Abstract
Benign familial neonatal epilepsy is a neuronal channelopathy most commonly caused by mutations in KCNQ2, which encodes the Kv7.2 subunit of the slow K+ channel. Kv7.2 is expressed in both central and peripheral nervous systems. Seizures occur in the neonatal period, often in clusters within the first few days of life, and usually remit by 12 months of age. The mechanism of involvement of Kv7.2 mutations in the process of seizure generation has not been established in vivo. In peripheral axons, Kv7.2 contributes to the nodal slow K+ current. The present study aimed to determine whether axonal excitability studies could detect changes in peripheral nerve function related to dysfunction or loss of slow potassium channel activity. Nerve excitability studies were performed on eight adults with KCNQ2 mutations and a history of benign familial neonatal epilepsy, now in remission. Studies detected distinctive changes in peripheral nerve, indicating a reduction in slow K+ current. Specifically, accommodation to long-lasting depolarizing currents was reduced in mutation carriers by 24% compared with normal controls, and the threshold undershoot after 100 ms depolarizing currents was reduced by 22%. Additional changes in excitability included a reduction in the relative refractory period, an increase in superexcitability and a tendency towards reduced sub-excitability. Modelling of the nerve excitability changes suggested that peripheral nerve hyperexcitability may have been ameliorated by upregulation of other potassium channels. We conclude that subclinical dysfunction of Kv7.2 in peripheral axons can be reliably detected non-invasively in adulthood. Related alterations in neuronal excitability may contribute to epilepsy associated with KCNQ2 mutations.
Keywords: epilepsy, channelopathy, nerve excitability, neuromyotonia, potassium channel
Introduction
Patients with benign familial neonatal epilepsy usually develop seizures within the first few weeks of life, although they typically remit by the age of 12 months, thereby obviating the need for long-term anti-convulsant treatment. A proportion of patients (estimated at 15%) may continue to have seizures beyond the first year of life, reflecting sustained susceptibility to aberrant neuronal excitability (Plouin et al., 2005; Reid et al., 2009; Berg et al., 2010; Bellini et al., 2011). Most patients with benign familial neonatal epilepsy carry heterozygous mutations in the KCNQ2 gene, which encodes the Kv7.2 subunit of low-threshold slowly activating K+ channels (Bievert et al., 1998; Singh et al., 1998, 2003). Less commonly, benign familial neonatal epilepsy is due to mutations in the KCNQ3 gene, which encodes the Kv7.3 subunit that co-assembles with Kv7.2 (Charlier et al., 1998; Dedek et al., 2003).
Kv7.2 is expressed in both the central nervous system (CNS) and peripheral nervous system (PNS) and is highly expressed at the nodes of Ranvier of peripheral myelinated nerve (Schwarz et al., 1995). Kv7.2-containing channels activate slowly upon depolarization and tend not to inactivate. Given that they remain open during prolonged depolarization, these channels serve to stabilize the nerve membrane and prevent repetitive firing, an effect sometimes referred to as a ‘neuronal brake’ (Maljevic et al., 2008). Blockade of slow K+ channels using tetraethylammonium or XE991 has shown that loss of slow K+ channel activity in peripheral nerve increases axonal excitability (Kocsis et al., 1983; Baker et al., 1987; Schwarz et al., 2006) and predisposes to repetitive discharges, particularly when fast K+ channels are blocked by 4-aminopyridine (Eng et al., 1988). Although some rare KCNQ2 mutations that affect channel activation kinetics are associated with myokymia (Dedek et al., 2001), most individuals with benign familial neonatal epilepsy do not have symptoms attributable to altered peripheral nerve excitability.
The aim of this study was to test whether a specific channel dysfunction can be detected in subjects with benign familial neonatal epilepsy in remission using nerve excitability studies, despite an absence of signs and symptoms of peripheral nerve dysfunction. This study reports the clinical, genetic and nerve excitability studies on eight subjects from three families with KCNQ2 mutations and a history of benign familial neonatal epilepsy, now in remission, and provides insight into the effects of the mutation in vivo.
