significant (p = 0.14) but may suggest that small amounts of hydrophobic recovery was occurring, although some of this may be driven by the exposure to UHV during XPS analysis as noted earlier. The table also shows that single ppTTg coated PDMS changed significantly with the silicon signal nearly doubling (6% to 11.4%) and the carbon content decreasing over 8%, suggesting large changes in the surface, most probably due to increased hydrophobic recovery when aged in air. In addition, the ether carbon content was reduced by nearly 14%, again showing that large changes in surface composition were occurring over the 100-day period.
TABLE III.
XPS elemental composition of survey spectra and ether carbon content after various aging time points. All values are percentage values to two decimal points and represent the mean percentage calculated from 3 replicates for each sample.
| Sample | Day | %C | %O | %Si | O/C | % C-O |
|---|---|---|---|---|---|---|
| ppTTg coated Si wafer | 0 | 67.5 | 32.5 | 0 | 0.48 | 81.9 |
| 10 | 67.9 | 32.1 | 0 | 0.47 | 80.5 | |
| 100 | 68.7 | 31.3 | 0 | 0.45 | 79.8 | |
| Single ppTTg coated PDMS | 0 | 65.8 | 28.2 | 6.0 | 0.43 | 65.1 |
| 10 | 60.4 | 30.1 | 9.5 | 0.51 | 57.4 | |
| 100 | 57.7 | 30.8 | 11.4 | 0.58 | 51.7 | |
| Double ppTTg coated PDMS | 0 | 68.2 | 29.9 | 1.9 | 0.45 | 79.5 |
| 10 | 69.1 | 28.5 | 2.4 | 0.41 | 77.4 | |
| 100 | 66.2 | 30.7 | 3.1 | 0.46 | 77.2 | |
| Native PDMS | 0 | 45.5 | 26.6 | 27.9 | 0.58 | 0 |
| 10 | 46.3 | 26.3 | 27.4 | 0.57 | 0 | |
| 100 | 47.9 | 27.0 | 25.1 | 0.56 | 0 |
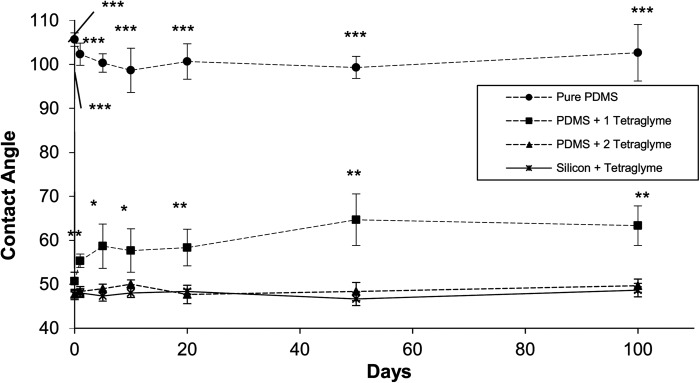
Water contact angles were also used to study the ppTTg film stability. As shown in Figure 2, the ppTTg coatings displayed a relatively low water contact angle (∼47°) compared to PDMS (∼110°), meaning that any small amounts of PDMS on the surface would significantly increase the contact angle. This technique has been used in other surface modification of PDMS studies to test stability.28, 55, 56 Positive and negative control samples were produced as well as the single and double coated PDMS samples. All the samples were produced and then left to “age” at room temperature in air for up to 100 days, while changes in hydrophobicity were monitored using contact angle measurements at regular intervals.
Figure 2.
Sessile water contact angles measured on uncoated PDMS, single and double ppTTg coated PDMS and ppTTg coated Si wafers. All values represent the mean optical density measured at 405 nm + SD, calculated from 3 replicates for each sample. All samples have been tested against the relevant positive control (ppTTg coated Si wafers) result by student's t test where *p<0.05, **p<0.01 and ***p<0.001.
