Abstract
Children with a history of a prolonged febrile seizure show signs of acute hippocampal injury on magnetic resonance imaging. In addition, animal studies have shown that adult rats who suffered febrile seizures during development reveal memory impairments. Together, these lines of evidence suggest that memory impairments related to hippocampal injury may be evident in human children after prolonged febrile seizures. The current study addressed this question by investigating memory abilities in 26 children soon after a prolonged febrile seizure (median: 37.5 days) and compared their results to those of 37 normally developing children. Fifteen patients were reassessed at a mean of 12.5 months after their first assessment to determine the transiency of any observed effects. We used the visual paired comparison task to test memory abilities in our group, as this task does not depend on verbal abilities and also because successful performance on the task has been proven to depend on the presence of functional hippocampi. Our findings show that patients perform as well as controls in the absence of a delay between the learning phase and the memory test, suggesting that both groups are able to form representations of the presented stimulus. However, after a 5-min delay, patients’ recognition memory is not different from chance, and comparison of patients and controls points to an accelerated forgetting rate in the prolonged febrile seizure group. The patients’ performance was not related to the time elapsed from the acute event or the duration of the prolonged febrile seizure, suggesting that the observed effect is not a by-product of the seizure itself or a delayed effect of medication administered to terminate the seizure. By contrast, performance was related to hippocampal size; participants with the smallest mean hippocampal volumes revealed the biggest drop in performance from the immediate to the delayed paradigm. At follow-up, children were still showing deficiencies in recognizing a face after a 5-min delay. Similarly, this suggests that the observed memory impairments are not a transient effect of the prolonged febrile seizures. This is the first report of such impairments in humans, and it is clinically significant given the links between mesial temporal sclerosis and prolonged febrile seizures. The persistence of these impairments a year onwards signals the potential benefits of intervention in these children who run the risk of developing episodic memory deficits in later childhood.
Keywords: memory, hippocampus, prolonged febrile seizures
Introduction
There is a long-standing debate in the literature regarding the association between prolonged febrile seizures and mesial temporal sclerosis (Falconer, 1971; Cendes et al., 1993; Tarkka et al., 2003). In humans, evidence linking prolonged febrile seizures to mesial temporal sclerosis consists mainly of retrospective associations, such as the finding that 40–60% of individuals with mesial temporal sclerosis identified in epilepsy surgery programmes have a history of prolonged febrile seizures (Cendes et al., 1993). Although hippocampal abnormalities have been prospectively identified in patients following prolonged febrile seizures (van Landingham et al., 1998; Scott et al. 2002, 2003; Tanabe et al., 2011), the majority of these do not qualify as mesial temporal sclerosis. Animal studies have shown that a syndrome resembling human temporal lobe epilepsy can arise from inducing hyperthermic seizures in normal newborn rats, but these animals do not show major hippocampal injury (Dubé et al., 2006). Nevertheless, a subset of these rats has memory impairments in adulthood that are linked to the functional integrity of their hippocampi following the seizure (Dubé et al., 2009).
In humans, it remains uncertain whether prolonged febrile seizures are associated with memory impairments or whether any such impairments are related to the presence and magnitude of structural hippocampal abnormalities. The present study was designed to investigate memory functions in children following prolonged febrile seizures shortly after their seizure, which in the majority of cases occurs during the first 3 years of life (Chin et al., 2006). One of the major constraints in the study of memory processes during development is the limited expressive and receptive language in young infants. Many paradigms used to study memory in adults or older children require both the comprehension of complex verbal instructions and the production of verbal output. One way to circumvent this issue has been to use memory paradigms drawn from the animal literature or those designed specifically for infants. The visual paired comparison task, a task specifically developed to test recognition memory abilities across the lifespan (Fantz, 1964), is one such task. In this task, subjects are familiarized with two identical items followed by the presentation of the familiar item coupled with a novel one. Attending to the novel stimulus for a longer duration is taken as a sign of recognition. Introducing a delay between the familiarization and the test phases transforms the paradigm into a memory test.
Infants as young as 3-days-old have been shown to orient to novel stimuli following a 2-min retention period (Pascalis and de Schonen, 1994). As infants grow older, they are able to withstand longer delays, require shorter familiarization periods and begin to form abstract representations of the stimuli presented (Pascalis et al., 1998; Robinson and Pascalis, 2004). Risk factors, such as prematurity, specifically when associated with hypoxic events, compromise performance on this task (Rose 1983). Most importantly for our current purposes, the visual paired comparison task has been shown to be sensitive to the presence of hippocampal damage, especially when a delay is introduced between familiarization and test (Clark et al., 2000; Pascalis et al., 2004; Munoz et al., 2010; Zeamer et al., 2010). For example, hippocampal lesions during adulthood result in novelty detection deficits following a delay (McKee and Squire, 1993; Pascalis et al., 2004). In addition, developmental amnesic patients, i.e. patients who are associated with early circumscribed hippocampal injury, have been shown to be impaired on this task when the delay between familiarization and test is >30 s (Munoz et al., 2010). The above results suggest that recognition memory as measured by the visual paired comparison is compromised in adulthood regardless of age of hippocampal injury.
The present study is the first to specifically investigate hippocampal-dependent memory in children following prolonged febrile seizures. A previous study investigating the effects of febrile seizures (including eight cases with prolonged febrile seizures) on memory functions used a predominantly working memory task to assess these children when they reached the age of 6 years (Chang et al., 2001). They found that children with a history of febrile seizures outperform their normal peers on a number of working memory tasks. However, worse performance on the delayed recognition measure of this task was predicted by an age of onset of <1 year. A more recent study specifically investigating episodic memory processes and recognition memory after simple febrile seizures (seizures lasting <5 min) discovered a different event-related potentials pattern in the febrile seizures group compared with a control group, in the absence of any behavioural differences (Kipp et al., 2010). Despite the absence of volumetric hippocampal differences between the two groups, correlations between left and right hippocampal volumes and performance on the recognition memory task were observed in this study when the volumes were collapsed together for both groups.
While the aforementioned results may be of interest, both studies focused on children with simple febrile seizures, despite indications that seizures of the prolonged kind are the ones that carry an association with mesial temporal sclerosis and the subsequent development of temporal lobe epilepsy (Baram and Shinnar, 2001). In the present study, we have assessed children with prolonged febrile seizures in order to examine the integrity of their memory faculties early on in the cascade of events that may in certain cases lead to hippocampal attrition. We hypothesized that children after prolonged febrile seizures will perform worse than normal controls in the delayed memory measure of the visual paired comparison task, but not the immediate memory measure because the latter relies less, if at all, on the presence of functionally intact hippocampi.
Materials and methods
Patient recruitment
This study was approved by the Great Ormond Street Hospital ethics committee, and the participants’ consent was obtained according to the Declaration of Helsinki. Recruitment of prolonged febrile seizures cases was undertaken as part of a larger study investigating the structural and functional effects of convulsive status epilepticus in a paediatric population (Yoong et al., 2012). We prospectively recruited a cohort of children who had experienced at least one episode of prolonged febrile seizures between December 2006 and March 2010 through our existing North London epilepsy research network. Recruitment practices were similar to those used in our North London epidemiological study (Chin et al., 2006). Having obtained parental consent, we collected baseline clinical data from parents and arranged for the children to have MRI and neuropsychological assessments at Great Ormond Street Hospital for Children NHS Foundation trust. Participating families were invited back 1 year later for repeat assessments. We defined prolonged febrile seizures as seizures that lasted >30 min, and occurred in a previously neurologically healthy child aged between 6 months and 5 years during a febrile (temperature: >38°C) illness, in the absence of defined CNS infection.
