Abstract
Lewy bodies are common in the ageing brain and often co-occur with Alzheimer’s disease pathology. There is little known regarding the independent role of Lewy body pathology in cognition impairment, decline and fluctuations in community-dwelling older persons. We examined the contribution of Lewy body pathology to dementia, global cognition, cognitive domains, cognitive decline and fluctuations in 872 autopsied subjects (mean age = 87.9 years) from the Rush Religious Order Study (n = 491) and Memory and Aging Project (n = 381) longitudinal community-based clinical–pathological studies. Dementia was based on a clinical evaluation; annual cognitive performance tests were used to create a measure of global cognition and five cognitive domains. Lewy body type was determined by using α-synuclein immunostained sections of substantia nigra, limbic and neocortical regions. Statistical models included multiple regression models for dementia and cognition and mixed effects models for decline. Cognitive fluctuations were estimated by comparing standard deviations of individual residuals from mean trajectories of decline in those with and without Lewy bodies. All models controlled for age, sex, education, Alzheimer’s disease pathology and infarcts. One hundred and fifty-seven subjects (18%) exhibited Lewy body pathology (76 neocortical-type, 54 limbic-type and 27 nigra-predominant). One hundred and three (66%) subjects with Lewy body pathology had a pathologic diagnosis of Alzheimer’s disease. Neocortical-type, but not nigral-predominant or limbic-type Lewy body pathology was related to an increased odds of dementia (odds ratio = 3.21; 95% confidence interval = 1.78–5.81) and lower cognition (P < 0.001) including episodic memory function (P < 0.001) proximate to death. Neocortical-type Lewy body pathology was also related to a faster decline in global cognition (P < 0.001), decline in all five specific cognitive domains (all P-values < 0.001), and to fluctuations in decline of working and semantic memory (P-values < 0.001). Limbic-type Lewy body pathology was related to lower and faster decline in visuospatial skills (P = 0.042). The relationship of Lewy body pathology to cognition and dementia was not modified by Alzheimer’s disease pathology. Neocortical-type Lewy body pathology is associated with increased odds of dementia; lower and more rapid decline in all cognitive domains including episodic memory and fluctuations in decline in semantic and working memory. Limbic-type Lewy body pathology is specifically associated with lower and more rapid decline in visuospatial skills. The effect of Lewy body pathology on cognition appears to be independent of Alzheimer’s disease pathology.
Keywords: Lewy body pathology, cognition, dementia, cognitive decline, fluctuations
Introduction
Lewy bodies are common in the ageing brain (Zaccai et al., 2005), frequently co-occur with Alzheimer’s disease pathology and are considered the central and pathognomonic pathology of one of the most common neurodegenerative dementias (Perry et al., 1990). Much of the current knowledge regarding the relationship between Lewy bodies and dementia and cognition is derived from clinical–pathological studies of atypical cases of dementia and specialty clinic samples (Gibb et al., 1989; Hansen et al., 1990; Hamilton et al., 2008). We are not aware of community-based clinical–pathological studies that have examined the relationship of Lewy bodies with both dementia and the different domains of cognition in older subjects.
An important issue in unravelling the relationship between Lewy bodies and dementia and cognitive phenotypes is the common coexistence of Alzheimer’s disease and other pathology. While it is generally accepted that Lewy bodies alone can be associated with a dementia syndrome (Kosaka et al., 1993), the common co-occurrence of Lewy bodies with Alzheimer’s disease pathology have led some to designate a Lewy body variant of Alzheimer’s disease pathology (Heyman et al., 1999). It has also been suggested that Alzheimer’s disease pathology must be present with Lewy bodies to induce severe cognitive impairment (Nelson et al., 2009) and that the presence of Alzheimer’s disease pathology obscures a unique Lewy body pathology cognitive phenotype (McKeith et al., 2005). For these reasons, and because mixed pathologies account for most cases of dementia in the community (Schneider et al., 2007a) a clear understanding of the separate and combined effect of Lewy bodies in older community-dwelling persons requires the simultaneous consideration of Lewy bodies in the presence of Alzheimer’s disease and other admixed pathologies.
The relationship between Lewy bodies and cognition may also be affected by the regional localization and stage of Lewy body pathology. Lewy body pathology is believed to often follow a caudal to rostral pattern of progression in older subjects (Braak et al., 2003) from the nigra (nigra-predominant) to the limbic regions (limbic-type) and finally to the neocortex (neocortical-type Lewy body pathology) (McKeith et al., 1996). While it is commonly accepted that neocortical Lewy bodies are associated with a dementia syndrome, some studies suggest that brainstem Lewy bodies may also be associated with dementia (Keage et al., 2012), and there is limited data on limbic-type Lewy body pathology and cognition.
In two community-based clinical–pathological studies, we explored the specific role of nigral-predominant, limbic-type and neocortical-type of Lewy body pathology on dementia, global and specific cognitive functions proximate to death, and rate and fluctuations of cognitive decline over multiple years prior to death, controlling for Alzheimer’s disease and cerebral infarct pathology. Clinical and pathologic data were used from 872 older subjects participating in the Religious Orders Study and the Memory Aging Project (Bennett et al., 2005, 2006)
Materials and methods
Subjects
The subjects in this study are deceased and autopsied participants from the Religious Orders Study (n = 491) and Memory and Aging Project (n = 381), longitudinal clinical–pathological studies of ageing and dementia. Participants of the Religious Orders Study are older nuns, priests and brothers from more than 40 sites across the US. Participants of the Memory and Aging project are older community-dwelling subjects from retirement communities and other housing units in the Chicago-land area. Both studies enrol older subjects without known dementia, who agree to annual clinical evaluation and brain donation at the time of death. These studies were approved by the Institutional Review Board of Rush University Medical Centre and each participant signed an informed consent and an Anatomical Gift Act. Since January 1994, 1165 people have enrolled in the Religious Orders study; and since 1997, 1515 people have enrolled in the Memory and Aging Project. Of the 2680 persons enrolled, 158 subsequently withdrew from the studies. Participation in the annual follow-up evaluations has exceeded 90% of survivors in both studies. Details of both studies have been previously reported (Bennett et al., 2005, 2006). As of December 2011, 557 participants of the Religious Orders Study have died, of whom 537 (93%) had undergone brain autopsy. As of the same time period, 531 subjects of the Memory and Aging Project have died, of whom 425 (80%) had an autopsy. Among a total of 962 autopsied subjects from both cohorts, 57 had Parkinson’s disease and were excluded from the current study and post-mortem data were available in a total of 872 consecutive deceased and autopsied participants (491 Religious Orders Study and 381 Memory and Aging Project participants), who were included in analyses. The final diagnosis of Parkinson’s disease was determined by an expert neurologist blinded to the pathologic diagnosis. The neurologist reviewed all clinical data consisting of baseline and annual medical histories (including annual questions regarding a diagnosis of Parkinson’s disease), medications and clinical examinations (including annual Unified Parkinson’s Disease Rating Scale), which are administered by trained nurses and reviewed by physicians as described previously (Buchman et al., 2012).