The results clearly complement the conclusions from the XPS data, showing ppTTg coated on silicon wafer maintained a stable contact angle throughout the 100-day period. Figure 2 also displays that PDMS samples that were prepared with a double ppTTg coating showed almost no change in contact angle over time. Within the accuracies of the sessile water contact angle measurements, the data from the double coated PDMS surfaces matche with the positive control at each time point. The results suggest that the level of silicon migration, if not induced by the exposure to UHV, is insufficient to disturb the contact angle measurements. By comparison, data collected from the single layer ppTTg coatings illustrated rapid changes in contact angle from 49° to 60° over the first few days of storage, with the contact angle increasing gradually and stabilizing at 64°.
Functionality of ppTTg coatings
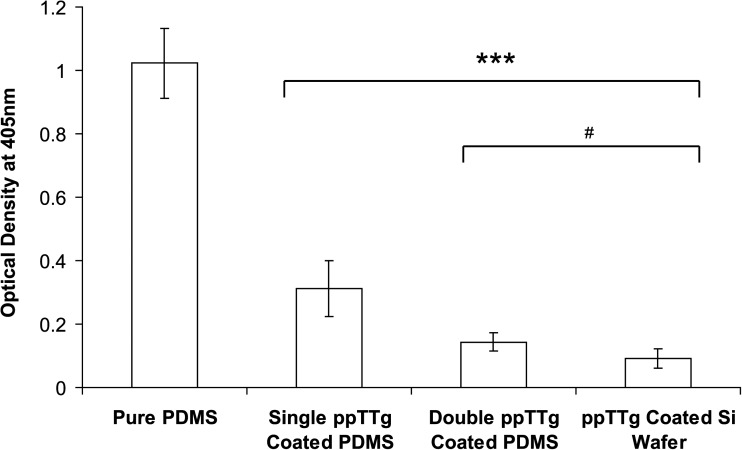
As ppTTg coatings aim to reduce protein adsorption, it was critical to test the coating functionality, as well as its functional stability. Tetraglyme samples were prepared using the enhanced curing and multi-layer plasma polymerization conditions before being analyzed using an adaptation of an ELISA for fibrinogen to enable detection of adsorbed protein directly from the surface. Figure 3 shows that uncoated PDMS avidly adsorbed fibrinogen, a material property characterized in previous studies.22, 42, 44, 57 The ppTTg coated silicon wafer again showed significant reductions in protein adsorption compared to uncoated PDMS, although not complete protein resistance. Double ppTTg clearly demonstrated very similar properties to those achieved on the positive control samples. Finally, single ppTTg coated PDMS significantly reduced protein adsorption when compared to the uncoated negative control samples but did not reduce adsorption to the levels of either double coated PDMS or the positive control samples. All ppTTg coated sample results were tested against the uncoated PDMS results using a student's t test which indicated that the results were highly significant (p < 0.001). Comparing the double ppTTg coated PDMS and ppTTg coated Si wafers with the single coated ppTTg samples also indicated that there was a significant difference between these data sets (p < 0.05). Overall, the ELISA results suggest that the double-coated PDMS samples resisted protein adsorption to an equivalent level as coated silicon wafers samples.
Figure 3.
A bar chart showing optical density measurements at 405 nm for fibrinogen ELISA experiments for uncoated PDMS, single and double ppTTg coated PDMS and ppTTg coated Si wafers. Values shown are background corrected using the values obtained from negative control ELISA experiments (i.e., ELISA experiments completed without protein). All values represent the mean optical density measured at 405 nm ± SD, calculated from 4 replicates for each sample. Student's t test ***p < 0.001 and #p < 0.05.