Control recruitment
We recruited control participants of a similar age range in whom parents and carers had no concerns about their development before the assessment. Control participants were recruited through parenting groups, cinema screenings for mothers and their babies and through acquaintances with children in that age range. Three of the patients’ siblings were also assessed, and they have been included in the analyses. As it is unethical to sedate young children for MRI investigations in the absence of clinical concerns, control MRI data were collected only for a subset of control subjects (n = 11) who were willing and able to cooperate for scanning unsedated. Indices of multiple deprivation were calculated for all participants to provide a proxy of socio-economic status (http://www.ons.gov.uk). The indices of multiple deprivation are a combination of a number of other indices that give an overall score for the relative level of multiple deprivation experienced in every neighbourhood in England. Control participants were only assessed once on the visual paired comparison.
Neurodevelopmental assessments
Cognitive composites were derived from the Bayley Scales of Infant and Toddler Development (3rd edition) for children aged <42 months (Bayley, 2005) (n = 45). Children aged>42 months were tested using the Wechsler Preschool and Primary Scale of Intelligence (WPPSI)-III UK edition (Wechsler, 2002) (n = 16). Two of 63 children did not provide cognitive scores.
Quantitative magnetic resonance imaging measures
All MRI of children was carried out using a Siemens Avanto 1.5 T whole body system (Erlangen, Germany). Brain volume (including CSF in ventricles) was calculated using the automatic brain extraction method available at FSL (http://www.fmrib.ox.ac.uk/fsl/). All scans were then manually inspected and adjusted as necessary. Quantitative measurements of hippocampal volumes were performed using the images obtained from the 3D-FLASH (fast low angle shot) sequences. This provided 1-mm isometric voxels. The images were rotated parallel to the long axis of the hippocampus, and a region of interest was manually drawn to encompass the entire hippocampus using MRIcroN (Rorden et al., 2007) (http://www.sph.sc.edu/comd/rorden/mricron/). This was done by two independent observers, M.Y. and M.M.M., who were blinded to the child’s status. The average of all available hippocampal measurements was computed to arrive at the present hippocampal volumes. Mean hippocampal ratios were computed by dividing the mean hippocampal volume by total brain volume.
The visual paired comparison task
Stimuli
Two sets of stimuli depicting female faces were developed for the visual paired comparison task. The female faces were taken from the NimStim face stimulus set (Tottenham et al., 2009). Half of the patients were tested on Set A first, then Set B on their follow-up visit, and vice versa for the remaining half. This was done to avoid any trace of recollection for the faces (especially in older children) in the follow-up visit. Controls were evenly split between Set A or Set B to control for potential differences between the stimuli sets. The size of the images was 19 cm in width and 22 cm in height, and the images subtended a visual angle of 23.5° from the viewing distance of 60 cm. No articles of clothing appeared in the images, and these were obtained under standard lighting conditions.
Apparatus and procedure
Stimuli were presented on a flat screen located within a dark cubicle to minimize peripheral visual distractions. Children viewed the stimuli from a chair placed ∼60 cm from the display screen. Depending on their age, the children either sat on their caregiver’s lap or by themselves. A digital camera positioned on top of the screen recorded their eye movements throughout the experiment. The output from the camera was visible to the experimenter online on their control screen. Parents were instructed to ensure that children were looking centrally at the initiation of each trial, but to allow them to gaze freely thereafter. During the recognition memory component of the visual paired comparison task, the children had a 5-min break, during which they were allowed to play freely with a toy of their choice. The whole experiment lasted ∼8 min.
Novelty preference task
In the first stage of the visual paired comparison task, subjects were tested to determine whether they are able to show a novelty preference in the context of minimal memory demands. The novelty preference task consisted of twelve 10-s trials, during which familiarization trials alternated with test trials (Fig. 1). A previous study using the same procedure showed that normal 1- and 3-month old children are able to show a novelty preference in this time frame (de Haan et al., 2001). Thus, we expected to be able to detect such preferences if present even in the youngest infants tested.
Figure 1.
Example of a familiarization (A) and a novelty (B) trial used in the visual paired-comparison paradigm. Familiarization and novelty trials were interchanged with the position of the novel face counterbalanced between left and right. A cartoon character was displayed between trials to engage the child before initiation of the upcoming trial. Corneal reflection was used to determine positioning of gaze on the left or right part of the screen.
At the beginning of each trial, a cartoon character was shown centrally on the screen to attract the child’s attention. Trials were initiated once it was determined that the child was looking centrally. Six trials in the novelty preference task consisted of the presentation of an identical face placed left and right of the fixation point. The remaining six displayed the original face presented alongside a trial-unique novel face. Position of the novel and the familiar face were counterbalanced across trials. The presentation of the familiarization and test trials was alternated, that is, the identical pair was followed by the novel/familiar pair followed by the identical pair and so on.
Recognition memory task
In the second stage of the visual paired-comparison task, participants were familiarized to a single face with the aim of determining whether a single face can be remembered over a 5 min delay. For learning, the child was exposed to five 10-s trials. On each trial, the to-be-learned face was presented at the left and right of fixation. After a 5-min delay, the subjects were shown the to-be-remembered face with a novel face for two 10-s trials, counterbalancing for the left/right position of the novel/familiar face. The identity of the familiar and novel face was counterbalanced across participants.
Coding
Half of the visual paired comparison recordings were coded by an investigator blind to patient diagnosis (M.D.) and the other half by the current author (M.M.M.). The experimenters were blind with regards to the positioning of the novel face throughout coding. The subjects’ were judged to be looking left or right of the fixation point according to the corneal reflection of the images in their eyes (Fig. 1). To obtain a measure of intra-rater reliability, four randomly selected cases were recoded. Cronbach’s alpha was found to range between 0.998 and 1 for the four recoded participants.
Experimental variables
For each novelty preference trial, the proportion of novelty preference was computed by dividing looking time to the novel face by the total looking time for the trial (i.e. attendance to the novel and the familiar face). Averaging the proportions of novelty preference across the six novelty preference trials in the immediate novelty preference component and the two test trials in the recognition memory component provided an overall novelty preference proportion for each task. Total familiarization time and total looking time in the recognition memory paradigm were also calculated and used as dependent variables in our group analysis.
Statistical analysis
One sample t-tests were used to determine attendance to novelty against the chance level of 0.50. Consistently being found to achieve scores above this benchmark signifies attendance to novelty, whereas the opposite signifies attendance to the familiar face. No difference to chance level signals that there is no preference for either the novel or the familiar face on the part of the participant. To show a mean difference of 0.1 between our observations and chance, assuming a standard deviation of 0.12 (Fagan, 1972; Rose, 1981, 1983; Rose and Wallace, 1985; Colombo et al., 1988; Diamond, 1990; McCall and Carriger, 1993; Pascalis et al., 1998; Nelson et al., 2000; Robinson and Pascalis, 2004; Jones et al., 2011) and α = 0.8, 20 cases per group were required.
