Case 1
A nine-month-old female infant presented with a two-day history of vomiting, diarrhea and decreased urine output, along with a three-month history of lethargy and reduced tone. Her early development had been normal, but regression of skills had begun three months before presentation, with a loss of gross motor skills progressing to a loss of head control. The child had been exclusively breastfed until solids were slowly introduced over the last month. Her family was of South-East Asian ethnic origin, and her mother was a strict lifelong vegan who took prenatal vitamins during pregnancy.
On examination, the baby was sleepy and pale. Her weight was 6.65 kg (< 3rd percentile), height was 69 cm (25th percentile) and head circumference was 41 cm (< 3rd percentile). The liver edge was 3 cm below the costal margin. The splenic tip was palpable. Her axial and peripheral muscle tone was decreased, with frog-like posture of both legs. No antigravity power was exhibited. Reflexes were 3+ in her lower extremities and 2+ in her upper extremities. She was able to fix visually but did not follow.
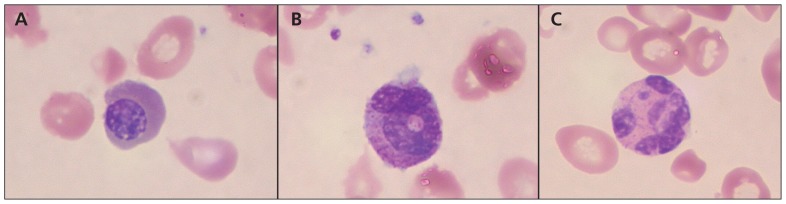
Laboratory investigations showed a hemoglobin level of 35 (range 103–135) g/L, leukocyte count of 3.4 (range 5–16) × 109/L and platelet count of 149 (range 200–550) × 109/L. The mean corpuscular volume was 95.3 (range 70–86) fL and reticulocyte count was 35 (range 20–80) × 109/L. The blood smear showed pancytopenia with severe leukoerythroblastic change, dysplastic red blood cells and rare hypersegmented neutrophils; it appeared severely megaloblastic overall (Figure 1). The albumin level was 18 (range 34–42) g/L. Vitamin B12 level was less than 37 (range 133–695) pmol/L (lower reporting limit), and the folate level was 14 (range 7–36) nmol/L. A bone marrow biopsy showed morphological changes consistent with megaloblastic anemia. Magnetic resonance imaging (MRI) of the patient’s brain showed generalized atrophy. Metabolic and biochemical investigations, including acylcarnitine profile, plasma amino acid and urine organic acids, showed abnormalities consistent with dietary protein deficiency.
Figure 1:
Blood smear from the patient described in Case 1, showing characteristics of megaloblastic anemia. (A) Megaloblastic erythroblast, (B) giant metamyelocyte and (C) hypersegmented neutrophil.
Because the infant had been mostly breastfed with limited solid intake, we examined the mother. Her complete blood count was normal, but her vitamin B12 level was low at 63 (adult reference range 133–695, deficient < 107) pmol/L. Review of the mother’s prenatal blood work indicated a normal hemoglobin level, with a normal mean corpuscular volume.
The infant’s anemia was managed initially with a slow transfusion of packed red blood cells. Intramuscular injections of vitamin B12 (1000 μg) were given daily for seven days, then weekly for the next month, along with oral iron supplementation. Nasogastric feeding with formula was initiated because of poor suck, and breastfeeding was maintained for comfort. The mother was started on oral B12 supplementation.
Five months later, the infant was consuming solid baby food and infant formula, and her growth parameters had improved. Her muscle tone and neurologic status had also improved. The complete blood count and B12 levels were normal.
Case 2
A seven-month-old male infant presented with a two-week history of lethargy and a loss of previously acquired milestones, as well as a two-month history of diarrhea and being generally unwell. He had been seen by his family doctor on multiple occasions and treated with antibiotics with no identified source of infection. He was exclusively breastfed. The family was white, and the mother had no dietary restrictions.
On examination, the child was lethargic. His weight was 7.5 kg (10th–25th percentile), and his head circumference was 43.75 cm (25th–50th percentile). His length was within the normal limits. On neurologic examination, he had generalized decreased tone, with brisk reflexes in the extremities. The rest of the examination was unremarkable.