To explore the effect of sample ageing on protein adsorption, a separate set of samples were aged and the surfaces incubated with protein at day 0, day 10, and day 100. Table TABLE IV. shows the XPS surface analysis results for each surface after protein incubation. Unsurprisingly, the uncoated PDMS adsorbs significant and constant amounts of protein over the course of the 100-day period, with little change evident in the nitrogen signal. After 10 days, both the ppTTg on silicon wafer and double ppTTg coated PDMS remain relatively protein resistant, with only very low levels of nitrogen detected on the surface. However, after 100 days there was a small increase to values of approximately 0.5% for both sample types. From the literature, we can estimate that this level of nitrogen on the surface corresponds to <10 ng/cm2 of protein.47 These values are commensurate to results from a number of previous studies using ppTTg coatings44, 47, 49 and match with the ELISA results discussed previously. Together this data suggest that coatings are able to retain significant functionality on PDMS even after extended periods of dry shelf storage. It should also be noted that there were no significant differences between the nitrogen content in the double ppTTg coated and positive control samples at any time point (p > 0.05 for each time point).
TABLE IV.
XPS elemental composition of the coated and uncoated surfaces at various aging time points, after exposure to fibrinogen solutions. n = 3. The nitrogen content for both ppTTg coated PDMS sample results were tested against the ppTTg coated Si wafer results by student's t test, where *p < 0.05; **p < 0.01; and ***p < 0.001.
| Sample | Day | %C | %O | %Si | %N |
|---|---|---|---|---|---|
| ppTTg coated Si wafer | 0 | 68.5 | 31.7 | 0 | 0 |
| 10 | 67.8 | 32.1 | 0 | 0.1 | |
| 100 | 67.9 | 31.6 | 0 | 0.5 | |
| Single ppTTg coated PDMS | 0 | 63.7 | 28.4 | 5.7 | 2.2*** |
| 10 | 58.3 | 29.9 | 6.9 | 4.9*** | |
| 100 | 58.8 | 24.5 | 10.9 | 5.8*** | |
| Double ppTTg coated PDMS | 0 | 67.9 | 30.0 | 2.0 | 0.1 |
| 10 | 65.7 | 31.6 | 2.5 | 0.2 | |
| 100 | 66.8 | 29.8 | 2.8 | 0.6 | |
| Native PDMS | 0 | 52.4 | 23.3 | 17.8 | 6.5 |
| 10 | 54.4 | 23.2 | 15.7 | 6.7 | |
| 100 | 53.8 | 23.2 | 16.8 | 6.2 |
Single ppTTg coated PDMS samples reduce protein adsorption compared to uncoated PDMS at day 0. However, the single coated PDMS samples contained significantly more nitrogen for every time point when compared to the positive control samples (p < 0.001). After 10 days, the surface had nitrogen levels almost as high as the negative control samples, suggesting only negligible reductions in protein adsorption. This suggested that these coatings have lost almost all of their protein resistance within the first 10 days of storage. It is critical to note that although the protein adsorption behavior as measured by the at. % N are virtually identical on the wafer and double coated PDMS surfaces, there is some silicon detected on at the surface of all of the PDMS samples. The protein adsorption rose with increasing levels of silicon detected on the PDMS with the single coating but little variation in at. % Si or N were detected on the doubled coated PDMS as the samples were aged in air. Coupled with the contact angle data discussed previously, this suggests that the low levels of Si detected on the double coated samples may be due to the UHV migration, whereas the higher levels of Si coupled with increases in protein adsorption detected on the single coated samples suggest migration due to storage in air.
DISCUSSION
The principal objective of this study was to explore methods for producing stable, functional plasma polymer films on PDMS. The challenge with coating PDMS is to restrict the ability of the hydrophobic polymer to migrate and/or reptate over time, a process that can render any coating ineffectual. Results from this study clearly demonstrated that combining PDMS heat curing with a double layer plasma polymer deposition, it is possible to produce a plasma polymer film capable of significantly reducing protein adsorption. Plasma polymerization gives us the opportunity to tailor surface chemistry by selecting the appropriate monomer. With all plasma polymer coatings our first aim is to incorporate as much of the precursor monomer chemistry on the surface as possible. This similarity between the precursor and deposited film composition is particularly important for the ethylene oxide-based protein resistant films used here, as the non-fouling properties have been directly linked to the ether carbon content of the film. Shen et al.47, 49 and Johnston et al.58 have shown that the higher the content of ether carbon, the more functional the coatings become, and the less protein they adsorb.