Repeated measures analysis of variance (ANOVA) were conducted to investigate familiarization and novelty preferences in the two groups. Spearman correlations were carried out to investigate any relationships between duration and days elapsed from prolonged febrile seizures and performance. Paired sample t-tests were used to detect changes in performance from baseline to follow-up in the patient group. The structural–functional analysis was conducted separately for our patient and control groups to avoid obscuring important relationships in the prolonged febrile seizures group.
Results
Description of the study sample
Table 1 describes characteristics of the current prolonged febrile seizures sample in comparison with the sample obtained in our North London epidemiological study (Chin et al., 2006). During our recruitment period, 225 cases of convulsive status epilepticus were referred to us through our referral network. Sixty-eight of these cases were classifiable as a prolonged febrile seizure (30.2%). This constitutes about one-third of convulsive status epilepticus cases, a proportion that is representative of the overall prolonged febrile seizures incidence according to our previous epidemiological study (Chin et al. 2006). Of the 68 cases with prolonged febrile seizures, 34 agreed to participate in our study. Non-participation was due to one of the following reasons: (i) eight children were non-contactable owing to missing or incorrect details; (ii) 19 parents declined participation; (iii) five children lived a considerable distance away and did not want to travel to our study centre; and (iv) two children were unsuitable for sedation because of existing comorbidities.
Table 1.
Comparison between the current sample of children recruited after a first prolonged febrile seizures episode and the Chin et al. (2006) sample
| Characteristics | Prolonged febrile seizures sample | Chin et al. (2006) |
|---|---|---|
| n | 24 | 56 |
| Male (%) | 7 (29.2) | 34 (60.7) |
| 30–60 min duration (%) | 11 (45.8) | 27 (48.2) |
| >60 min duration (%) | 13 (54.2) | 29 (52.8) |
| Continuous prolonged febrile seizures (%) | 10 (41.7) | 32 (57.1) |
| Intermittent prolonged febrile seizures (%) | 14 (58.3) | 24 (43.9) |
| Tonic (%) | 3 (12.5) | 3 (5.4) |
| Clonic (%) | 1 (4.2) | 1 (1.8) |
| Tonic–clonic (%) | 20 (83.3) | 52 (92.8) |
Our total sample size of prolonged febrile seizures cases was 26 patients. In this table, we report characteristics of the 24 that were recruited after their first prolonged febrile seizure, as the sample described in the Chin et al. (2006) study was one of first episodes.
Date of birth and indices of multiple deprivation details were available for 31 of 34 non-participants, and gender information was available for all. There were no differences in age at prolonged febrile seizure (P = 0.818) and index of multiple deprivation (P = 0.922) between the two groups. There was a trend for a significant difference in gender between participants (23 females) and non-participants (15 females) [χ2(1) = 3.82, P = 0.09]. Clinical data were also available for non-participants with respect to the occurrence of previous seizures (n = 34), whether prolonged febrile seizures were focal or generalized (n = 25), duration of prolonged febrile seizures (n = 26) and whether prolonged febrile seizures were continuous or intermittent (n = 34). The median duration for participants and non-participants was 53.5 min (range 30–190 min); therefore, we categorized children based on a median split into those who had seizures lasting <53.5 min and those who had seizures lasting >53.5 min. Comparisons of participants and non-participants on all clinical variables showed no significant differences between the two groups. Of the 34 children that underwent neuropsychological investigations, 26 children were tested on the visual paired comparison task. A Mann–Whitney test revealed no differences in age (P = 0.858), days from seizure (P = 1), seizure duration (P = 0.510) and cognition (P = 0.636) between those assessed on the visual paired comparison task (n = 26) and those that were not (n = 8).
Participating patients were seen a median of 37.5 days following their seizure (range 10–254 days). However, the child assessed 254 days post-seizures constitutes an outlier with the remaining children seen in <120 days post-seizures. Prolonged febrile seizures lasted a median of 75 min (range 30–190 min). Only one child from the entire group was receiving medication (phenytoin) at the time of assessment. Phenytoin was started because of parental anxiety and evidence of a pituitary hemorrhage on the MRI performed at the local hospital. Review of the MRI scan at Great Ormond Street Hospital raised no concerns. The remaining children were receiving no medication. Thus, any results reported here could not be related to an effect of medication. Visual inspection of MRI scans showed that only one patient’s scan was found to contain abnormalities that were deemed minor (deep white matter lesions). Seven of 26 children had experienced short febrile seizures before the prolonged febrile seizure episode, and two children had experienced a previous prolonged febrile seizure event. Mann–Whitney tests revealed no differences in immediate (P = 0.569) and delayed novelty preference (P = 0.711) between those who had and those who had not experienced previous episodes; therefore, the two groups were investigated together.
Patients were compared with 37 normally developing children at baseline. The two groups were similar in gender [χ2(1) = 3.01, P = 0.12], the representation of preterms within each sample [χ2(1) = 3.36, P = 0.15], mean hippocampal ratio [t(35) = 0.70, P = 0.49] and the indices of multiple deprivation [t(58) = −0.57, P = 0.57]. However, the prolonged febrile seizures group obtained a significantly lower cognitive composite score (mean 95.36) than the controls (mean 110.58) [t(59) = −3.85, P < 0.01], and they revealed a trend for being younger than controls [t(61) = −1.85, P = 0.07].
Are the groups able to show novelty preference without a delay?
Table 2 contains the means and standard deviations for the total novelty preference proportions exhibited by each group during the novelty preference task. One sample t-tests revealed that both groups showed a mean novelty preference when virtually no delay was imposed between familiarization and test (P < 0.001).
Table 2.
Characteristics of the two groups and their performance on the visual paired-comparison task
| Characteristics and performance | Prolonged febrile seizures | Control subjects |
|---|---|---|
| n | 26 | 37 |
| Male (%) | 7 (26.9) | 18 (48.6) |
| Age at test (months) | 23.5 (12.66) | 30.89 (17.36) |
| Prematurity (<36 weeks) | 3 | 1 |
| Index of mean deprivation (SD) | 27.56 (15.10) | 29.47 (10.86) |
| Median days from prolonged febrile seizure (range) | 37.5 (10–254) | Not applicable |
| Median duration of prolonged febrile seizure (range) | 75 (30–190) | Not applicable |
| Immediate novelty preference (SD) | 0.62 (0.11)a | 0.58 (0.10)a |
| Mean familiarization looking time (SD) | 27.77 (8.54) | 30.76 (7.85) |
| Mean looking time during test (SD) | 11.37 (5.24) | 12.93 (3.94) |
| Delayed novelty preference (SD) | 0.49 (0.11) | 0.55 (0.12)a |
| Cognition (SD) | 95.36 (14.63)b | 110.58 (15.58)b |
| Mean hippocampal ratio (SD) | 16.87 (1.52) | 16.49 (1.45) |
a Significant difference from chance level at the P < 0.05 level in novelty preference.
b Significant difference between the two groups at the P < 0.05 level. SD = standard deviation.
Do the groups show familiarization?
The mean and standard deviations for the total familiarization times in the recognition memory task for the two groups are described in Table 2. A repeated measures ANOVA with the amount of time spent attending to the to-be-remembered face during the five familiarization trials and group as a between subjects’ factor revealed a main effect of change in familiarization proportions from trial to trial [F(4,57) = 8.434, P < 0.001] suggesting that children became increasingly familiarized with the presented face. There was no group by familiarization interaction (P = 0.69), which points to a similar familiarization pattern in both groups.