Materials and methods
Ethical approval was obtained for the studies from the Human Research Ethics Committees of the University of Sydney, Australia. All subjects provided written informed consent to participate and were recruited from a database held at the University of Melbourne Epilepsy Research Centre. Individuals were eligible for inclusion if they were over the age of 18, with a history of benign familial neonatal epilepsy and genetic confirmation of a KCNQ2 mutation. Eight mutation-positive subjects from three families were recruited.
Peripheral nerve excitability studies were performed on the median nerve using the Trond protocol (Kiernan et al., 2000), which is summarized below. Results were compared with measurements from 30 healthy volunteers previously recorded by the same operator (S.T.) at the Institute of Neurology, University College London [benign familial neonatal epilepsy subjects: 33.9 ± 9.5 years (mean ± SD), controls: 39.1 ± 13.2 years].
Statistical analysis was performed using unpaired t-tests. Student’s t-test is sensitive to the assumptions of normality and equality of variance when sample values are small. However, of the 18 excitability variables in Table 1, only one failed the Lilliefors test of normality, which is no more than expected by chance, and for this variable, TEh40 (90–100 ms) in Table 1, the non-parametric Mann–Whitney U-test indicated lack of statistical significance (P > 0.05). Three of the 20 variables exhibited a significantly higher variance in the patient group (i.e. stimulus for 50% compound muscle action potential, rheobase and hyperpolarizing current-voltage (I/V) slope in Table 1), but these variables were not significantly different between the two groups, whether Student’s t-test, the Welch test for unequal variances or Mann-Whitney U-test was employed. A glossary of terms used in this study is available in the online Supplementary material.
Table 1.
Nerve excitability measurements
| Variable | Controls | BFNE | t (df = 36) | P |
|---|---|---|---|---|
| Mean ± SE (n = 30) | Mean ± SE (n = 8) | |||
| Stimulus response and strength–duration properties | ||||
| Stimulus for 50% Compound muscle action potential (CMAP) (mA) | 4.29 ×/÷ 1.04† | 4.47 ×/÷ 1.14† | 0.44 | 0.66 |
| Strength–duration time constant (ms) | 0.481 ± 0.018 | 0.493 ± 0.047 | 0.29 | 0.77 |
| Rheobase (mA) | 2.80 ×/÷ 1.04† | 2.94 ×/÷ 1.14† | 0.51 | 0.88 |
| Threshold electrotonus (%) | ||||
| TEd40(10–20 ms) | 68.69 ± 0.74 | 69.33 ± 0.96 | 0.41 | 0.64 |
| TEd40(40–60 ms) | 50.66 ± 0.67 | 55.98 ± 1.25 | 3.68 | 0.00085*** |
| TEd40(90–100 ms) | 43.2 ± 0.66 | 50.09 ± 1.36 | 4.20 | 0.00021*** |
| TEh40(90–100 ms) | −114.5 ± 3.5 | −127.0 ± 6.5 | 1.83 | 0.075 |
| TEd40 undershoot | −18.78 ± 0.60 | −14.65 ± 1.55 | 2.93 | 0.0058** |
| TEh40 overshoot | 14.06 ± 0.60 | 10.18 ± 1.75 | 2.67 | 0.011* |
| S2 accommodation | 24.21 ± 0.53 | 18.61 ± 1.30 | 4.60 | 0.000073**** |
| Accommodation half time (ms) | 40.1 ± 0.78 | 38.8 ± 1.15 | 0.82 | 0.42 |
| Current–voltage relationship | ||||
| Depolarizing I/V slope | 1.317 ± 0.026 | 1.145 ± 0.030 | 3.27 | 0.0025** |
| Resting I/V slope | 0.607 ± 0.014 | 0.561 ± 0.033 | 1.43 | 0.16 |
| Minimum I/V slope | 0.246 ± 0.008 | 0.228 ± 0.018 | 0.99 | 0.33 |
| Hyperpolarizing I/V slope | 0.341 ± 0.011 | 0.355 ± 0.037 | 0.49 | 0.63 |
| Recovery cycle | ||||
| Relative refractory period (ms) | 2.95 ×/÷ 1.02† | 2.68 ×/÷ 1.05† | 2.14 | 0.038* |
| Superexcitability (%) | −23.05 ± 0.93 | −27.83 ± 1.03 | 2.54 | 0.015* |
| Sub-excitability (%) | 14.40 ± 0.66 | 12.84 ± 1.15 | 1.12 | 0.27 |
†These values expressed as geometric mean and geometric SE, since they were log converted to normalize before applying t-test; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. BFNE = benign familial neonatal epilepsy; CMAP = compound muscle action potential; TEd = depolarizing threshold electrotonus; TEh = hyperpolarizing threshold electrotonus.