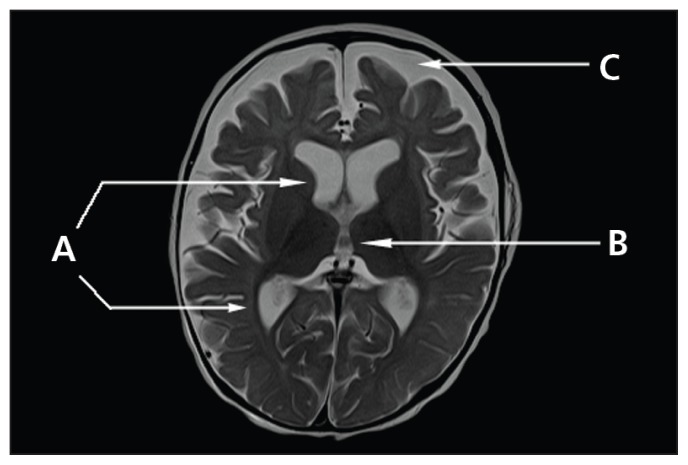
Laboratory investigation showed a hemoglobin level of 46 (range 103–135) g/L, leukocyte count of 4.4 (range 5–16) × 109/L, platelet count of 88 (range 200–550) × 109/L and a neutrophil count of 0.37 (range 1.0–8.5) × 109/L. The mean corpuscular volume was 91.3 (range 70–86) fL, and the reticulocyte count was 15 (range 20–80) × 109/L. The blood smear showed pancytopenia with evidence of red blood cell macrocytosis. No blast cells were seen. A bone marrow biopsy showed megaloblastic anemia in keeping with vitamin B12 deficiency. The vitamin B12 level was less than 37 (133–695) pmol/L, and the folate level was 30 (range 7–36) nmol/L. An MRI of the patient’s brain showed generalized symmetric cortical atrophy and incomplete subcortical myelination (Figure 2). The metabolic investigations did not show evidence of an inborn error of metabolism.
Figure 2:
Axial t2-weighted magnetic resonance scan of the patient’s brain (Case 2) showing enlarged (A) lateral and (B) third ventricles and (C) cortical subarachnoid spaces consistent with atrophy, with microcephaly. On T2, myelination was within normal limits, but on T1 (not shown) there was incomplete subcortical myelination.
Because the patient was exclusively breastfed, the mother was examined and found to have antibodies to intrinsic factor and gastric parietal cells, and undetectable vitamin B12 levels. A diagnosis of maternal pernicious anemia was made.
Intramuscular injections of vitamin B12 (1000 μg) were administered daily to the child for 10 days, followed by monthly injections, along with iron supplementation. His muscle tone slowly improved. After one week of treatment, the infant was consuming solid food, and breastfeeding was decreased. The infant’s complete blood count and vitamin B12 levels normalized. The mother was referred to a hematologist and began injections of vitamin B12 subcutaneously for four weeks, followed by monthly injections indefinitely.
Worldwide, vitamin B12 deficiency is an important cause of infant morbidity, especially in the Indian subcontinent, Mexico, Central America and certain regions of Africa.1 Although relatively rare in the developed world, vitamin B12 deficiency in infants is important to recognize because early treatment can prevent potentially devastating neurologic and developmental sequelae, such as hypotonia and developmental regression.2
Infant vitamin B12 deficiency, in most cases, results from a maternal deficiency. Maternal causes of infant deficiency can be broadly divided into deficient maternal diet or maternal pernicious anemia in a breastfed infant (Table 1), because infant formulas are supplemented with B12. Other more rare causes in children include surgical removal of the stomach and/or distal ileum, autoimmune gastritis, bacterial overgrowth, ileal disease (Crohn disease), exocrine pancreatic insufficiency and Whipple disease.
Table 1:
Characteristics of B12 deficiency in infants
| Causes | Infants whose mothers’ diets are deficient in B12* | Infants whose mothers have pernicious anemia |
|---|---|---|
| Presentation | ||
| Diagnosis |
|
|
| Treatment |
|
|
| Prevention |
|
|
For example, vegan or vegetarian diets.
We are unaware of recommendations on oral B12 supplementation for prevention in infants whose mothers have pernicious anemia.
Maternal dietary deficiency is usually due to vegetarian diet (which excludes meat, fish and fowl) or vegan diet (excluding also all dairy and eggs), dietary practices that are becoming increasingly popular for religious, humanitarian and health reasons in the developed world.3 Maternal pernicious anemia is less common as a cause. In both situations, if the mother has vitamin B12 deficiency during pregnancy, the newborn will have low B12 stores, and depletion will occur rapidly without supplementation. If maternal breast milk is deficient in B12, the infant will have inadequate daily intake to maintain B12 stores.