Clinical evaluation
Both the Religious Orders Study and the Memory and Aging Project have an identical core of uniform and structured baseline neuropsychological performance testing and annual neurologic examinations. Trained and certified research assistants performed the testing and a board-certified neuropsychologist reviewed the cognitive performance tests as previously described (Bennett et al., 2005). Participants were evaluated or clinical findings were reviewed by a neurologist or geriatrician with expertise in the evaluation of older people with and without dementia. The diagnosis of dementia and Alzheimer’s disease followed the recommendations of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA) (McKhann et al., 1984) as previously described (Bennett et al., 2006). Annual follow-up evaluations were essentially identical to baseline examinations and were performed by examiners blinded to previously collected data. After death, all clinical data were reviewed by a board-certified neurologist blinded to post-mortem data who then rendered a summary diagnostic opinion establishing the most likely clinical diagnosis proximate to death.
Neuropsychological performance testing
Both studies used a core of 19 cognitive function tests to assess a broad range of cognitive abilities. In addition, the Mini-Mental State Examination was used to describe the cohort but was not used in analyses (Wilson et al., 2002; Bennett et al., 2005, 2006). Seven tests were used to assess episodic memory: Word List Memory, Word List Recall and Word List Recognition from CERAD (Consortium to Establish a Registry for Alzheimer’s Disease); immediate and delayed recall of Story A from the Logical Memory subtest of the Wechsler Memory Scale-Revised; and immediate and delayed recall of the East Boston Story. Four tests were used to assess semantic memory: Verbal Fluency and Boston Naming Test from CERAD, subsets of items from the Extended Range Vocabulary Test and National Adult Reading Test. There were three tests of working memory: Digit Span subtests forward and backward of the Wechsler Memory Scale-Revised, and Digit Ordering. Two tests were used to assess perceptual speed: the oral version of the Symbol Digit Modalities Test and Number Comparison. Finally, we used two tests of visuospatial ability: items from Judgment of Line Orientation and Standard Progressive Matrices. Data were collected on laptop computers with forms programmed in Blaise (Central Bureau of Statistics, the Netherlands), a Pascal-based data entry program and scored in SAS [SAS Institute Inc. SAS/STAT® user guide (Version 9.2): SAS Institute Inc. 2009] on a SunUltraSparc workstation.
To reduce floor and ceiling effects and other types of measurement error, summary measures were created instead of using individual test scores. Raw scores from individual tests were converted to z-scores, using the mean and standard deviation from the baseline evaluation from all participants in both cohorts, and the z-scores were averaged to create the summary measure. Valid summary measures required valid scores on at least half of the component tests. We created summary measures of episodic memory, semantic memory, working memory, perceptual speed and visuospatial ability, and a global measure based on all tests. Studies characterizing cognitive function using this methodology have previously been reported (Wilson et al., 2002; Bennett et al., 2004; Schneider et al., 2004). Annual neuropsychologist performance tests were used for longitudinal modelling. The average number of annual neuropsychological assessments in the subjects used for this study was 7.0 (SD = 3.56; range 2–17).
Neuropathological evaluation
Brains were removed in Chicago and participating autopsy sites in a standard fashion as previously described (Schneider et al., 2009). The average post-mortem interval was 8.5 h (SD = 8.0). After weighing, each brain was cut coronally using a Plexiglas jig into 1-cm slabs. Slabs from one hemisphere and slabs from the other hemisphere not designated for rapid freezing were fixed for at least 3 days in 4% paraformaldehyde. Uniform examination for gross pathology including cerebral infarcts was conducted on slabs and/or pictures from both hemispheres, as previously described (Schneider et al., 2003). After gross examination, blocks of midfrontal, midtemporal, inferior parietal, anterior cingulate, entorhinal and hippocampal cortices, basal ganglia and midbrain were dissected from the 1-cm slabs of fixed tissue. Blocks were processed and embedded in paraffin. Sections (6 µm) were stained for haematoxylin and eosin for the assessment of general pathology including microscopic infarcts, modified Bielshowsky silver stain for assessment of Alzheimer’s disease pathology, and α-synuclein immunostain (Zymed; 1:50) for assessment of Lewy bodies. Immunohistochemistry was performed using the VECTASTAIN® ABC method with alkaline phosphatase as the colour developer. All immunohistochemical runs included a positive control. Nigral Lewy bodies were identified as round, intracytoplasmic structures with a darker halo. In the cortex, Lewy bodies were identified as round intracytoplasmic structures, often lacking any halo and with an eccentric nucleus. Only intracytoplasmic Lewy bodies were used as an indicator of positive staining. To simplify criteria for the different types of Lewy body pathology, the McKeith criteria (McKeith et al., 1996) were modified such that nigral predominant Lewy body pathology included cases with Lewy bodies in the substantia nigra without evidence of Lewy bodies in the limbic or neocortical regions. Limbic-type Lewy body disease included cases with either anterior cingulate or entorhinal positivity (typically also with nigral pathology) without neocortical Lewy body pathology. Finally neocortical-type Lewy body pathology required Lewy bodies in either midfrontal, temporal, or inferior parietal cortex with either nigral or limbic positivity, but often with both. Each case, therefore, could only be considered: 0 = no, 1 = nigral-predominant, 2 = limbic-type or 3 = neocortical-type Lewy body pathology. Each category was mutually exclusive and therefore could be considered as an indicator for each condition (see ‘Statistical analysis’ section).
Alzheimer’s disease pathology was defined by NIA-Reagan criteria (Hyman and Trojanowski, 1996) with intermediate and high likelihood cases indicating a pathologic diagnosis of Alzheimer’s disease as previously described (Schneider et al., 2007a, 2009). Manual counts of neuritic plaques, diffuse plaques and neurofibrillary tangles were used to create summary measures of Alzheimer’s disease pathology. The slides were scanned at low power and counts were performed at a total magnification of × 100. Each Alzheimer’s disease marker (e.g. neuritic plaques, diffuse plaques and neurofibrillary tangles) was counted within each region by finding the greatest density of that marker and counting within the 1 mm2 area of the graticule. A summary measure of Alzheimer’s disease pathology was constructed and used for analysis, as previously described (Bennett et al., 2003; Schneider et al., 2004). Counts were standardized by dividing by the standard deviation of the mean for all counts in that region for that particular marker. Each marker was than averaged across regions to obtain a neurofibrillary tangles score, neuritic plaques score and diffuse plaques score for each case. These scores were averaged to obtain an overall Alzheimer’s disease summary score.