Do the groups recognize the to-be-remembered face after a 5-min delay?
Table 2 contains the means and standard deviations for the total looking time during test trials and the novelty preference proportions following the 5-min delay for the two groups. There were no differences between the two groups in the total amount of time they spent looking at the stimuli during familiarization [t(60) = −1.42, P = 0.16] or during test [t(61) = −1.35, P = 0.18]. One sample t-tests revealed that only the control group showed a preference for the novel face following the 5 min delay [t(36) = 2.31, P = 0.03]. The prolonged febrile seizures group revealed no preference for the novel face following the delay [t(25) = 0.49, P = 0.65], a finding that was supported even after the exclusion of the preterms [t(21) = −0.30, P = 0.77], who have been associated with deficits on this task.
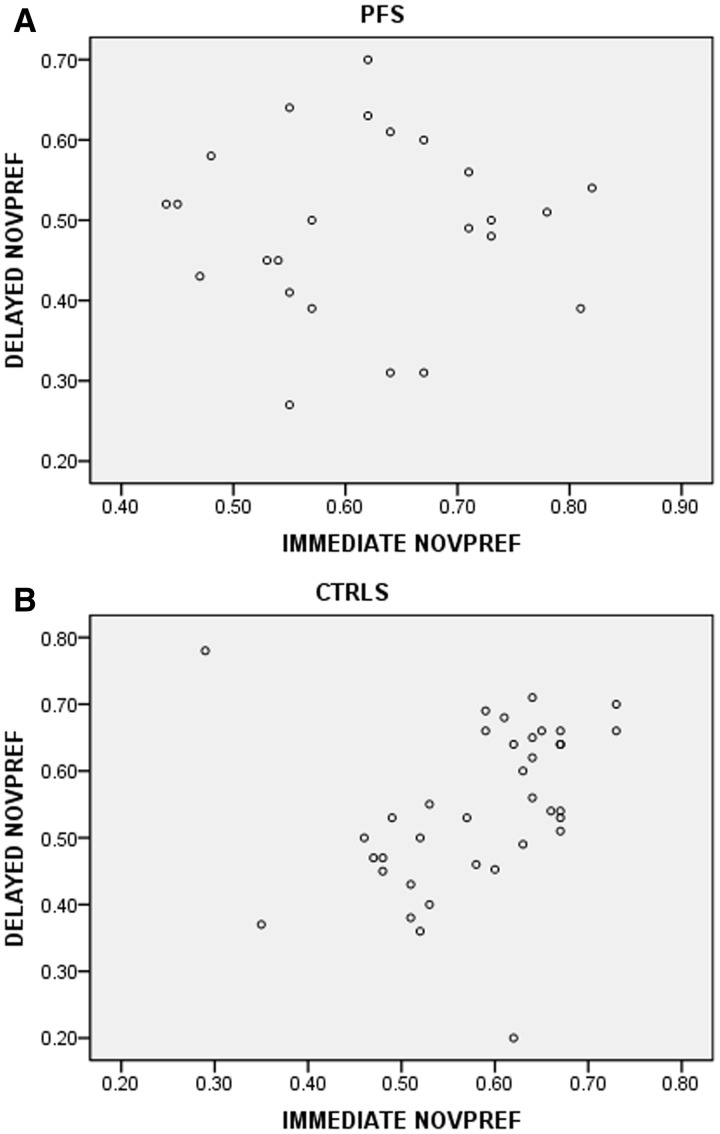
Importantly, in the control group, performance on the immediate novelty preference task was highly correlated with performance on the delayed novelty task (r = 0.494, P = 0.002). This relationship was absent in the prolonged febrile seizures group (r = 0.009, P = 0.969) (Fig. 2).
Figure 2.
Relationship between immediate and delayed novelty preference in subjects with prolonged febrile seizures (A) (B). CTRL-control subjects; NOVPREF = novelty preference; PFS = prolonged febrile seizures.
Is there an effect of delay on the performance of the two groups?
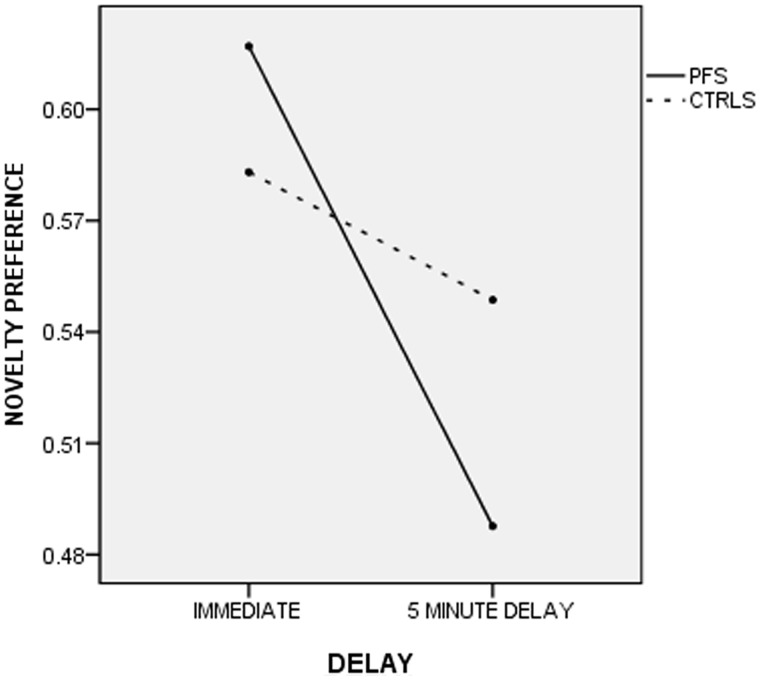
A repeated measures ANOVA with novelty preference proportions in the immediate and the delayed conditions and age, cognition, total familiarization and total looking times as covariates and gender as a fixed factor revealed a trend for an overall effect of delay [F(1, 52) = 3.30, P = 0.08]. There was a significant group by delay interaction [F(1, 52) = 7.52, P = 0.008], suggesting that the two groups behave differently following a delay (Fig. 3), with the prolonged febrile seizures group showing a big drop in memory performance from the immediate to the delayed paradigm that was not observed in the control group. There was also an interaction between delay and cognition [F(1,52) = 6.54, P = 0.014].
Figure 3.
Performance of subjects with prolonged febrile seizures and control subjects on the visual paired comparison in the immediate and the delayed conditions. A repeated measures ANOVA with age and cognition as covariates revealed a significant interaction between group and delay [F(1,52) = 7.52, P = 0.008] suggesting that the prolonged febrile seizures group suffered more than the control group after the 5 min delay.
Is the observed drop of performance in the prolonged febrile seizures group a result of their lower cognitive capacity relative to controls?