Nerve excitability studies
The non-invasive nerve excitability tests are similar in application to the nerve conduction studies routinely used in clinical practice, but differing in the information obtained. Each study was well tolerated and took ∼15 min to complete. The median nerve was stimulated at the wrist, with the anode placed 10 cm more proximally over muscle, off the course of the median nerve. The compound muscle action potential was recorded over the abductor pollicis brevis. The reference electrode was placed distally near the interphalangeal joint of the thumb. In all experiments, temperature was monitored at the stimulus site and was kept at ∼33°C (mean ± SEM: controls 33.2 ± 0.2°C; mutation carriers 33.1 ± 0.2°C).
Stimuli were delivered from a laptop computer running the QtracS program (©UCL Institute of Neurology). They were converted to voltage waveforms by a data acquisition board (National Instruments; NI USB 6221) and then to currents by a DS5-isolated linear bipolar constant-current stimulator (Digitimer). The Trond protocol uses the principle of ‘threshold tracking’, which involves determining the effects of conditioning stimuli on the threshold current required to produce a target response. The target response was set to 40% of the maximal compound muscle action potential, determined by first recording a stimulus–response function. Nerve excitability was then tracked by making continuous automated trial and error adjustments to the stimulus current to keep the amplitude of the target response constant, using the slope of the stimulus–response function to optimize the stimulus adjustments. The Trond protocol measures nerve excitability in the following ways.
Strength–duration properties
As stimulus duration increases, a smaller current is required to produce a compound muscle action potential of constant size. Threshold current measurements were made for stimuli lasting 0.2, 0.4, 0.6, 0.8 and 1.0 ms. Rheobase and strength duration time constant (τsd) were then calculated from a plot of stimulus charge against stimulus duration. Strength–duration measurements reflect properties of the nodes of Ranvier (Mogyoros et al., 2000), particularly persistent Na+ currents (Bostock and Rothwell, 1997).
Threshold electrotonus
During threshold electrotonus, subthreshold conditioning currents of pre-set intensities were applied for 100 ms, and the change in threshold was tested at different time points during and up to 100 ms after the 100-ms conditioning current. The conditioning currents were applied in both hyperpolarizing (TEh) and depolarizing (TEd) directions and were set at strengths of ±20 and ±40% of the unconditioned threshold current, which was monitored continuously. Threshold electrotonus provides information regarding the state of membrane polarization and ion channel activity, including nodal Na+ channels, fast and slow K+ channels and hyperpolarization-activated, cyclic nucleotide-gated channels (Ih current).
Current/threshold relationship
The current/threshold relationship measured the change in threshold 200 ms into a conditioning current lasting 220 ms, and, thereby, assessed the rectifying currents responsible for the accommodation to the polarizing current. The prolonged hyperpolarizing currents evoked inward rectification due to the activation of Ih. Depolarizing currents evoked outward rectification due to outward K+ currents (Trevillion et al., 2007). In both cases, greater accommodation would provide an increase in the slope of the curve (analogous to conductance).
Recovery cycle
The recovery cycle was measured by giving a supramaximal conditioning stimulus and tracking the change in threshold at time points up to 200 ms afterwards. Recovery cycles comprise the relative refractory period (reflecting recovery of nodal Na+ channels from inactivation), superexcitable period (due to the depolarizing afterpotential, which is affected by the activity of fast K+ channels) and the late sub-excitable period (reflecting hyperpolarization due to slow K+ channels activated by the conditioning stimulus and whose activity outlasts the depolarizing afterpotential).
Mathematical modelling
The ‘Bostock’ mathematical model of the human motor axon, recently described in detail by Howells et al. (2012), was used to assess the likely biophysical basis of the altered nerve excitability properties in subjects with benign familial neonatal epilepsy. This two-compartment (node + internode) model is incorporated in the MEMFIT function of the QtracP (©UCL Institute of Neurology) data analysis program (Bostock, 2006). MEMFIT uses an iterative procedure to minimize the ‘discrepancy’ (normalized least squares difference) between simulated excitability measures and all the recorded excitability measurements of the Trond protocol, by adjusting single parameters or combinations of parameters of the model (Kiernan et al., 2005a; Farrar et al., 2011).