Statistical analysis
Chi-square or t-statistics were used to test for unadjusted differences in demographics, Alzheimer’s disease pathology and infarcts between subjects with and without Lewy body disease. Logistic regression models were used to examine whether the odds of clinically diagnosed dementia proximate to death differ between subjects with and without Lewy body pathology. In these models, the main predictor of Lewy body pathology was coded as three indicator variables, representing neocortical-type, limbic-type and nigral-predominant Lewy body pathology separately. We added appropriate terms to account for the separate effects of Alzheimer’s disease pathology and infarcts. Interaction terms were added to test for effect modification by Alzheimer’s disease pathology. All the models were additionally adjusted for age, sex and years of education.
Next, multiple linear regression models were applied to examine the relation between Lewy body pathology and the global measure of cognitive function proximate to death, adjusting for infarcts, Alzheimer’s disease pathology, age, sex and education. This series of analyses followed the same steps as those with logistic regression. Briefly, nigral-predominant, limbic-type and neocortical-type Lewy body pathology were coded as indicator variables and the interaction terms was used to test effect modification by Alzheimer’s disease pathology. Models were repeated for the separate outcome measures of episodic memory, semantic memory, working memory, perceptual speed and visual–spatial skills.
Linear mixed models were applied to examine the association of Lewy body disease with the rate of change in cognition, adjusting for infarcts, Alzheimer’s disease pathology, age, sex and education. In these models, our outcome of interest was rate of change over time (in years since baseline), that is the slope, and the interaction between time and indictor variables for Lewy body disease. If the interaction terms were statistically significant and the associated coefficient is negative, then subjects with Lewy body disease tend to have slopes that decline faster.
To assess fluctuations we investigated the variability of longitudinal decline in cognition between subjects with and without Lewy body disease. To do so, we first characterized linear trajectories (marginal means) of cognitive decline from the linear mixed models, adjusted for Lewy body pathology, infarcts, Alzheimer’s disease pathology, age, sex and education. Individual residuals (deviations) were calculated from these mean trajectories, and the metric of fluctuation was approximated by the standard deviation of subject specific residuals. Larger SDs suggests more fluctuation from the mean trajectories. These individual standard deviations of residuals, square-rooted to account for the right skewness were compared between subjects with and without Lewy body pathology using ANOVA, and the results were subsequently confirmed by the non-parametric Kruskal–Wallis test.
All models were validated graphically and analytically. All analyses were carried out using SAS/STAT software (Version 9.2: SAS Institute Inc.) on a SunUltraSparc workstation. A nominal threshold of P < 0.05 was imposed for statistical significance throughout.
Results
Lewy bodies were present in 157 (18%) subjects (27 nigral predominant, 54 limbic type and 76 neocortical type). There was a trend for subjects with Lewy bodies to be older, but they did not differ in sex or education compared with those without pathology (Table 1). A pathological diagnosis of Alzheimer’s disease (intermediate or high likelihood Alzheimer’s disease by NIA-Reagan diagnostic criteria) was present in ∼61% of those without Lewy body pathology compared with 66% of those with Lewy body pathology. The proportion with infarcts was also similar across groups with and without Lewy body pathology.
Table 1.
Clinical pathologic characteristics in 872 subjects with and without Lewy bodies
| No Lewy bodies, n = 715 | Lewy body disease, n = 157 | P-value | |
|---|---|---|---|
| Age, in years* | 87.7 (6.4) | 88.8 (6.5) | 0.062 |
| Gender, female (n, %) | 469 (54) | 98 (11) | 0.45 |
| Education, years | 16.5 (3.6) | 16.2 (3.8) | 0.39 |
| Mini-mental state examination | 22.3 (8.5) | 17.8 (10.3) | <0.001 |
| Global cognition (summary score**) | −0.78 (1.12) | −1.31 (1.36) | <0.001 |
| Dementia, (n, %)*** | 267 (37) | 89 (57) | <0.001 |
| Interval last exam, months | 7.1 (4.4) | 7.0 (4.8) | 0.84 |
| Post-mortem interval, in hours | 8.5 (8.1) | 8.0 (7.8) | 0.43 |
| NIA-Reagan diagnosis of Alzheimer’s disease (pathological diagnosis) | 436 (61) | 103 (66) | 0.28 |
| Cerebral infarction (n, %) | 350 (49) | 73 (47) | 0.58 |
*Numbers are mean (SD), unless otherwise specified.
**See text for description of scores. ***See text for diagnostic criteria.
Lewy bodies pathology and dementia
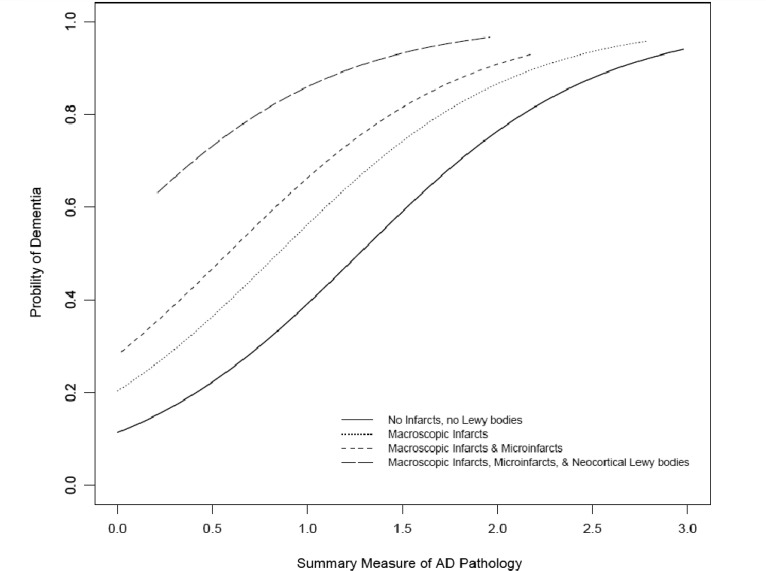
Over half of subjects with Lewy body pathology had evidence of dementia at death (53 neocortical, 26 limbic and 10 nigral) compared with a little over a third of older persons without Lewy bodies (Table 1). The increase in proportion of dementia appeared to be specific for those with neocortical Lewy body pathology; 53 of 75 persons with neocortical Lewy body pathology had dementia compared with 26 of 54 with limbic Lewy body pathology and 10 of 27 with nigral-predominant disease. To determine if this effect was independent of age and Alzheimer’s disease pathology as well as other potential confounders, a logistic regression analysis was performed controlling for age, sex and education, and terms for Alzheimer’s disease pathology and infarcts. In this model each unit of Alzheimer’s disease pathology increased the odds of dementia by ∼5-fold, whereas macroscopic infarcts increased the odds of dementia by ∼2-fold and microscopic infarcts by 1.5-fold (Table 2). Next terms for nigral-predominant, limbic-type and neocortical-type Lewy body pathology were added to the model. Neither nigral-predominant nor limbic-type Lewy body pathology increased the odds of dementia; however, the presence of neocortical-type Lewy body pathology increased the odds of dementia by >3-fold (odds ratio = 3.21; 95% confidence interval = 1.78–5.81; Table 2). The increased probability of dementia for a person with neocortical-type Lewy body disease for each level of Alzheimer’s disease pathology in addition to the additive influences of macroscopic and microscopic infarcts is illustrated in Fig. 1. The association of each type of Lewy body disease and dementia was not modified by the amount of Alzheimer’s disease pathology (P-values for interaction >0.27) suggesting an independent effect.