To determine to what extent the aforementioned effect is a result of lowered cognition in the prolonged febrile seizures group or an isolated memory deficit, we conducted the above analyses on a cognitively matched sample of patients with prolonged febrile seizures (n = 18) and controls (n = 27). To do this, we excluded the bottom and top 15th percentiles on the cognitive composite continuum. Namely, we excluded everyone with a cognitive score of <95 and >122. The resulting groups were matched on all variables apart from gender where the prolonged febrile seizures group was found to contain a significantly larger proportion of females than the control group [χ2(1) = 7.20, P = 0.01] (Table 3). A repeated measures ANOVA with novelty preference proportions in the immediate and the delayed conditions and age, cognition, total familiarization and total looking times as covariates and gender and group as fixed factors revealed an interaction between delay and group [F(1,36) = 7.43, P = 0.01]. No other main effects or interactions were shown to be significant in the matched-group analysis.
Table 3.
Characteristics of the two matched subgroups and their performance on the visual paired-comparison task
| Characteristics and performance | Prolonged febrile seizures | Control subjects |
|---|---|---|
| n | 18 | 27 |
| Male (%) | 4 (22.2)a | 18 (63)a |
| Age at test (months) | 24.1 (12.1) | 29.55 (12.79) |
| Prematurity (<36 weeks) | 1 | 1 |
| Index of mean deprivation (SD) | 25.81 (14.25) | 29.61 (11.6) |
| Median days from prolonged febrile seizures (range) | 33.5 (10–90) | Not applicable |
| Median duration of prolonged febrile seizures (range) | 80 (30–190) | Not applicable |
| Immediate novelty preference (SD) | 0.65 (0.11)b | 0.57 (0.10)b |
| Mean familiarization looking time (SD) | 28.57 (9.09) | 31.79 (6.14) |
| Mean looking time during test (SD) | 10.69 (5.26) | 12.23 (3.86) |
| Delayed novelty preference (SD) | 0.49 (0.12) | 0.55 (0.11)b |
| Cognition (SD) | 103 (7.73) | 105.63 (9.07) |
| Mean hippocampal ratio (SD) | 16.91 (1.51) | 16.96 (1.97) |
a Significant difference between the two groups at the P < 0.05 level.
b Significant difference from chance level at the P < 0.05 level in novelty preference.
Moreover, one sample t-tests confirmed the results obtained in the larger sample. Namely, the prolonged febrile seizures group was able to show a novelty preference in the immediate condition [t(16) = 5.78, P < 0.001], but not in the delayed condition [t(17) = −0.47, P = 0.642], whereas the control group evinced a novelty preference in both conditions [immediate: t(26) = 3.31, P = 0.003; delayed: t(26) = 2.381, P = 0.025].
Correlation between performance and clinical variables
Spearman correlations revealed no relationship between days elapsed from the prolonged febrile seizure or duration of the event to immediate and delayed novelty preference on our task. This suggests that the recognition memory impairments observed in the children with prolonged febrile seizures are not affected by these two seizure-related characteristics. A repeated measures ANOVA with novelty preference proportion in the immediate and the delayed conditions as the within subjects’ factor and duration as a covariate revealed no relationship between change in performance and duration [F(1,22) = 0.45, P = 0.51]. The same was true when we looked separately at the relationship between days from prolonged febrile seizure and performance. [F(1,22) = 0.02, P = 0.89).
The relationship between hippocampal volumes and performance
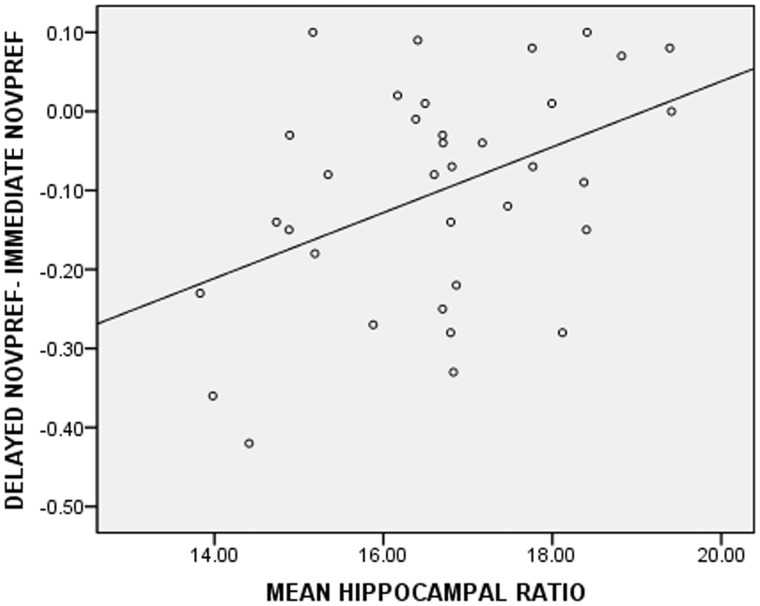
Mean hippocampal ratios were not correlated to novelty preference in the immediate (r = −0.32, P = 0.121) or the delayed (r = 0.28, P = 0.165) condition in the prolonged febrile seizures group. A repeated measures ANOVA with novelty preference proportions in the immediate and the delayed conditions as the repeated measure and mean hippocampal ratios as a covariate revealed an overall effect of delay [F(1,22) = 11.87, P = 0.002] and an interaction between mean hippocampal ratio and the difference between performance on the immediate and the delayed conditions [F(1,22) = 9.28, P = 0.006]. This means that a bigger drop in novelty preference from the immediate to the delayed condition was associated with smaller hippocampal ratios in the prolonged febrile seizures group (Fig. 4). This effect persisted [F(1,16) = 6.60, P = 0.021] even after the inclusion of age (P = 0.432), gender (P = 0.134), cognition (P = 0.153), total familiarization time (P = 0.432) and total looking time (P = 0.706) in the model. Finally, cognitive composites were not found to be associated with mean hippocampal ratios (r = −0.134, P = 0.523) pointing to the specificity of the aforementioned finding.
Figure 4.
Relationship between the mean hippocampal ratio and performance in the prolonged febrile seizures group. The biggest reductions in novelty preference were related to the smallest mean hippocampal ratios. Conversely, no change in performance or an increase in novelty preference was observed in the children with the biggest mean hippocampal ratios.
In the control group, the mean hippocampal ratio was not related to the immediate (r = 0.032, P = 0.926) or the delayed (r = 0.146, P = 0.669) novelty preference. A repeated measures ANOVA revealed no main effect of delay [F(1,9) = 0.99, P = 0.347] or an interaction between delay and mean hippocampal ratio [F(1,9) = 0.73, P = 0.416]. Akin to the prolonged febrile seizures group, hippocampal ratios were not shown to be correlated with cognitive composite scores in the control group (r = −0.267, P = 0.455).
How are the patients performing on the visual paired comparison at follow-up?