A recent study of the prevalence of B12 deficiency in an unselected group of pregnant women in Canada found that about 1 in 20 women were biochemically deficient for vitamin B12 in early pregnancy.4 Similarly, results of the Canadian Health Measures Survey revealed that about 5% of women aged 20–45 years were deficient in vitamin B12, and 20% had marginal stores.5 Another study involving pregnant adolescents found that about half had suboptimal vitamin B12 levels.6 The cause of deficiency in these women, and the sequelae in infants, was not reported in these studies. There is no literature on the prevalence of vitamin B12 deficiency in infants.
In a 2001 survey by the National Institute on Nutrition, 4% of Canadians identified themselves as vegetarians; there are little data, however, on the rate of vegan diets. The prevalence of vegetarian or vegan diets in pregnant and lactating mothers in Canada is unknown.7 In an Ontario study involving predominantly lacto–ovo vegetarian East Indian women (mean age 33 ± 7 yr), 7.8% were found to be deficient in vitamin B12.8 Vegetarian mothers have been shown to have evidence of vitamin B12 deficiency, regardless of whether or not they were lactating.9
There is a positive relation between maternal and breastfed infant vitamin B12 deficiency linked to low B12 levels in breast milk. According to a study by Specker and colleagues, vitamin B12 milk concentrations less than 360 pmol/L, approximately corresponding to a maternal serum B12 of less than 300 pmol/L, could result in an infant who is biochemically deficient in vitamin B12.10 It remains to be determined what maternal serum vitamin B12 level is sufficient for exclusive breastfeeding.
The prevalence of pernicious anemia in Canada is not reported in the literature. In the United States, it has been estimated to affect 150 per 100 000 adults, with peaks between 50 and 60 years of age.11 These numbers do not reflect the prevalence of pernicious anemia in women of child-bearing age.
Mechanism of action
Vitamin B12 (cobalamin) is essential for folate metabolism and DNA synthesis, acting as a cofactor for two key enzymatic reactions. Deoxyadenosylcobalamin, one of the coenzyme forms of cobalamin, is a cofactor for methionine synthase, the enzyme which converts homocysteine to methionine. If this coenzyme is lacking, homocysteine accumulates, leading to megaloblastic anemia and neurologic sequelae. Cobalamin is also a cofactor for methylmalonyl CoA mutase, the enzyme that converts methylmalonyl CoA to succinyl CoA. The latter is needed for the metabolism of odd-chain fatty acids and purine and pyrimidine synthesis. Methylmalonic aciduria and defective amino acid synthesis results from the lack of the cobalamin cofactor, clinically leading to pancytopenia, metabolic acidosis and hypotonia.12
Vitamin B12 is not synthesized by the body and must therefore be ingested through diet. In the stomach, it binds to intrinsic factor, a protein synthesized by parietal cells, which is lacking in pernicious anemia because of autoimmune injury. The B12–intrinsic factor complex is then absorbed into the circulation in the distal ileum.
Clinical presentation
The clinical features of infant vitamin B12 deficiency from either maternal dietary deficiency or maternal pernicious anemia do not appear to depend on cause, but rather on the severity of B12 deficiency, with many children being asymptomatic and presenting only with megaloblastic anemia found on blood work.13 When infants show clinical effects, the effects usually present between 2 and 12 months of age, when neonatal stores have been depleted and dietary vitamin B12 is inadequate. Typically, symptoms of vitamin B12 deficiency include poor feeding, weight loss and irritability; glossitis and infections have also been reported.14,15
It is the neurologic manifestations, however, that are the most concerning. As illustrated by the two infants we described, central nervous system symptoms can be severe, beginning with irritability, apathy, lethargy and gross motor developmental regression. Head circumference measurements fall off the growth curve, indicating slowing of brain growth. Patients progress to hypotonia with hyperreflexia, and exhibit choreoathetoid movements. The mechanisms of central nervous system involvement are not clearly understood. Demyelination, delayed myelination, impaired methylation and lactate accumulation in peripheral nerves, spinal cord and cerebrum have all been proposed.2,15
Diagnosis
The diagnosis of vitamin B12 deficiency in infants can be easily suspected when macrocytic anemia is seen on a complete blood count. This test is usually part of the initial battery in the workup of an ill child. The diagnosis is confirmed by determining serum vitamin B12 concentration. Treatment should not be delayed to wait for the vitamin B12 results, because the neurologic sequelae can be corrected. In our patients, bone marrow aspiration was also performed, which confirmed the diagnosis, but this would not be necessary for mildly affected patients.