Table 2.
Lewy bodies and dementia
| Multiple variable regression, model predictors* | Odds of dementia* | 95% confidence interval |
|---|---|---|
| Model 1 | ||
| Alzheimer’s disease pathology | 5.27 | 3.92−7.09 |
| Macroscopic infarct pathology | 1.96 | 1.40−2.74 |
| Microinfarct pathology | 1.52 | 1.06−2.16 |
| Model 2 | ||
| Alzheimer’ disease pathology | 5.01 | 3.72−6.76 |
| Macroscopic infarct pathology | 2.01 | 1.43−2.82 |
| Microinfarct pathology | 1.55 | 1.08−2.21 |
| Lewy body disease | ||
| Nigral predominant | 1.26 | 0.48−3.28 |
| Limbic type | 1.40 | 0.73−2.66 |
| Neocortical type | 3.21 | 1.78−5.81 |
*Models 1 and 2 represent separate logistic regression models controlling for age, sex and education.
Figure 1.
Probability of dementia by increasing levels of Alzheimer’s disease (AD) pathology, showing additive effects of macroscopic infarcts, microinfarcts and neocortical Lewy bodies.
Lewy body pathology and global cognitive function
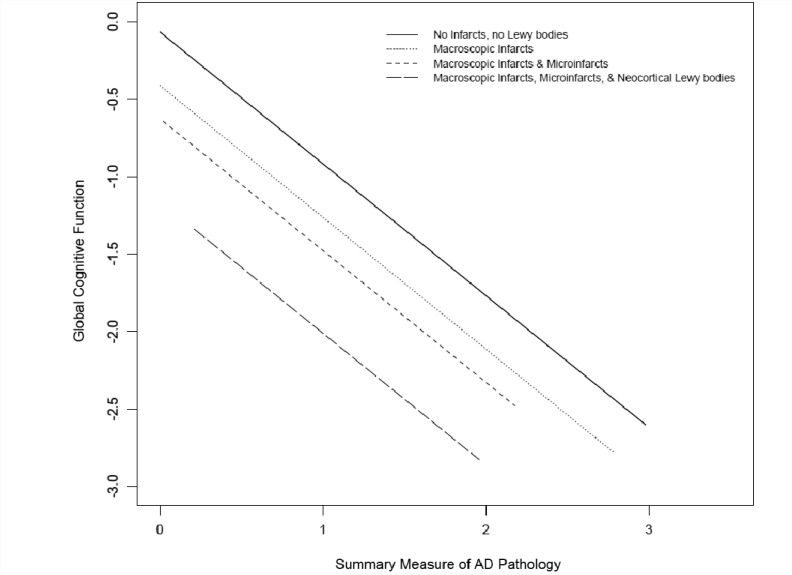
A similar set of analyses were conducted with level of global cognitive function proximate to death, rather than clinical diagnosis of dementia. In the core linear regression model, age, sex, education, one unit of Alzheimer’s disease pathology lowered cognition by 0.89 units, macroinfarct pathology lowered cognition by 0.34 units and microinfarcts lowered cognition by 0.21 units (Table 3). In the subsequent model including terms for Lewy body pathology, we found that neocortical-type Lewy body pathology was related to global cognitive summary score (reduction of 0.55 units, P < 0.001; Table 3); however, nigral-predominant and limbic-type Lewy body pathology were not related to global cognition (P-values > 0.163). The further lowering of cognitive function by neocortical-type Lewy body pathology, after accounting for Alzheimer’s disease pathology and infarcts, is shown in Fig. 2. Models with term for an interaction between Lewy body pathology and Alzheimer’s disease pathology were performed and showed no evidence of an interaction between the two pathologies (P > 0.26).
Table 3.
Lewy bodies and global cognitive function
| Multiple variable regression, model predictors* | Cognitive function* | P-value |
|---|---|---|
| Model 1 | ||
| Alzheimer’s disease pathology | −0.89 (0.06) | <0.001 |
| Macroscopic infarct pathology | −0.34 (0.07) | <0.001 |
| Microinfarct pathology | −0.21 (0.08) | 0.006 |
| Model 2 | ||
| Alzheimer’s disease pathology | −0.85 (0.06) | <0.001 |
| Macroscopic infarct pathology | −0.35 (0.07) | <0.001 |
| Microinfarct pathology | −0.22 (0.08) | 0.004 |
| Lewy body disease | ||
| Nigral predominant | −0.15 (0.19) | 0.420 |
| Limbic type | −0.19 (0.14) | 0.164 |
| Neocortical type | −0.55 (0.12) | <0.001 |
*Models 1 and 2 represent separate linear regression models controlling for age, sex and education; values are estimates (SE).
Figure 2.
Additive effects of Alzheimer’s disease (AD) pathology, macroscopic infarcts, microinfarcts and neocortical Lewy bodies on impairment in global cognition.