Participants were tested on a different set of stimuli at follow-up; therefore, any significant changes between baseline and follow-up performance reported here cannot be interpreted as a test–retest effect. Fifteen children out of 26 (57.7%) seen originally at baseline were reassessed on the visual paired comparison a mean of 12.5 months after their first assessment. Four out of 15 patients experienced short febrile seizures in the interim period, but none of them were put on medication. Independent sample t-tests revealed no differences between the follow-up and the no follow-up sample (Table 4). One sample t-tests replicated the baseline results, that is, patients showed an immediate novelty preference [t(14) = 5.217, P < 0.001], but not a delayed novelty preference [t(14) = −0.538, P = 0.599]. Immediate novelty preference was not predictive of delayed novelty preference (r = −0.366, P = 0.180), akin to baseline findings. Paired sample t-tests revealed no significant differences between baseline and follow-up performance in the immediate [t(14) = −1.099, P = 0.290] and the delayed conditions [t(14) = 0.418, P = 0.682]. Finally, a paired sample t-test revealed a drop in novelty preference from the immediate to the delayed condition [t(14) = 2.801, P = 0.014] in the patients’ follow-up performance. Excluding the four cases that experienced short febrile seizures during the follow-up period made no changes to the results. Taken together, these findings suggest that the prolonged febrile seizures group as a whole continue to have memory deficits a mean of 12.5 months following their first assessment. A repeated measures ANOVA with immediate and delayed novelty preference as the repeated measure and mean hippocampal ratio as a covariate revealed no main effects or interactions, in contrast to the baseline results where mean hippocampal ratio was shown to be predictive of the drop in memory performance after the delay.
Table 4.
Comparison of follow-up and no follow-up prolonged febrile seizures sample
| Characteristics and performance | Follow-up prolonged febrile seizures | No follow-up prolonged febrile seizures |
|---|---|---|
| n | 15 | 11 |
| Male (%) | 5 (33.3) | 2 (18.2) |
| Age at test (months) | 22.4 (11.74) | 25.02 (14.25) |
| Mean days from prolonged febrile seizures (SD) | 55.66 (61.68) | 40.18 (19.58) |
| Mean duration of prolonged febrile seizures (SD) | 76 (29.79) | 72.73 (49.92) |
| Immediate novelty preference (SD) | 0.60 (0.13) | 0.64 (0.07) |
| Mean familiarization looking time (SD) | 28.27 (7.29) | 27.02 (10.52) |
| Mean looking time during test (SD) | 12.51 (5.58) | 9.81 (4.53) |
| Delayed novelty preference (SD) | 0.5 (0.10) | 0.48 (0.12) |
| Cognition (SD) | 94.57 (17.08) | 96.36 (11.52) |
| Mean hippocampal ratio (SD) | 16.9 (1.4) | 16.84 (1.75) |
Discussion
The present study has shown that a median of 37.5 days following prolonged febrile seizures, children show recognition memory impairments. Importantly, these children have no problem identifying novelty in itself, which suggests that their impairments are circumscribed to their memory functions. Moreover, taking an intellectually matched sample of controls and patients replicated the above results confirming that the patients’ memory impairments are not simply a reflection of their overall lower cognitive functioning. In addition, their deficits do not seem to be related to the seizure itself or to the medication administered to suppress the seizure at the time of admission, as there were no significant correlations between duration and time from seizure and their performance on the visual paired comparison task. Instead, the magnitude of the decline in performance from the immediate to the delayed paradigms was linked to the size of their hippocampi at the time of testing. One year later, the prolonged febrile seizures group is still showing impairments in remembering a face after a 5-min delay, suggesting that what we observed in our baseline assessments was not a transient effect of the seizure. Below we discuss the implications of our findings along with certain limitations.
Why are the patients with prolonged febrile seizures failing to remember a face after the 5-min delay?
There are several possible explanations for the lack of a novelty preference in the prolonged febrile seizures group following the 5-min delay. One possibility is that these children are faster at forgetting the encoded face than their normal peers. Thus, while similarly capable of committing a face to memory as their peers, retaining that face for 5 min appears to be difficult for this group. A second explanation would be that the prolonged febrile seizures group had insufficiently encoded the presented face in the first place, thus, resulting in a null preference following the delay. The third explanation is that while children in the prolonged febrile seizures group are similarly capable at encoding and retaining the presented face as normal controls, they have problems with retrieving the retained construct after a delay. As we observed no differences between the two groups in their familiarization patterns, and, more importantly, the prolonged febrile seizures group was able to show a novelty preference in the immediate condition, the second explanation is not supported. We think it more likely that these children have problems in retaining the memory construct for 5 min or have problems retrieving it following the delay. More studies in this direction may shed light on which memory mechanisms are failing in this instance.
Are the memory impairments observed in the prolonged febrile seizures group a result of selection bias?
Our North London epidemiological study, which had a recruiting period of 2 years, recorded 56 cases of first ever prolonged febrile seizures episodes during that time (Chin et al., 2006). Therefore, one would expect a referral of at least 91 cases of prolonged febrile seizures during the current recruitment period that lasted 39 months. Instead, 68 cases of prolonged febrile seizures were referred to us in that period. There are two possibilities for this: (i) some cases were missed; and (ii) the incidence of prolonged febrile seizures has gone down since the Chin et al. (2006) study due to better clinical management. Given that the comparison between the studied and the Chin et al. (2006) sample as well as the one between participants and non-participants revealed no significant differences between the samples, we feel that the present sample is adequately representative of the population as a whole. Moreover, gender, which showed a trend for being significantly different between participants and non-participants, did not have an effect on our results, suggesting that our current findings apply to both genders.
To investigate the possibility that lower cognitive functioning is responsible for the observed memory impairments, we replicated our analysis in a subgroup of patients and controls that were matched for cognition. This resulted in no differences in our pattern of results, with patients showing no difficulties in the immediate measure, but significant deficits on the delayed measure. In addition, our control and patient groups were matched on socio-economic status, which further corroborates the notion that this is not a result of selection bias but of an actual performance difference between the two groups that pertains to a circumscribed memory deficit.
Age of onset and memory impairments
The present study has not found an effect of age on recognition memory akin to the Chang et al. (2001) study, suggesting that such impairments manifest themselves regardless of age of onset. Nevertheless, it remains possible that if these children were reassessed in late childhood a relationship between an earlier age of onset and memory performance could still emerge, as some children may outgrow their deficits by that point. However, there are other potential reasons for the discrepancies between the two studies. Specifically, the present study and the Chang et al. (2001) study have investigated different memory functions in different populations at different time points; our study examines recognition memory functions within 1 year of prolonged febrile seizures, and the Chang et al. (2001) study examines working memory functions in children with a history of simple febrile seizures at the age of 6 years. While recognition memory functions have been shown to be mediated by the hippocampus, i.e. one of the main structures implicated in prolonged febrile seizures, working memory processes are known to be supported mainly by frontal lobe structures (Owen et al., 1990; Zanto et al., 2011), although there have been some recent suggestions that the hippocampus may also be playing a role in the development of working memory functions during infancy (Beauchamp et al., 2008).
What causes the observed memory impairments: the chicken, the egg or their offspring?
Results in the literature are mixed with regards to the long-term outcome of children following prolonged febrile seizures, with some studies reporting a normal development (Nelson and Ellenberg, 1978; Verity et al., 1993) and other studies reporting impairments (Schiottz-Christensen and Bruhn, 1973; Kölfen et al., 1998). The present study has found that children shortly following prolonged febrile seizures show impairments in a task of recognition memory. There are at least three possible explanations for the reduced performance in the prolonged febrile seizures group.