It is clear from the results of this study that while the single ppTTg coatings on PDMS were able to reduce protein adsorption initially, over time the surface chemistry changed and protein resistance diminished. These results were due to hydrophobic recovery of the PDMS that can be explained via LMW hydrophobic recovery migration.26, 27, 28, 29, 30 Increasing the curing time of the PDMS following the work of Eddington et al.,26 aimed to reduce the level of pre-polymer present in the sample, but once the first plasma polymer layer was deposited, residual LMW siloxane molecules in the substrate were still able to migrate to the surface due to their motility and hydrophobic nature. This migration partially engulfed the plasma polymer layer, creating a patchy or incomplete ppTTg surface. This process also occurred during the preparation of double-coated PDMS samples. The intermediate high temperature post deposition dry bake step aimed to encourage this process. This high temperature and dry environment were designed to increase the rate of any LMW siloxane migration to the surface. Then when the samples were placed in to the vacuum environment for their second coating it ensured that as much of the LMW molecules were present on the surface, again further driven by the vacuum environment. Finally, when the high power initial plasma deposition occurred, it is postulated that the high-energy plasma molecules hit the surface with higher energy, integrating these molecules into the surface where much of the motile siloxane was present. It is well known in plasma processes that bonds on the substrate surface are continually broken and re-formed throughout the deposition process, so integration of these LMW molecules in to the subsequent film is highly likely. After this initial high power deposition period the motile PDMS molecules which were present on the surface were then cross-linked and integrated into the plasma polymer layer, stabilizing the system and creating a barrier for further migration. The subsequent lower power (10 W) deposition period allowed a functional and optimal ppTTg plasma film to be formed. After this deposition had been completed, the samples were not as prone to hydrophobic recovery as the majority of the motile, hydrophobic monomers from the PDMS bulk were now integrated in the primary plasma polymer layer, and no longer motile enough to migrate onto the new surface and engulf the secondary ppTTg layer. In effect, there is a 4-step process to producing a stable and chemically functional film:
-
(a)
Baking of the initial PDMS substrate to optimize the cross linking and reduce the initial LMW polymer;
-
(b)
The primary plasma polymer layer was deposited onto the surface, LMW molecules on the surface became cross-linked within this initial layer;
-
(c)
The samples were placed in a dry oven to encourage more of the residual LMW polymer in the bulk to be drawn out/encouraged to migrate to the surface, partially engulfing the plasma polymer layer;
-
(d)
A second plasma polymer layer is deposited and the LMW molecules on the surface became cross-linked within this layer. This creates a barrier to further migration and enables a functional film to be deposited.
We have also recently utilized this approach to coat other plasma polymers (acrylic acid) into open PDMS microfluidic devices for protein digestion.59 While the requirement for the devices to be open for the coating process to occur may appear to be a limitation, all devices tested were sealed using a clamp system and operational pressures readily obtained without leakage. This work, alongside a number of other application-based studies in our laboratory, clearly demonstrated the applicability of this approach.
CONCLUSIONS
By combining a modification of the PDMS curing regime with a sequence of plasma polymerization steps, this study has clearly demonstrated that it is possible to produce shelf stable protein resistant coatings on PDMS. This approach appears to both reduce the amount of mobile siloxane in the system and trap what is present within the plasma polymer layers reducing its ability to migrate to the interface. Using this method, tetraglyme coatings were deposited on PDMS and were demonstrated to be able to resist protein adsorption while being stable to shelf storages for periods up to 100 days. This methodology can be readily translated to any plasma polymer system, enabling a wide range of surface functionalities to be introduced to PDMS surfaces and devices.