Lewy body pathology and different cognitive systems
Evidence from clinical studies has suggested that Lewy bodies may affect some domains of cognition more than others. In idiopathic Parkinson’s disease, where pathology is most evident in the substantia nigra, Lewy bodies have been associated with executive dysfunction (Kao et al. 2009); whereas Lewy body dementia where pathology is more diffuse has been associated with impairment of executive function (Kraybill et al., 2005; Kao et al., 2009) and visuospatial skills (Aarsland et al., 2003; Hamilton et al., 2008; Kao et al., 2009). Therefore, we next conducted a set of analyses to examine the association between nigral-predominant, limbic-type and neocortical-type Lewy body pathology and level of function proximate to death in five different cognitive abilities: episodic memory, semantic memory, working memory, perceptual speed and visuospatial ability. In multiple linear regression analyses after controlling for age, sex, education, Alzheimer’s disease pathology and infarcts, neocortical-type Lewy body pathology was related to all five domains of cognition (Table 4). In addition, limbic-type Lewy body pathology was specifically associated with a lower level of visuospatial function. No other relations were found of limbic-type or nigral-predominant Lewy body pathology with impairment in cognitive domains (all P-values >0.16). Alzheimer’s disease pathology did not modify the effect of Lewy body pathology on specific cognitive functions (all P-values for interaction >0.10).
Table 4.
Lewy bodies and cognitive domain scores
| Lewy body disease |
|||
|---|---|---|---|
| Nigral predominant | Limbic type | Neocortical type | |
| Episodic memory | −0.19 (0.22), 0.379 | −0.15 (0.16), 0.350 | −0.48 (0.14), <0.001 |
| Working memory | 0.07 (0.20), 0.713 | −0.01 (0.14), 0.923 | −0.54 (0.12), <0.001 |
| Semantic memory | −0.24 (0.24), 0.311 | −0.24 (0.17), 0.163 | −0.43 (0.15), 0.004 |
| Perceptual speed | −0.15 (0.21), 0.487 | −0.09 (0.15), 0.533 | −0.47 (0.13), <0.001 |
| Visuospatial abilities | −0.26 (0.21), 0.227 | −0.31 (0.15), 0.042 | −0.32 (0.13), 0.017 |
Values are: parameter estimates (SE) for cognitive domain scores, P-values. Model controlling for age, sex, education, Alzheimer's disease pathology, macroscopic and microinfarcts.
Lewy body pathology and cognitive decline
Some clinic-based studies (Byrne et al., 1989; Armstrong et al., 1991; Olichney et al., 1998; Galasko et al., 2000) have suggested that persons with Lewy body disease have a faster pace of cognitive decline. To examine the association of Lewy body pathology with the rate of change in cognitive function in community-dwelling older persons, linear mixed models were constructed with the repeated measures of cognition as the outcome and terms to adjust for Lewy body pathology, Alzheimer’s disease pathology, infarcts, age, sex and education. The first model included the global measure of cognition as the outcome; this was followed by models with each of the five cognitive domains. Compared to persons without Lewy bodies, persons with neocortical-type Lewy body pathology had a faster rate of cognitive decline in the measure of global cognition (P < 0.001), and all five cognitive domains (P-values <0.001 for episodic, semantic, working memory and perceptual speed and P = 0.011 for visuospatial abilities). Limbic-type Lewy body pathology was related to a faster rate of decline in visuospatial abilities (P = 0.046) and not to decline of other cognitive domains or global cognition (P-values > 0.13). There were no relations of nigral predominant Lewy body pathology with decline in any of the cognitive measures (all P-values >0.091).
Lewy bodies and variability in cognitive decline (fluctuations)
Cognitive fluctuations are among the clinical characteristics used to establish the diagnosis of dementia associated with Lewy bodies during life (McKeith et al., 1996, 2005). Fluctuations in cognitive decline were examined using the residuals from mean trajectories estimated by the linear mixed model, adjusted for Lewy body disease, Alzheimer’s disease pathology, infarcts, age, sex and education. The square-root of the standard deviations of subject specific residuals in persons with and without Lewy body pathology were compared using parametric and non-parametric one-way ANOVA. Subjects with neocortical-type Lewy body pathology (but not nigral-predominant or limbic-type Lewy body pathology) had more variability across mean decline in global cognition compared with subjects without Lewy body pathology after adjusting for age, sex, education, Alzheimer’s disease pathology and infarct pathology [F(3,794) = 7.96, P < 0.001]. Analyses, specifically investigated variability of decline separately across each cognitive domain, showed that persons with neocortical-type Lewy body pathology had more variability in performance compared to those without Lewy body pathology specifically in semantic memory and working memory (both P-values < 0.001) but not in the other cognitive domains (P-values > 0.10).
Discussion
In this prospective clinical–pathological study of nearly 900 older community-dwelling older persons we found that Lewy body pathology is common and deleterious. Region was a key factor in the relation of Lewy body pathology with cognition and dementia. Neocortical-type but not limbic-type or nigral-predominant Lewy body pathology was related to dementia, overall cognition and five cognitive domains, including episodic memory, the clinical hallmark of Alzheimer’s disease. Neocortical-type Lewy body pathology was also related to global decline, and annual cognitive fluctuations which were most pronounced in working and semantic memory. Limbic-type Lewy body pathology was related to lower and more rapid decline of visual–spatial skills, whereas nigral-predominant Lewy body pathology was not independently related to any specific cognitive impairments. Alzheimer’s disease pathology had a separate and additive effect with Lewy body pathology and did not modify the clinical expression of Lewy body pathology.
There are relatively few studies of Lewy body pathology in community-based cohorts (White et al., 2005; Sonnen et al., 2007; Zaccai et al., 2008; Matthews et al., 2009; O’Brien et al., 2009; Keage et al., 2012). The proportion of older persons with Lewy body pathology in the current study (18%) was within the range (11.2–25%) reported by most of the other community-based studies (White et al., 2005; Sonnen et al., 2007; O’Brien et al., 2009), though at least one study reported < 10% (Matthews et al., 2009) and another > 35% with Lewy body pathology (Zaccai et al., 2008). Both the extent of sampling and recent improvements in staining methods for Lewy body pathology likely play a role in these disparate findings (Matthews et al., 2009). Α-synuclein immunohistochemistry is sensitive for Lewy body pathology, but is a relatively recent technique and thus has not been used uniformly across all studies (Matthews et al., 2009; Keage et al., 2012). Selection biases in enrolment and autopsy may also play a role. Lewy body pathology may be associated with atypical clinical phenotypes which may bias participation in clinical studies or agreement for autopsy (Bower, et al., 2002).
There has been controversy on the link between Lewy body pathology and dementia in older subjects (Parkkinen et al., 2005a). Most (White et al., 2005; Sonnen et al., 2007), but not all (Zaccai et al., 2008), community-based studies have demonstrated a strong link between Lewy body pathology and dementia). Previous studies have focused on the role of neocortical Lewy body disease and dementia and few have systematically studied nigral predominant or limbic-type Lewy body disease and dementia. In one study neocortical but not nigral Lewy body disease were independently related to dementia, but limbic-type Lewy body disease was not explored (Sonnen et al., 2007). By contrast, a recent study suggested a specific role for nigral Lewy body in dementia (Keage et al., 2012), though cortical Lewy bodies were not simultaneously studied. Our data indicate that Lewy body pathology is common in the ageing brain; and that neocortical but not nigral or limbic Lewy bodies are strongly related to cognitive ageing and dementia.