The first is that prolonged febrile seizures lead to a brain injury that causes the observed deficits. Long-term memory impairments have been identified in an animal model of prolonged febrile seizures, where animals with previously normal brains were made to seize under high-temperature conditions (Dubé et al., 2006, 2009). The second is that the factors, which predispose a human to develop prolonged febrile seizures are also responsible for the memory impairments we observed in the present study. Consistent with this idea is that, in contrast to rats that uniformly develop seizures when exposed to a fever, only ∼5% of children will have a febrile seizure, even though almost all will experience a significant fever at the age at which febrile seizures occur (Baulac et al., 2004). This suggests that it is more than just a fever that is required to generate a febrile seizure and that some children have brains that are predisposed to having a seizure when exposed to a fever. The third option would be a combination of the above two alternatives, whereby there is a cumulative effect of having a brain-at-risk along with an effect of the seizure itself. Recent animal studies support this view by showing that experiencing seizures during early development while having a brain with malformations of cortical development may worsen cognitive outcomes in the short but not the longer term (Lucas et al., 2011).
The current study has not been directly designed to address this issue. To be able to causally identify the cascade of events that leads to impairments and mesial temporal sclerosis one would need to investigate these children premorbidly. The incidence of paediatric convulsive status epilepticus in the UK has been calculated to be in the region of 17 to 23 per 100 000 yearly, with prolonged febrile seizures cases constituting about one-third of overall convulsive status epilepticus cases (Chin et al., 2006). Therefore, one would need to image and assess a large group of children to prospectively identify prolonged febrile seizures cases and follow them up.
Nevertheless, the current study is in a position to provide some insights on the presence and expression of impairments within the first year after prolonged febrile seizures, as children were seen within 6 weeks of the seizure and assessments were repeated 1 year later. We believe that the absence of a relationship between duration of the seizure (also reported in other studies, e.g. Hesdorffer et al., 2011) and the days elapsed from the acute event and performance points to a lesser effect of the seizure on performance and argues more strongly for a premorbid tendency to perform lower than controls. Secondly, on their baseline assessment, patients with prolonged febrile seizures with smaller hippocampal ratios showed the biggest drop in performance after the delay compared with children with larger hippocampal ratios. As the first child was seen 10 days after the event and children were seen on average within 6 weeks of prolonged febrile seizures, it is unlikely that this is an effect of underlying oedema on the current results, since oedema will have subsided by that time point (Scott et al., 2002, 2003). This finding further strengthens the view that the recognition memory impairments detected in this cohort are more strongly driven by brain morphology rather than seizure characteristics. Thirdly, 15 children were reassessed on the visual paired comparison 1 year onwards, and showed no differences from their baseline assessment. Namely, they were still showing circumscribed recognition memory impairments. While these children constituted only 57% of the original sample, there were no differences between the two groups in baseline clinical and performance characteristics that would make them qualitatively different. Moreover, the absence of follow-up data for our controls does not call these findings into question as visual recognition memory has been shown to be a reliable measure in normal infants (Rose et al., 2004), and we would, therefore, expect controls to continue to show a novelty preference on our task. However, in contrast to our baseline findings, the mean hippocampal ratio at the follow-up assessment was not found to be associated with the drop in novelty preference from the immediate to the delayed condition, which can be either due to a lack of a relationship a year onwards or a sample size issue. The current data cannot distinguish between these two alternatives.
Conclusion
Overall, our results agree with findings stemming from the animal literature that associate prolonged febrile seizures with memory impairments. In contrast to animal studies, the present study cannot conclude whether the prolonged febrile seizures have caused the observed impairments or not. We can, however, conclusively rule out the possibility that such impairments are a temporary effect of the prolonged febrile seizures and seem to either be a result of a more permanent functional network alteration or of a premorbid condition. Moreover, these recognition memory impairments have been shown to be associated with the average size of the patients’ hippocampi at baseline and are present even when controls and patients are intellectually matched. These data point to a dissociation between overall cognitive status and memory status in the prolonged febrile seizures group. Determining the long-term trajectory of these impairments requires follow-up of these children and probing specific memory functions known to be hippocampally mediated, such as episodic memory processes. However, if the observed memory impairments are permanent, then early recognition may lead to early remediation in the school setting, and this in turn is likely to positively impact school performance in these children.
Funding
This work was undertaken at GOSH/UCL Institute of Child Health, which received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme. The Centre for Paediatric Epidemiology and Biostatistics also benefits from funding support from the Medical Research Council in its capacity as the MRC Centre of Epidemiology for Child Health. R.F.M.C. held a National Institute for Health Research (NIHR) Academic Clinical Lectureship. R.C.S. is supported by Great Ormond Street Hospital Children’s Charity. This work was supported by the Great Ormond Street Hospital Children’s Charity [2011-LDR-02]; the National Centre for Young People with Epilepsy and the Wellcome Trust [060214 to R.C.S.].
Acknowledgements
The authors thank the participants and their families for giving their time so generously to participate in this study. The authors would also like to thank our local collaborators for helping us with the recruitment phase of our study.
References
- Baram TZ, Shinnar S. Do febrile seizures improve memory? Neurology. 2001;57:7–8. doi: 10.1212/wnl.57.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulac SC, Gourfinkel-An I, Nabbout R, et al. Fever, genes and epilepsy. Lancet Neurol. 2004;3:421–30. doi: 10.1016/S1474-4422(04)00808-7. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley scales of infant development. 3rd edn. San Antonio: The Psychological Corporation; 2005. [Google Scholar]
- Beauchamp MH, Thompson DK, Howard K, Doyle LW, Egan GF, Inder TE, et al. Preterm infant hippocampal volumes correlate with later working memory deficits. Brain. 2008;131:2986–94. doi: 10.1093/brain/awn227. [DOI] [PubMed] [Google Scholar]
- Cendes F, Andermann F, Dubeau F, Gloor P, Evans A, Jones-Gotman M, et al. Early childhood prolonged febrile convulsions, atrophy and sclerosis of mesial structures, and temporal lobe epilepsy: an MRI volumetric study. Neurology. 1993;43:1083–7. doi: 10.1212/wnl.43.6.1083. [DOI] [PubMed] [Google Scholar]
- Chang YC, Guo NW, Wang ST, Huang CC, Tsai JJ. Working memory of school-aged children with a history of febrile convulsions: a population study. Neurology. 2001;57:37–42. doi: 10.1212/wnl.57.1.37. [DOI] [PubMed] [Google Scholar]