Results
Patient phenotype and genotype
Clinical and genetic information for the eight mutation carriers is summarized in Table 2 (Biervert et al., 1998; Richards et al., 2004; Heron et al., 2007). Family 1 carried a heterozygous point mutation c.1910T>G resulting in the Leu637Arg mutation that lies in the cytoplasmic C-terminal domain of the channel. Previous in vitro studies of the mutant channel demonstrated an increase in calmodulin binding to the C-terminus by ∼5-fold, probably due to a conformational change in the protein (Richards et al., 2004).
Table 2.
Clinical and genetic features of patients with KCNQ2 mutations
| Family, mutation | Sex; age (years) | Onset of seizures | Phenotype | Medication | Offset of seizures |
|---|---|---|---|---|---|
| Family 1, c.1910T>G p.L637R (Richards et al., 2004) | M; 38 | Neonatal period | Apnoeic spells, convulsions | PHB; stopped by 3 years | First few months of life |
| F; 26 | Neonatal period | Apnoeic spells, convulsions | PHB; stopped by 1 year | First few months of life | |
| Family 2, c.1684insGCCCT p.Y562fsX566 (Biervert et al., 1998) | M; 50 | First few weeks of life | Convulsions | None known | First few weeks of life |
| M; 29 | Day 5 | Apnoeic spells, convulsions | None known | Day 10 | |
| F; 27 | Day 3 | Convulsions; FC at 2 months | PHB (briefly) | Age 2 months | |
| F; 27 | Day 3 | Convulsions; FC at 2 months | PHB (briefly) | Age 2 months | |
| Family 3, deletion of exons 1–5 (Heron et al., 2007) | F; 58 | 5 days | Convulsions | PHB, PHT (stopped at 13 years) | Age 12 years |
| F; 30 | 4 months | Apnoeic spells; Convulsions | PHB (stopped at 18 months) | 5 months |
F = female; FC = febrile convulsions; M = male; PHB = phenobarbitone; PHT = phenytoin.
Family 2 members carry a 5 bp insertion resulting in the frameshift mutation Y562fsX566. This mutation lies in the calmodulin binding region and translates to a premature stop codon, truncating the protein in the C terminus by 300 amino acids. In vitro expression studies in this family found that no current was carried by mutant channels in functional expression studies performed in Xenopus oocytes (Biervert et al., 1998). When co-expressed with wild-type channels, a dominant negative effect was not seen.
Family 3 carried a deletion of exons 1–5 detected by multiplex ligase-dependent probe amplification. Although in vitro expression data are not available, the deletion segregated with benign familial neonatal epilepsy in affected family members was not detected in control DNA (Heron et al., 2007).
All patients studied from the present cohort had typical features of benign familial neonatal epilepsy. Seizures remitted in infancy in seven out of eight mutation carriers. The eighth patient experienced seizures until the age of 8 years. All patients had been seizure free for >25 years and had stopped anti-convulsant treatment for a minimum of 20 years before testing. No subject had clinical evidence of neuromyotonia.
Nerve excitability studies
The measures most affected by the activity of slow K+ channel activity include the response to depolarizing conditioning stimuli during threshold electrotonus (Fig. 1A) and the degree of accommodation to prolonged depolarizing stimuli (Table 1). There were no significant differences in stimulus-response or strength-duration properties (Table 1).
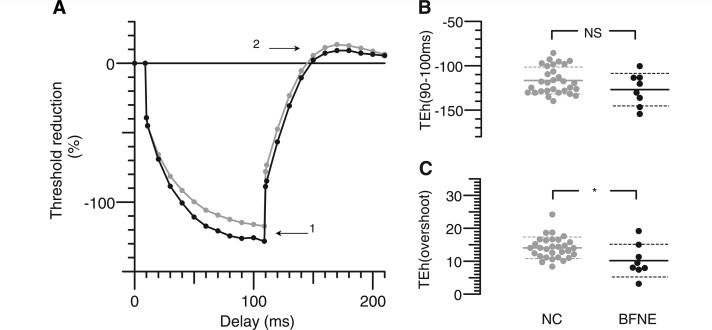
Figure 1.