It is recognized that Alzheimer’s disease pathology commonly co-occurs with Lewy body pathology in the brains of older persons. In this study, over half of persons with or without Lewy body pathology had a pathologic diagnosis of Alzheimer’s disease (66% and 61%, respectively). This overall proportion of older persons with a pathologic diagnosis of Alzheimer’s disease was very similar to other community-based studies (Snowdon et al. 1997; MRC-CFAS, 2001). We found that neocortical-type Lewy body pathology has an additive rather than synergistic effect on the lowering of cognition in the presence of Alzheimer’s disease pathology. This is consistent with some (Samuel et al., 1996) but not all previous studies (Stern et al., 2001; Walker et al., 2012). In one study, subjects with Lewy body pathology needed less Alzheimer’s disease pathology to reach the same level of cognitive impairment (Samuel et al., 1996). However, other studies found no relationship between the presence of Lewy body pathology and clinical phenotype (Stern et al., 2001) or rapidity of cognitive decline (Stern et al., 2001; Walker et al., 2012). The null findings in these latter studies may be attributable to restricting inclusion to subjects with clinically probable Alzheimer’s disease (Stern et al., 2001), clinically probable Alzheimer’s disease and dementia with Lewy bodies (Walker et al., 2012), or shorter follow-up times (Walker et al., 2012). Our data support the contention that neocortical-type Lewy body pathology lowers both the level and increases the pace of cognitive function in persons with Alzheimer’s disease pathology. The additional effect of Lewy body pathology appears to be highly deleterious, lowering global cognition by a full standard deviation and increasing the odds of dementia by > 3-fold. Yet, this Lewy body pathology effect does not appear to be modified by the presence of Alzheimer’s disease pathology. Alzheimer’s disease and Lewy body pathology are both independently related to cognition and Lewy body pathology deleteriously affects many aspects of cognition in older persons. By contrast, a previous study suggested that Alzheimer’s disease pathology was necessary for Lewy body pathology to cause severe cognitive impairment (Nelson et al., 1999). The authors reported on 1500 autopsied subjects, derived from 31 Alzheimer’s disease centres, many recruited from clinics rather than communities and consequently with more severe cognitive impairment proximate to death compared to the current study. Further longitudinal clinical–pathological study in community subjects will be needed to clarify the separate and combined roles of Lewy body and Alzheimer’s disease pathology.
Overall, these studies show Lewy body pathology, particularly neocortical-type Lewy body pathology, affects a broad range of cognitive skills, including episodic memory. Previous studies have shown that macroscopic and microscopic infarcts also have separate effects on episodic memory (Arvanitakis et al., 2011). Together, these data show that impaired episodic memory in older persons is not a specific indicator of Alzheimer’s disease pathology. While Alzheimer’s disease pathology certainly has a profound effect on episodic memory, both Lewy body as well as infarct pathology (Schneider et al. 2004, 2007b; Arvanitakis et al., 2011) also lower episodic memory in older persons. While co-existing Alzheimer’s disease pathology may obscure the diagnostic clinical profile of Lewy body pathology, e.g. dementia with Lewy body (McKeith et al. 2005); the current data suggest that the clinical expression of Lewy body pathology can overlap with that of Alzheimer’s disease pathology by directly affecting episodic memory, the clinical hallmark of Alzheimer’s disease pathology. In spite of this apparent association with episodic memory, in a post-mortem neuroimaging study using these cohorts, hippocampal atrophy was specifically related to Alzheimer’s disease but not Lewy body pathology (Dawe et al., 2011). Further investigation of the neuroimaging characteristics of Lewy body pathology with larger numbers using ante or post-mortem neuroimaging will be important.
The diffuse deposition of Lewy body pathology in the brain has been associated with cognitive impairment marked by hallucinations, parkinsonism and fluctuations (Perry et al., 1990; McKeith et al., 1996; 2005; Ferman and Boeve, 2007), i.e. dementia with Lewy bodies. In previous studies we showed a relationship between nigral Lewy bodies and parkinsonism in these cohorts (Buchman et al., 2011). To study cognitive fluctuations we investigated variability of decline in annual performance in persons with and without Lewy body pathology. Compared with subjects without Lewy body pathology, patients with neocortical-type Lewy body pathology exhibited more evidence of variability in cognitive performance, specifically semantic and working memory, but not episodic memory. Semantic memory refers to acquired knowledge of general concepts and meanings, whereas working memory refers to the ability to temporarily store, manipulate, and process information in time and space. Thus, subjects with Lewy body pathology may show more variation, i.e. fluctuations, in their ability to access knowledge of fundamental concepts and meanings and to process and manipulate new information. Interestingly, deficits in attention and fluency have been specifically cited as early neurocognitive changes associated with Lewy body pathology (Hansen 1990; Kao 2009).
Clinic-based studies suggest a specific pattern of Lewy body-related cognitive profiles including prominent deficits in attention, fluency and visuospatial skills (Hansen 1990; Kao 2009). These profiles and patterns have not been well studied in community-based cohorts. Data from the current study suggests that in community-dwelling older persons neocortical Lewy bodies are associated with a wide spectrum of cognitive impairments and prominent annual fluctuations in semantic and working memory. Limbic Lewy body disease was specifically and only related to lower of visuospatial skills, but not to other domains of cognition, suggesting that these might mark the one of the earliest stages of cognitive impairment marking Lewy body pathology. This is consistent with clinical samples, where cortical Lewy bodies have been associated with early deficits in visuospatial skills (Hansen 1990; Kao 2009). The timing and profile of cognitive impairment in the early progression of Lewy body disease will be important to further delineate in future studies. Clinic-based studies have also shown that cortical Lewy body pathology is associated with more rapid decline in cognition (Byrne et al., 1989; Armstrong et al., 1991; Stern et al., 1994; Olichney et al., 1998; Galasko et al., 2000); however, this has not been noted in all studies (Ballard et al., 2001; Stern et al., 2001; Helmes et al., 2003; Hanyu et al., 2009; Walker et al., 2012). In an investigation in a community-based autopsy sample of dementia more rapid decline was found in those with Lewy bodies and coexisting Alzheimer’s disease pathology (Kraybill et al., 2005). Our current study shows that there is a faster rate of decline in all cognitive domains and global cognition in persons with neocortical-type Lewy body pathology independent of Alzheimer’s disease and other age-related pathologies. This acceleration in cognitive decline may be an important biomarker of Lewy body pathology. Characterizing the clinical timing, profile and whether there is a change point associated with the onset of this acceleration will be important to investigate in future studies.