There are case reports of infant vitamin B12 deficiency being diagnosed on newborn screening. In maternal vitamin B12 deficiency, the upstream products homocysteine and methylmalonic acid accumulate and can be transmitted to the fetus.16 Raised levels of these products have been detected on newborn screening of infants with low vitamin B12 stores, before the development of megaloblastic anemia in the infant.16 Both of our patients had normal newborn screens, however. Thus, screening for methylmalonic acid cannot be relied upon to detect infants with low vitamin B12 stores. Indeed, the role of neonatal screening for vitamin B12 deficiency remains unclear.16 A normal maternal blood count is not a reliable marker for deficiency in the infant.
If maternal vitamin B12 deficiency is suspected, a complete blood count and serum vitamin B12 assay in the mother are diagnostic. If the mother’s history does not suggest a dietary deficiency, a Schilling test should be completed for pernicious anemia.
Treatment
Diverse recommendations exist for initial and maintenance therapy of vitamin B12 deficiency in adults, and no clear guidelines are available for children. Studies involving adults have reported equivalent efficacy of oral and intramuscular replacement therapy.17 Oral replacement therapy, however, is likely to be less reliable in infants because they are more prone to regurgitation and vomiting.
In adults with severe B12 deficiency with neurologic manifestations, experts recommend 1000 μg cyanocobalamin given via intramuscular injection daily for a week, followed by weekly injections for a month, then every third month.18 The use of oral cyanocobalamin in patients with neurologic symptoms, adult or pediatric, has not been adequately assessed.17 Consensus guidelines for optimal replacement therapy in deficient pediatric patients are required. Replacement therapy at adult doses was administered by the intramuscular route in both of the infants described in this article, according to common practice for severe B12 deficiency with neurologic symptoms.
In patients with pernicious anemia, adult or pediatric, lifelong replacement with cyanocobalamin is required. Common practice for adults in North America and Europe is monthly 1000 μg cyanocobalamin via intramuscular injection, which was the treatment regimen for the mother in Case 2. Treatment regimens vary widely, however, because oral cyanocobalamin has also been shown to be efficacious for pernicious anemia.17 The British Columbia Guidelines and Protocols Advisory Committee recommends oral B12 replacement of 1000 μg daily.19 Considerations when initiating B12 therapy include providing adequate iron supplementation for the increased red blood cell production and also the potential for unmasking a concurrent folate deficiency.
Prevention
To prevent vitamin B12 deficiency, health care providers caring for pregnant or lactating mothers and their newborns should take a full dietary history and consider vitamin B12 status, particularly in vegetarian mothers and their infants.
Health Canada recommends a daily intake of 2.4 μg vitamin B12 for all women.20 Dietary guidelines in North America recommend a daily dietary vitamin B12 intake for pregnant and lactating women of 2.6 and 2.8 μg B12, respectively.20,21 A new food guide for vegetarians, suggested by Messina and colleagues,22 recommends that vegetarians consume at least three servings of vitamin B12-rich foods daily or supplement with 5–10 μg vitamin B12 daily. Recommendations for vegetarian pregnant and lactating women are less specific.22 The recommendations for vitamin B12 intake for women are summarized in Table 2.
Table 2:
Recommendations for B12 intake in women
| Group | No risk factors for anemia | With dietary risk factors* for anemia22 | With pernicious anemia17 |
|---|---|---|---|
| Nonpregnant women | 2.4 μg/d19,20 | 3 servings of B12-rich foods or 5–10 μg/d | Monthly 1000 μg via IM injection |
| Pregnant women | 2.6 μg/d19,20 | ~4 servings per day of B12-rich foods† | Monthly 1000 μg via IM injection |
| Lactating women | 2.8 μg/d20 | ~4 servings per day of B12-rich foods/d† | Monthly 1000 μg via IM injection |
Note: IM = intramuscular.
For example, vegan or vegetarian diets. in vegetarian or vegan pregnant
No specific recommendations are made with regards to the recommended daily intake of B12 and lactating women.