- Chin RF, Neville BG, Peckham C, Bedford H, Wade A, Scott RC. Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet Neurol. 2006;368:222–9. doi: 10.1016/S0140-6736(06)69043-0. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–60. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo J, Mitchell DW, Horowitz FD. Infant visual attention in the paired-comparison paradigm: test-retest and attention-performance relations. Child Dev. 1988;59:1198–210. doi: 10.1111/j.1467-8624.1988.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Diamond A. Rate of maturation of the hippocampus and the developmental progression of children’s performance on the delayed non-matching to sample and visual paired comparison tasks. Ann N Y Acad Sci. 1990;608:394–433. doi: 10.1111/j.1749-6632.1990.tb48904.x. [DOI] [PubMed] [Google Scholar]
- de Haan M, Johnson MH, Maurer D, Perrett DI. Recognition of individual faces and average face prototypes by 1- and 3-month-old infants. Cogn Dev. 2001;16:659–78. [Google Scholar]
- Dubé C, Richichi C, Bender RA, Chung G, Litt B, Baram TZ. Temporal lobe epilepsy after experimental prolonged febrile seizures: a prospective analysis. Brain. 2006;129:911–22. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé C, Zhou JL, Hamamura M, Zhao Q, Ring A, Abrahams J, et al. Cognitive dysfunction after experimental febrile seizures. Exp Neurol. 2009;215:167–77. doi: 10.1016/j.expneurol.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan JF. Infants’ recognition memory for faces. J Exp Child Psychol. 1972;14:453–76. doi: 10.1016/0022-0965(72)90065-3. [DOI] [PubMed] [Google Scholar]
- Falconer MA. Genetic and related aetiological factors in temporal lobe epilepsy. A review. Epilepsia. 1971;12:13–31. doi: 10.1111/j.1528-1157.1971.tb03912.x. [DOI] [PubMed] [Google Scholar]
- Fantz RL. Visual experience in infants: decreased attention to familiar patterns relative to novel ones. Science. 1964;146:668–70. doi: 10.1126/science.146.3644.668. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Benn EKT, Bagiella E, Nordli D, Pellock J, Hinton V, et al. Distribution of febrile seizure duration and associations with development. Ann Neurol. 2011;70:93–100. doi: 10.1002/ana.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EJ, Pascalis O, Eacott MJ, Herbert JS. Visual recognition memory across contexts. Dev Sci. 2011;14:136–47. doi: 10.1111/j.1467-7687.2010.00964.x. [DOI] [PubMed] [Google Scholar]
- Kipp KH, Mecklinger A, Becker M, Reith W, Gortner L. Infant febrile seizures: Changes in declarative memory as revealed by event-related potentials. Clin Neurophy. 2010;121:2007–16. doi: 10.1016/j.clinph.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Kölfen W, Pehle K, König S. Is the long-term outcome of children following febrile seizures favourable? Dev Med Child Neurol. 1998;40:667–71. doi: 10.1111/j.1469-8749.1998.tb12326.x. [DOI] [PubMed] [Google Scholar]
- Lucas MM, Lenck-Santini P-P, Holmes GL, Scott RC. Impaired cognition in rats with cortical dysplasia: additional impact of early life seizures. Brain. 2011;134:1684–93. doi: 10.1093/brain/awr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall RB, Carriger MS. A meta-analysis of infant habituation and recognition memory performance as predictors of later IQ. Child Dev. 1993;64:57–79. [PubMed] [Google Scholar]
- McKee RD, Squire LR. On the development of declarative memory. J Exp Learn Mem Cogn. 1993;19:397–404. doi: 10.1037//0278-7393.19.2.397. [DOI] [PubMed] [Google Scholar]
- Munoz M, Chadwick M, Perez-Hernandez E, Vargha-Khadem F, Mishkin M. Novelty preference in patients with developmental amnesia. Hippocampus. 2010;21:1268–76. doi: 10.1002/hipo.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Wewerka S, Thomas KM, Tribby-Walbridge S, deRegnier R, Georgieff M. Neurocognitive sequelae of infants of diabetic mothers. Behav Neurosci. 2000;114:950–6. [PubMed] [Google Scholar]
- Nelson KB, Ellenberg JH. Prognosis of children with febrile seizures. Pediatrics. 1978;61:720–27. [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–34. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- Pascalis O, de Schonen S. Recognition memory in 3-to-4-day -old human neonates. Neuroreport. 1994;5:1721–4. doi: 10.1097/00001756-199409080-00008. [DOI] [PubMed] [Google Scholar]
- Pascalis O, de Haan M, Nelson CA, de Schonen S. Long-term recognition memory for faces assessed by visual paired comparison in 3- and 6-month-old infants. J Exp Psychol Learn Mem Cogn. 1998;24:249–60. doi: 10.1037//0278-7393.24.1.249. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Hunkin NM, Holdstock JS, Isaac CL, Mayes AR. Visual paired comparison performance is impaired in a patient with selective hippocampal lesions and relatively intact item recognition. Neuropsychologia. 2004;42:1293–300. doi: 10.1016/j.neuropsychologia.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Robinson AJ, Pascalis O. Development of flexible visual recognition memory in human infants. Dev Sci. 2004;7:527–33. doi: 10.1111/j.1467-7687.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H-O, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–8. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Rose SA. Developmental changes in infants’ retention of visual stimuli. Child Dev. 1981;52:227–33. [PubMed] [Google Scholar]
- Rose SA. Differential rates of visual information processing in full-term and preterm infants. Child Dev. 1983;54:1189–98. [PubMed] [Google Scholar]
- Rose SA, Wallace IF. Visual recognition memory: a predictor of later cognitive functioning in preterms. Child Dev. 1985;56:843–52. [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Infant visual recognition memory. Dev Rev. 2004;24:74–100. doi: 10.1037/0012-1649.39.3.563. [DOI] [PubMed] [Google Scholar]
- Schiottz-Christensen E, Bruhn P. Intelligence, behaviour and scholastic achievement subsequent to febrile convulsions: an analysis of discordant twin-pairs. Dev Med Child Neurol. 1973;15:565–75. doi: 10.1111/j.1469-8749.1973.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Scott RC, Gadian DG, King MD, Chong WK, Cox TX, Neville BG, et al. Magnetic resonance imaging findings within 5 days of status epilepticus in childhood. Brain. 2002;125:1951–9. doi: 10.1093/brain/awf202. [DOI] [PubMed] [Google Scholar]
- Scott RC, King MD, Gadian DG, Neville BG, Connelly A. Hippocampal abnormalities after prolonged febrile convulsion: a longitudinal MRI study. Brain. 2003;126:2551–7. doi: 10.1093/brain/awg262. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Hara K, Shimakawa S, Fukui M, Tamai H. Hippocampal damage after prolonged febrile seizure: one case in a consecutive prospective series. Epilepsia. 2011;52:837–40. doi: 10.1111/j.1528-1167.2010.02958.x. [DOI] [PubMed] [Google Scholar]
- Tarkka R, Pāākkö E, Pyhtinen J, Uhari M, Rantala H. Febrile seizures and mesial temporal sclerosis: no association in a long-term follow-up study. Neurol. 2003;60:215–18. doi: 10.1212/01.wnl.0000037482.55894.b1. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Todd Hare TA, Marcus DJ, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–9. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Landingham KE, Heinz ER, Cavazos JE, Lewis DV. Magnetic resonance imaging evidence of hippocampal injury after prolonged febrile seizure convulsions. Ann Neurol. 1998;43:413–26. doi: 10.1002/ana.410430403. [DOI] [PubMed] [Google Scholar]
- Verity CM, Greenwood R, Golding J. Outcome of childhood status epilepticus and lengthy febrile convulsions: findings of a national cohort study. BMJ. 1993;307:225–8. doi: 10.1136/bmj.307.6898.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 3rd edn. San Antonio, TX: The Psychological Corporation; 2002. Wechsler Preschool and Primary Scale of Intelligence. [Google Scholar]
- Yoong M, Mardari M, Martinos M, Clark C, Chong K, Neville B, et al. The role of magnetic resonance imaging in the follow-up of children with convulsive status epilepticus. Dev Med Child Neurol. 2012;54:328–33. doi: 10.1111/j.1469-8749.2011.04215.x. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci. 2011;14:656–61. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeamer A, Heuer E, Bachevalier J. Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions. J Neurosci. 2010;30:9157–65. doi: 10.1523/JNEUROSCI.0022-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]