There are several limitations to this study. First, these community-based studies do not currently collect data on hallucinations or (hourly or daily) fluctuations, diagnostic hallmarks of dementia with Lewy body. Instead, variation across annual mean decline was used to assess fluctuations. While this may not capture the hourly and daily fluctuations found in dementia with Lewy bodies, subjects with Lewy body pathology had greater variation in annual decline supporting this measure as surrogate marker of fluctuations. Second, neuropsychological testing included five tests for executive functions (working memory and perceptual speed) but did not include a more comprehensive panel of tests for executive functions, e.g. mental flexibility, verbal reasoning and inhibition, which may also be vulnerable to Lewy body pathology. Further investigations of Lewy body pathology and the spectrum of executive function are warranted. Third, the current study measured the presence and absence of the three subtypes of Lewy body pathology but did not attempt to quantify the number of Lewy bodies which may be related to level of cognitive function (Samuel et al., 1996). Future work investigating quantitative measures may be informative. Finally, cohorts that agree to annual evaluations and autopsy at the end of life may not be representative of the population.
There are also many strengths to this study. Older persons with and without dementia were prospectively examined using structured and detailed clinical assessments yielding data on different cognitive domains. The relatively low withdrawal rates along with high autopsy rates lessen potential biases and increases generalizability. All clinical examiners were blinded to previous evaluations and the results of brain autopsy. All brain sections were evaluated with α-synuclein antibodies, whether or not substantia nigra Lewy bodies were present on haematoxylin and eosin, and analyses included the examination of separate effects of nigral, limbic and neocortical Lewy body disease. Finally, neuropathological evaluation was performed blinded to the clinical data, reducing the potential for bias.
Similar to other age-related brain pathologies, Lewy body pathology is commonly present in older non-demented community-dwelling persons. This is particularly true for nigral-predominant and limbic-types of pathology, and also, albeit less commonly, in those with neocortical disease. Indeed, > 25% of subjects with neocortical Lewy body pathology did not exhibit dementia in this cohort. This may suggest that there are currently unrecognized factors associated with Lewy body pathology responsible for dementia (Parkkinen et al., 2005b), or factors associated with resilience. In practice, the clinical manifestations of dementia from Lewy body pathology probably depend on multiple factors, including but limited to the severity and stage of the other two most common pathologies of ageing: Alzheimer’s disease and infarcts. Other factors including environmental, genetic and yet unknown pathologic factors may also play a role. It will be important to define these factors as well as factors that promote the progression from nigral to limbic to neocortical regions. Indeed, therapies aimed at promoting resilience, attenuating toxicity or preventing the progression of Lewy body pathology are likely to have a large impact on risk of dementia from Lewy body pathology.
Funding
This study was supported by National Institute on Aging grants P30AG10161, R01AG15819, R01AG17917 and R01AG34374.
Acknowledgements
The authors thank the participants in the Religious Orders Study and the Memory and Aging Project, and the staff of the Rush Alzheimer’s Disease Centre.
Glossary
Abbreviation
- CERAD
Consortium to Establish a Registry for Alzheimer’s Disease
References
- Aarsland D, Litvan I, Salmon D, Galasko D, Wentzel-Larsen T, Larsen JP. Performance on the dementia rating scale in Parkinson's disease with dementia and dementia with Lewy bodies: comparison with progressive supranuclear palsy and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:1215–20. doi: 10.1136/jnnp.74.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong TP, Hansen LA, Salmon DP, Masliah E, Pay M, Kunin JM, et al. Rapidly progressive dementia in a patient with the Lewy body variant of Alzheimer’s disease. Neurology. 1991;41:1178–80. doi: 10.1212/wnl.41.8.1178. [DOI] [PubMed] [Google Scholar]
- Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42:722–7. doi: 10.1161/STROKEAHA.110.595082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C, O'Brien J, Morris CM, Barber R, Swann A, Neill D, et al. The progression of cognitive impairment in dementia with Lewy bodies, vascular dementia and Alzheimer's disease. Int J Geriatr Psychiatry. 2001;16:499–503. doi: 10.1002/gps.381. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Aggarwal NT, Arnold SE, et al. Apolipoprotein E e4 allele, AD pathology, and the clinical expression of Alzheimer’s disease. Neurology. 2003;60:246–52. doi: 10.1212/01.wnl.0000042478.08543.f7. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Relation of amyloid load and neurofibrillary tangles to AD and level of cognitive function in older persons with and without dementia. Arch Neurol. 2004;61:378–84. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–75. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah R, Kelly JF, et al. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–76. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- Bower JH, Dickson DW, Taylor L, Maraganore DM, Rocca WA. Clinical correlates of the pathology underlying parkinsonism: a population perspective. Mov Disord. 2002;17:910–6. doi: 10.1002/mds.10202. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–78. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Shulman JM, Nag S, Leurgans SE, Arnold SE, Morris MC, et al. Nigral pathology and parkinsonian signs in elders without Parkinson disease. Ann Neurol. 2012;71:258–66. doi: 10.1002/ana.22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne EJ, Lennox G, Lowe J, Godwin-Austen RB. Diffuse Lewy body disease: clinical features in 15 cases. J Neurol Neurosurg Psychiatry. 1989;52:709–17. doi: 10.1136/jnnp.52.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe RJ, Bennett DA, Schneider JA, Arfanakis K. Neuropathologic correlates of hippocampal atrophy in the elderly: a clinical, pathologic, postmortem MRI study. PLoS One. 2011;6:e26–286. doi: 10.1371/journal.pone.0026286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferman TJ, Boeve BF. Dementia with Lewy Bodies. Neurol Clin. 2007;25:741–60. doi: 10.1016/j.ncl.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasko DR, Gould RL, Abramson IS, Salmon DP. Measuring cognitive change in a cohort of patients with Alzheimer’s disease. Stat Med. 2000;19:1421–32. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1421::aid-sim434>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Luthert PJ, Janota I, Lantos PL. Cortical Lewy body dementia: clinical features and classification. J Neurol Neurosurg Psychiatry. 1989;52:185–92. doi: 10.1136/jnnp.52.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JM, Salmon DP, Galasko D, Raman R, Emond J, Hansen LA, et al. Visuospatial deficits predict rate of cognitive decline in autopsy-verified dementia with Lewy bodies. Neuropsychology. 2008;22:729–37. doi: 10.1037/a0012949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L, Salmon D, Galasko D, Masliah E, Katzman R, DeTeresa R, et al. The Lewy body variant of Alzheimer's disease: a clinical and pathologic entity. Neurology. 1990;40:1–8. doi: 10.1212/wnl.40.1.1. [DOI] [PubMed] [Google Scholar]