Depolarizing threshold electrotonus. (A) Changes in threshold during and after a 100 ms depolarizing current, set to 40% of unconditioned threshold. Grey lines and dots: mean of 30 normal control subjects (NC). Black lines and dots: mean of eight subjects with benign familial neonatal epilepsy (BFNE). (B) Distribution of threshold reductions at the end of depolarizing current, as indicated by Arrow 1 in A. (C) Peak threshold increases after the end of depolarizing current, as indicated by Arrow 2 in A. Asterisks indicate probability of such a threshold difference occurring by chance: **P < 0.01, ***P < 0.001. In B and C, horizontal solid lines indicate means, and dashed lines indicate mean ± SD. Subjects with benign familial neonatal epilepsy show less accommodation to depolarizing currents and less undershoot, attributed to reduced activation of hyperpolarizing slow potassium currents.
Threshold electrotonus
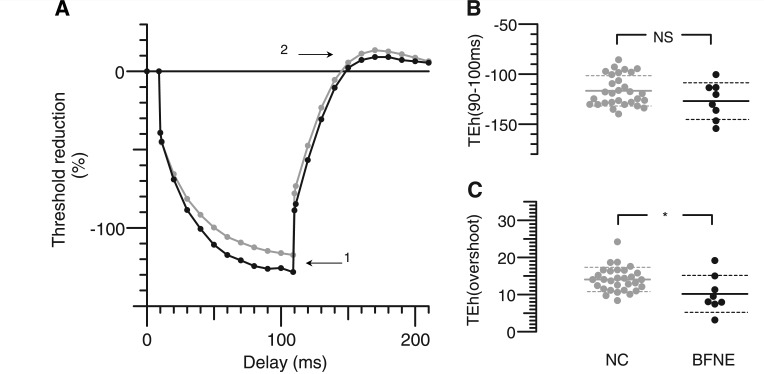
Increased nerve excitability in mutation carriers was detected during threshold electrotonus in response to depolarizing conditioning stimuli when compared with controls (Fig. 1). During depolarizing threshold electrotonus, the extent of threshold reduction in response to conditioning stimuli normally reaches a peak at ∼20 ms after the onset of the current pulse. As the conditioning stimulus continues, the threshold starts to return slowly towards the control level. This lessening of the degree of depolarization is due to activation of hyperpolarizing slow K+ channels and is referred to as ‘S2 accommodation’ (Baker et al., 1987; Bostock and Baker, 1988; Baker and Bostock, 1989). In subjects with benign familial neonatal epilepsy, there was a reduction in this accommodative response to long-lasting depolarizing stimuli so that excitability at TEd40 (90–100 ms) (Arrow 1) was increased in benign familial neonatal epilepsy by 12% (P = 0.006). The degree of accommodation to the 40% conditioning stimulus was reduced in benign familial neonatal epilepsy by an average of 24% (P = 7 × 10−5) compared with healthy controls (Table 1). The threshold undershoot when the 100-ms depolarizing current ended (Arrow 2) was 22% less in mutation carriers (P = 0.006). During hyperpolarizing threshold electrotonus (Fig. 2), there was a non-significant trend towards a greater increase in threshold in affected individuals with the stronger −40% current (Arrow 1), but on release of hyperpolarization, there was an overshoot (Arrow 2) that was significantly less in benign familial neonatal epilepsy subjects.
Figure 2.
Hyperpolarizing threshold electrotonus. (A) Changes in threshold during and after a 100-ms hyperpolarizing current, set to −40% of unconditioned threshold current, presented as in Fig. 1. (B) Distribution of threshold changes at the end of hyperpolarizing current, as indicated by Arrow 1 in A. (C) Peak threshold decreases after the end of depolarizing current, as indicated by Arrow 2 in A. Asterisks indicate probability of such a threshold difference occurring by chance: *P < 0.05; NS = P > 0.05. BFNE = benign familial neonatal epilepsy; NC = normal control.