References
- van der Woerd M., Ferree D., and Pusey M., J. Struct. Biol. 142, 180–187 (2003). 10.1016/S1047-8477(03)00049-2 [DOI] [PubMed] [Google Scholar]
- Li L., Mustafi D., Fu Q., Tereshko V., Chen D. L. L., Tice J. D., and Ismagilov R. F., Proc. Natl. Acad. Sci. U. S. A. 103, 19243–19248 (2006). 10.1073/pnas.0607502103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia S. K. and Whitesides G. M., Electrophoresis 24, 3563–3576 (2003). 10.1002/elps.200305584 [DOI] [PubMed] [Google Scholar]
- Vilkner T., Janasek D., and Manz A., Anal. Chem. 76, 3373–3385 (2004). 10.1021/ac040063q [DOI] [PubMed] [Google Scholar]
- Dittrich P. S. and Manz A., Nat. Rev. Drug Discovery 5, 210–218 (2006). 10.1038/nrd1985 [DOI] [PubMed] [Google Scholar]
- Hong J., BioChip J. 2, 12–26 (2008). [Google Scholar]
- Figeys D. and Pinto D., Electrophoresis 22, 208–216 (2001). [DOI] [PubMed] [Google Scholar]
- Liu Y., Liu B. H., Yang P. Y., and Girault H. H., Anal. Bioanal. Chem. 390, 227–229 (2008). 10.1007/s00216-007-1664-6 [DOI] [PubMed] [Google Scholar]
- McDonald J. C. and Whitesides G. M., Acc. Chem. Res. 35, 491–499 (2002). 10.1021/ar010110q [DOI] [PubMed] [Google Scholar]
- Johnson T. J., Ross D., and Locascio L. E., Anal. Chem. 74, 45–51 (2002). 10.1021/ac010895d [DOI] [PubMed] [Google Scholar]
- Chen Y. H. and Chen S. H., Electrophoresis 21, 165–170 (2000). [DOI] [PubMed] [Google Scholar]
- Rhee S. W., Taylor A. M., Tu C. H., Cribbs D. H., Cotman C. W., and Jeon N. L., Lab Chip 5, 102–107 (2005). 10.1039/b403091e [DOI] [PubMed] [Google Scholar]
- Anderson J. R., Chiu D. T., Jackman R. J., Cherniavskaya O., McDonald J. C., Wu H. K., Whitesides S. H., and Whitesides G. M., Anal. Chem. 72, 3158–3164 (2000). 10.1021/ac9912294 [DOI] [PubMed] [Google Scholar]
- Duffy D. C., McDonald J. C., Schueller O. J. A., and Whitesides G. M., Anal. Chem. 70, 4974–4984 (1998). 10.1021/ac980656z [DOI] [PubMed] [Google Scholar]
- McDonald J. C., Duffy D. C., Anderson J. R., Chiu D. T., Wu H. K., Schueller O. J. A., and Whitesides G. M., Electrophoresis 21, 27–40 (2000). [DOI] [PubMed] [Google Scholar]
- Ng J. M. K., Gitlin I., Stroock A. D., and Whitesides G. M., Electrophoresis 23, 3461–3473 (2002). [DOI] [PubMed] [Google Scholar]
- Gates B. D. and Whitesides G. M., J. Am. Chem. Soc. 125, 14986–14987 (2003). 10.1021/ja0367647 [DOI] [PubMed] [Google Scholar]
- Jo B. H., Van Lerberghe L. M., Motsegood K. M., and Beebe D. J., J. Microelectromech. Syst. 9, 76–81 (2000). 10.1109/84.825780 [DOI] [Google Scholar]
- Butler J. E., Lu E. P., Navarro P., and Christiansen B., J. Mol. Recognit. 10, 36–51 (1997). [DOI] [PubMed] [Google Scholar]
- Eteshola E. and Leckband D., Sens. Actuator B 72, 129–133 (2001). 10.1016/S0925-4005(00)00640-7 [DOI] [Google Scholar]