- Hanyu H, Sato T, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Differences in clinical course between dementia with Lewy bodies and Alzheimer’s disease. Eur J Neurol. 2009;16:212–7. doi: 10.1111/j.1468-1331.2008.02388.x. [DOI] [PubMed] [Google Scholar]
- Helmes E, Bowler JV, Merskey H, Munoz DG, Hachinski VC. Rates of cognitive decline in Alzheimer's disease and dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2003;15:67–71. doi: 10.1159/000067969. [DOI] [PubMed] [Google Scholar]
- Heyman A, Fillenbaum GG, Gearing M, Mirra SS, Welsh-Bohmer KA, Peterson B, et al. Comparison of Lewy body variant of Alzheimer’s disease with pure Alzheimer’s disease: consortium to Establish a Registry for Alzheimer's Disease, Part XIX. Neurology. 1999;52:1839–44. doi: 10.1212/wnl.52.9.1839. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan InstituteWorking Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–7. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Kao AW, Racine CA, Quitania LC, Kramer JH, Christine CW, Miller BL. Cognitive and Neuropsychiatric Profile of the Synucleinopathies. Alzheimer Dis Assoc Disord. 2009;23:365–70. doi: 10.1097/WAD.0b013e3181b5065d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keage HA, Ince PG, Matthews FE, Wharton SB, McKeith IG, Brayne C. MRC CFAS and CC75C. Impact of less common and “disregarded” neurodegenerative pathologies on dementia burden in a population-based cohort. J Alzheimers Dis. 2012;28:485–93. doi: 10.3233/JAD-2011-111268. [DOI] [PubMed] [Google Scholar]
- Kosaka K. Dementia and neuropathology in Lewy body disease. Adv Neurol. 1993;60:456–63. [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–24. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachmann D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of AD. Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on AD. Neurology. 1984;34:939. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Kraybill JL, Larson EB, Tsuang DW, Teri L, McCormick WC, Bown JD, et al. Cognitive Differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology. 2005;64:2069–73. doi: 10.1212/01.WNL.0000165987.89198.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P. Epidemiological pathology of dementia: attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000180. e1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study. (MRC CFAS) Lancet. 2001;357:169–75. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Kryscio RJ, Jicha GA, Abner EL, Schmitt FA, Xu LO, et al. Relative preservation of MMSE scores in autopsy-proven dementia with Lewy bodies. Neurology. 2009;73:1127–33. doi: 10.1212/WNL.0b013e3181bacf9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RJ, Resnick SM, Zonderman AB, Ferrucci L, Crain BJ, Pletnikova O, et al. Neuropathologic studies of the Baltimore Longitudinal Study of Aging (BLSA) J Alzheimers Dis. 2009;18:665–75. doi: 10.3233/JAD-2009-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Galasko D, Salmon DP, Hofstetter CR, Hansen LA, Katzman R, et al. Cognitive decline is faster in Lewy body variant than in Alzheimer's disease. Neurology. 1998;51:351–7. doi: 10.1212/wnl.51.2.351. [DOI] [PubMed] [Google Scholar]
- Parkkinen L, Kauppinen T, Pirttilä T, Autere JM, Alafuzoff I. Alpha-synuclein pathology does not predict extrapyramidal symptoms or dementia. Ann Neurol. 2005a;57:82–91. doi: 10.1002/ana.20321. [DOI] [PubMed] [Google Scholar]
- Parkkinen L, Pirttilä T, Tervahauta M, Alafuzoff I. Widespread and abundant alpha-synuclein pathology in a neurologically unimpaired subject. Neuropathology. 2005b;25:304–14. doi: 10.1111/j.1440-1789.2005.00644.x. [DOI] [PubMed] [Google Scholar]
- Perry RH, Irving D, Tomlinson BE. Lewy body prevalence in the aging brain: relationship to neuropsychiatric disorders, Alzheimer-type pathology and catecholaminergic nuclei. J Neurol Sci. 1990;100:223–33. doi: 10.1016/0022-510x(90)90037-n. [DOI] [PubMed] [Google Scholar]
- Samuel W, Galasko D, Masliah E, Hansen LA. Neocortical lewy body counts correlate with dementia in the Lewy body variant of Alzheimer's disease. J Neuropathol Exp Neurol. 1996;55:44–52. doi: 10.1097/00005072-199601000-00005. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Wilson RS, Cochran EJ, Bienias JL, Evans DA, Bennett DA. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60:1082–9. doi: 10.1212/01.wnl.0000055863.87435.b2. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer's disease pathology. Neurology. 2004;62:1148–56. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007a;69:2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA. Subcortical infarcts, Alzheimer’s disease pathology, and memory function in older persons. Ann Neurol. 2007b;62:59–66. doi: 10.1002/ana.21142. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. Mixed pathologies in probable Alzheimer’s disease and mild cognitive impairment. Ann Neurol. 2009;66:200–8. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–7. [PubMed] [Google Scholar]
- Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–13. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- Stern Y, Jacobs D, Goldman J, Gomez-Tortosa E, Hyman BT, Liu Y, et al. An investigation of clinical correlates of Lewy bodies in autopsy-proven Alzheimer disease. Arch Neurol. 2001;58:460–5. doi: 10.1001/archneur.58.3.460. [DOI] [PubMed] [Google Scholar]
- Walker Z, McKeith I, Rodda J, Qassem T, Tatsch K, Booij J, et al. Comparison of cognitive decline between dementia with Lewy bodies and Alzheimer's disease: a cohort study. BMJ Open. 2012;2:e000380. doi: 10.1136/bmjopen-2011-000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White L, Small BJ, Petrovitch H, Ross W, Masaki K, Abbott RD, et al. Recent clinical-pathologic research on the causes of dementia in later life: update from the Honolulu-Asia Aging Study. J Geriatr Psychiatry Neurol. 2005;18:224–7. doi: 10.1177/0891988705281872. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002a;17:179–93. [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Mendes de Leon C, Aggarwal NT, Schneider JA, Bach J, et al. Depressive symptoms, Cognitive decline, and risk of Alzheimer’s disease in older persons. Neurology. 2002b;59:364–370. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- Zaccai J, McCracken C, Brayne C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing. 2005;34:561–6. doi: 10.1093/ageing/afi190. [DOI] [PubMed] [Google Scholar]