A recent position statement by the Canadian Paediatric Society suggests that all solely breastfed infants of vegan mothers should be given 5–10 μg vitamin B12 daily through oral vitamin supplements or at least three servings of food rich in vitamin B12 to prevent deficiency.23 Vitamin B12 is naturally found in animal products, especially meat, as well as in dairy products and eggs. Other sources include fortified foods, such as infant formulas, cereals and soy products (Table 3).24 There are no current recommendations for the supplementation of infants born to mothers with pernicious anemia.
Table 3:
Sources of dietary vitamin B12
| Food | Vitamin B12, μg |
|---|---|
| Fortified cereal, 1 oz | 0.6–6.0 |
| 1 large egg, 50 g | 0.5 |
| Cow’s milk, 1/2 cup | 0.4–0.5 |
| Fortified soy milk, 1/2 cup | 0.4–1.6 |
| Fortified nutritional yeast,*1 tbsp | 1.5 |
| Fortified meat analog | 0.5–1.2 |
Source: Package information and US Department of Agriculture’s Nutrient Database.24
Not all yeasts are fortified.
In the two patients described in this article, their mothers were following the current practice of exclusively breastfeeding for the first six months. The late introduction (after six months of age) of solid food rich in vitamin B12, such as fortified infant cereals and those mentioned above, may have contributed to the development of vitamin B12 deficiency in our patients. This may argue against the late introduction of solids (after six months of age) into infant diets.
Prognosis
Although vitamin B12 deficiency is relatively rare, it is important to recognize the disorder, given the potentially serious neurologic outcomes. As seen in our two patients and in multiple case reports, most clinical features of vitamin B12 deficiency resolve quickly with repletion of vitamin B12. The neurologic findings generally improve within weeks, and imaging (i.e., MRI of the brain) returns to normal within a few months. Long-term prognosis after B12 repletion and supplementation is less clear. Cognitive and developmental delays have been reported. In one case series of six infants with nutritional B12 deficiency, two had poor intellectual outcomes in terms of motor and language delay at four and five years.2,25 However, the developmental prognosis of these children months and years later is based only on a few case reports with an unclear duration of B12 supplementation; more follow-up research is needed to further clarify outcome.
Key points
One in twenty women of child-bearing age in Canada has inadequate levels of vitamin B12.
Infant B12 sufficiency is related to maternal levels via neonatal stores at birth and the amount in breast milk.
Vitamin B12 deficiency in infants, although rare, is important to recognize because treatment can prevent severe developmental delay and neurologic sequelae.
Prevention includes dietary supplementation for mothers and their breastfed infants who are at risk of vitamin B12 deficiency.
CMAJ remains committed to notifying readers in a timely way about advisories and warnings pertaining to serious adverse drug events. A collection of recent drug advisories from Health Canada and the US Food and Drug Administration is regularly updated at www.cmaj.ca/misc/advisories.xhtml.
Acknowledgements
The authors thank Dr. Louis Wadsworth, Dr. Jason Ford and Dr. Michael Sargent from BC Children’s Hospital for their help with this article.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: All of the authors contributed to the conception of this article. The manuscript was drafted by Nadia Roumeliotis and revised critically by Alisa Lipson and David Dix for clinical expertise and intellectual content. All of the authors approved the final version of the article submitted for publication.