- Huang B., Wu H. K., Kim S., and Zare R. N., Lab Chip 5, 1005–1007 (2005). 10.1039/b509251e [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay R., Anal. Chem. 77(21), 429A–432A (2005). 10.1021/ac053496h [DOI] [PubMed] [Google Scholar]
- Kenis P. J. A. and Stroock A. D., MRS Bull. 31, 87–94 (2006). 10.1557/mrs2006.21 [DOI] [Google Scholar]
- Owen M. J. and Smith P. J., J. Adhes. Sci. Technol. 8, 1063–1075 (1994). 10.1163/156856194X00942 [DOI] [Google Scholar]
- Morra M., Occhiello E., Marola R., Garbassi F., Humphrey P., and Johnson D., J. Colloid Interface Sci. 137, 11–24 (1990). 10.1016/0021-9797(90)90038-P [DOI] [Google Scholar]
- Eddington D. T., Puccinelli J. P., and Beebe D. J., Sens. Actuator B 114, 170–172 (2006). 10.1016/j.snb.2005.04.037 [DOI] [Google Scholar]
- Hillborg H. and Gedde U. W., Polymer 39, 1991–1998 (1998). 10.1016/S0032-3861(97)00484-9 [DOI] [Google Scholar]
- Kim J., Chaudhury M. K., and Owen M. J., J. Colloid Interface Sci. 226, 231–236 (2000). 10.1006/jcis.2000.6817 [DOI] [Google Scholar]
- Kim J., Chaudhury M. K., Owen M. J., and Orbeck T., J. Colloid Interface Sci. 244, 200–207 (2001). 10.1006/jcis.2001.7909 [DOI] [Google Scholar]
- Toth A., Bertoti I., Blazso M., Banhegyi G., Bognar A., and Szaplonczay P., J. Appl. Polym. Sci. 52, 1293–1307 (1994). 10.1002/app.1994.070520914 [DOI] [Google Scholar]
- Delamarche E., Bernard A., Schmid H., Bietsch A., Michel B., and Biebuyck H., J. Am. Chem. Soc. 120, 500–508 (1998). 10.1021/ja973071f [DOI] [Google Scholar]
- Xiao D. Q., Zhang H., and Wirth M., Langmuir 18, 9971–9976 (2002). 10.1021/la0205553 [DOI] [Google Scholar]
- Hu S. W., Ren X. Q., Bachman M., Sims C. E., Li G. P., and Allbritton N. L., Langmuir 20, 5569–5574 (2004). 10.1021/la049974l [DOI] [PubMed] [Google Scholar]
- Lahann J., Balcells M., Lu H., Rodon T., Jensen K. F., and Langer R., Anal. Chem. 75, 2117–2122 (2003). 10.1021/ac020557s [DOI] [PubMed] [Google Scholar]
- Hu S. W., Ren X. Q., Bachman M., Sims C. E., Li G. P., and Allbritton N. L., Anal. Chem. 76, 1865–1870 (2004). 10.1021/ac049937z [DOI] [PubMed] [Google Scholar]
- Wu Z. G. and Hjort K., Lab Chip 9, 1500–1503 (2009). 10.1039/b901651a [DOI] [PubMed] [Google Scholar]
- Barbier V., Tatoulian M., Li H., Arefi-Khonsari F., Ajdari A., and Tabeling P., Langmuir 22, 5230–5232 (2006). 10.1021/la053289c [DOI] [PubMed] [Google Scholar]
- Wu D. P., Zhao B. X., Dai Z. P., Qin J. H., and Lin B. C., Lab Chip 6, 942–947 (2006). 10.1039/b600765a [DOI] [PubMed] [Google Scholar]
- Lee J. N., Park C., and Whitesides G. M., Anal. Chem. 75, 6544–6554 (2003). 10.1021/ac0346712 [DOI] [PubMed] [Google Scholar]
- Siow K. S., Britcher L., Kumar S., and Griesser H. J., Plasma Processes Polym. 3, 392–418 (2006). 10.1002/ppap.200600021 [DOI] [Google Scholar]
- Lopez G. P., Ratner B. D., Tidwell C. D., Haycox C. L., Rapoza R. J., and Horbett T. A., J. Biomed. Mater. Res. 26, 415–439 (1992). 10.1002/jbm.820260402 [DOI] [PubMed] [Google Scholar]
- Salim M., Wright P. C., and McArthur S. L., Electrophoresis 30, 1877–1887 (2009). 10.1002/elps.200800619 [DOI] [PubMed] [Google Scholar]
- Mishra G. and McArthur S. L., Langmuir 26, 9645–9658 (2010). 10.1021/la100236c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim M., Mishra G., Fowler G. J. S., O'Sullivan B., Wright P. C., and McArthur S. L., Lab Chip 7, 523–525 (2007). 10.1039/b615328c [DOI] [PubMed] [Google Scholar]
- Seah M. P. and Dench W. A., Surf. Interface Anal. 1, 2–11 (1979). 10.1002/sia.740010103 [DOI] [Google Scholar]
- Chen H., Zhang Z., Chen Y., Brook M. A., and Sheardown H., Biomaterials 26, 2391–2399 (2005). 10.1016/j.biomaterials.2004.07.068 [DOI] [PubMed] [Google Scholar]
- Wagner M. S., McArthur S. L., Shen M. C., Horbett T. A., and Castner D. G., J. Biomater. Sci., Polym. Ed. 13, 407–428 (2002). 10.1163/156856202320253938 [DOI] [PubMed] [Google Scholar]
- Shen M. C., Pan Y. V., Wagner M. S., Hauch K. D., Castner D. G., Ratner B. D., and Horbett T. A., J. Biomater. Sci., Polym. Ed. 12, 961–978 (2001). 10.1163/156856201753252507 [DOI] [PubMed] [Google Scholar]
- Shen M. C., Wagner M. S., Castner D. G., Ratner B. D., and Horbett T. A., Langmuir 19, 1692–1699 (2003). 10.1021/la0259297 [DOI] [Google Scholar]
- See supplementary material at http://dx.doi.org/10.1063/1.4754600 for the XPS survey scans and contact angle data before and after exposure to vacuum.
- Gengenbach T. R. and Griesser H. J., J. Polym. Sci. Pol. Chem. 36, 985–1000 (1998). [DOI] [Google Scholar]
- Selli E., Mazzone G., Oliva C., Martini F., Riccardi C., Barni R., Marcandalli B., and Massafra M. R., J. Mater. Chem. 11, 1985–1991 (2001). 10.1039/b101360m [DOI] [Google Scholar]
- Unger W. E. S., Swaraj S., Oran U., and Lippitz A., Surf. Interface Anal. 38, 522–525 (2006). 10.1002/sia.2158 [DOI] [Google Scholar]
- Mishra G., Ph.D. dissertation, University of Sheffield, 2008. [Google Scholar]
- Fritz J. L. and Owen M. J., J. Adhes. 54, 33–45 (1995). 10.1080/00218469508014379 [DOI] [Google Scholar]
- Vickers J. A., Caulum M. M., and Henry C. S., Anal. Chem. 78, 7446–7452 (2006). 10.1021/ac0609632 [DOI] [PubMed] [Google Scholar]
- Toepke M. W. and Beebe D. J., Lab Chip 6, 1484–1486 (2006). 10.1039/b612140c [DOI] [PubMed] [Google Scholar]
- Johnston E. E., Bryers J. D., and Ratner B. D., Langmuir 21, 870–881 (2005). 10.1021/la036274s [DOI] [PubMed] [Google Scholar]
- Pereira-Medrano A. G., Forster S., Fowler G. J. S., McArthur S. L., and Wright P. C., Lab Chip 10, 3397–3406 (2010). 10.1039/c0lc00147c [DOI] [PubMed] [Google Scholar]