References
- 1.Stabler SP, Allen RH. Vitamin B12 deficiency as a worldwide problem. Annu Rev Nutr 2004;24:299–326 [DOI] [PubMed] [Google Scholar]
- 2.Graham SM, Arvela OM, Wise GA. Long-term neurological consequences of nutritional Vitamin B12 deficiency in infants. J Pediatr 1992;121:710–4 [DOI] [PubMed] [Google Scholar]
- 3.Sabbaté J, Duk A, Lee CL. Publication trends of vegetarian nutrition articles in biomedical literature 1966–1995. Am J Clin Nutr 1999;70(3 suppl):601S–607S [DOI] [PubMed] [Google Scholar]
- 4.Ray JG, Goodman J, O’Mahoney PRA. High rate of maternal vitamin B12 deficiency nearly a decade after Canadian folic acid flour fortification. QJM 2008;101:475–7 [DOI] [PubMed] [Google Scholar]
- 5.MacFarlane AJ, Greene-Finestone LS, Shi Y. Vitamin B12 and homocysteine status in a folate-replete population: results from the Canadian Health Measures Survey. Am J Clin Nutr 2011;94: 1079–87 [DOI] [PubMed] [Google Scholar]
- 6.Gadowsky SL, Gale K, Wolfe SA. Biochemical folate, B12, and iron status of a group of pregnant adolescents accessed through the public health system in Southern Ontario. J Adolesc Health 1995;16:465–74 [DOI] [PubMed] [Google Scholar]
- 7.National Institute of Nutrition Tracking nutrition trends. RAPPORT 2002;17:1 Available: www.ccfn.ca/pdfs/rap-vol17-1.pdf (accessed 2011 Dec). [Google Scholar]
- 8.Bindra GS, Gibson RS, Berry M. Vitamin B12 and folate status of east Indian immigrants living in Canada. Nutr Res 1987;7: 365–74 [Google Scholar]
- 9.Specker BL. Nutritional concerns of lactating women consuming vegetarian diets. Am J Clin Nutr 1994;59(suppl):1182–6S [DOI] [PubMed] [Google Scholar]
- 10.Specker BL, Black A, Allen L. Vitamin B12: low milk concentrations are related to low serum concentrations in vegetarian women and methylmalonic aciduria in their infants. Am J Clin Nutr 1990;52:1073–6 [DOI] [PubMed] [Google Scholar]
- 11.Jacobson DL, Grange SJ, Rose NR, et al. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopath 1997. 84:223–43 [DOI] [PubMed] [Google Scholar]
- 12.Kapadia CR. Vitamin B12 in health and disease: part I — inherited disorders of function, absorption, and transport. Gastroenterologist. 1995;3:329–44 [PubMed] [Google Scholar]
- 13.Wong MP, Wadsworth L, Wu J, et al. Case 2: A pale infant — not a typical case of iron deficiency. Paediatr Child Health 2008; 13:507–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaikov Y, Wadsworth LD, Hall CA, et al. Transcobolamin II deficiency: case report and review of the literature. Eur J Pediatr 1991; 150:841–3 [DOI] [PubMed] [Google Scholar]
- 15.Dror DK, Allen LH. Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutr Rev 2008;66:250–5 [DOI] [PubMed] [Google Scholar]
- 16.Campbell CD, Ganesh J, Ficicioglu C. Two newborns with nutritional vitamin B12 deficiency: challenges in newborn screening for vitamin B12 deficiency. Haematologica 2005; 90:ECR45. [PubMed] [Google Scholar]
- 17.Lane LA, Rojas-Fernandez C. Treatment of vitamin b12-deficiency anemia: oral versus parenteral therapy. Ann Pharmacother 2002;36:1268–72 [DOI] [PubMed] [Google Scholar]
- 18.Hvas A-M, Nexo E. Diagnosis and treatment of vitamin b12 deficiency. An update. Haematologica 2006;91:1506–12 [PubMed] [Google Scholar]
- 19.British Columbia Medical Association B12 Deficiency — investigation and management of B12 and folate deficiency. Guidelines and protocols. Vancouver (BC): The Association; 2006 [Google Scholar]
- 20.Dietary reference intakes reference values for vitamins. Ottawa (ON): Health Canada; 2006. Available: www.hc-sc.gc.ca/fn-an/alt_formats/hpfb-dgpsa/pdf/nutrition/dri_tables-eng.pdf (accessed 2012 Mar. 12). [Google Scholar]
- 21.Institute of Medicine. Food and Nutrition Board Dietary reference intakes: thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): National Academy Press; 1998 [PubMed] [Google Scholar]
- 22.Messina V, Melina V, Mangels AR. A new food guide for North American vegetarians. Can J Diet Pract Res 2003;64:82–6 [DOI] [PubMed] [Google Scholar]
- 23.Amit M; Canadian Paediatric Society, Community Paediatrics Committee Vegetarian diets in children and adolescents. Paediatr Child Health 2010;15:303–14 [PMC free article] [PubMed] [Google Scholar]
- 24.US Department of Agriculture USDA Nutrient Database for Standard, Reference, Release 15. Washington (DC): Agricultural Research Service; 2002 [Google Scholar]
- 25.von Schenck U, Bender-Götze C, Koletzko B. Persistance of neurological damage induced by dietary vitamin B12 deficiency in infancy. Arch Dis Child 1997;77:137–9 [DOI] [PMC free article] [PubMed] [Google Scholar]