Current–threshold relationship
At 200 ms into long subthreshold polarizing currents, the threshold approximates to a steady state, related to the membrane potential change, and the slope of this relationship (change in current/change in threshold) provides a threshold analogue of input conductance, which increases on depolarization due to outward rectification by potassium channels and also on hyperpolarization due to inward rectification (Fig. 3). Consistent with a reduction in GKs in the subjects with benign familial neonatal epilepsy, there was a 13% reduction in depolarizing I/V slope (Fig. 3B and Arrow 1 in Fig. 3A; P = 0.0025), but no significant abnormality in hyperpolarizing I/V slope (Fig. 3C and Arrow 2 in Fig. 3A).
Figure 3.
Current/threshold slope. (A) Slope of the current/threshold relationship, recorded 200 ms after application of polarizing currents, and representing a threshold analogue of input conductance. Mean control recordings are in grey, and subjects with benign familial neonatal epilepsy are in black. (B) Distributions of slopes at the depolarizing end of the recording, as indicated by Arrow 1 in A. (C) Distributions of hyperpolarizing slopes, indicated by Arrow 2 in A. Subjects with benign familial neonatal epilepsy (BNFE) show less increase in conductance on depolarization, attributed to reduced activation of slow potassium channels, but no significant difference on hyperpolarization. **P < 0.01, NS = P > 0.05. NC = normal control.
Recovery cycle
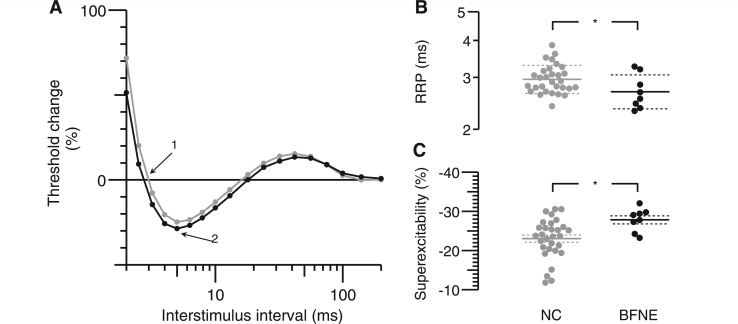
The relative refractory period was 9% shorter in patients compared with controls (Fig. 4B; P = 0.037). Excitability during the superexcitable period was increased by 17.2% (Fig. 4C; P = 0.015). The mean hyperpolarizing threshold change during the late sub-excitable period was ∼12% smaller in individuals with benign familial neonatal epilepsy during the recovery cycle compared with normal controls, but this was not statistically significant (P = 0.27).
Figure 4.
Recovery cycle. (A) Changes in threshold currents at different times after a supramaximal conditioning stimulus (note that logarithmic scales of interstimulus intervals are used). Means of 30 controls are in grey, and eight subjects with benign familial neonatal epilepsy are in black. (B) Distribution of relative refractory periods (RRPs), at which threshold returns to control value, is indicated by Arrow 1 in A (note logarithmic relative refractory period scale). (C) Distribution of maximum threshold reductions is indicated by Arrow 2 in A. Relative refractory period was slightly reduced and super-excitability increased in subjects with benign familial neonatal epilepsy. The small reduction in late sub-excitability was not significant. BFNE = benign familial neonatal epilepsy; NC = normal control.
Modelling of nerve excitability changes
Table 3 lists the best fits obtained to the mean benign familial neonatal epilepsy excitability data obtained by altering either one or two membrane parameters. Reduction in the slow potassium conductance GKs on its own did not produce as good a fit to the recordings as an alteration in membrane potential alone. A much better fit was obtained, however, when a 38% reduction in GKs was combined with a 32% increase in the fast potassium conductance, GKf.
Table 3.
Modelling the nerve excitability data from subjects with benign familial neonatal epilepsy
| Parameter | Change | Discrepancy reduction (%) | |||
|---|---|---|---|---|---|
| Best fits obtained by changing single parameters | |||||
| 1 | Er | −0.7 mV | 34.8 | ||
| 2 | GKs | −12% | 25.8 | ||
| 3 | GBB | +7.4% | 24.3 | ||
| 4 | GLk | −19% | 9.3 | ||
| 5 | PNap | +5.9% | 5.3 | ||
| 6 | PNa | +6.7% | 3.1 | ||
| 7 | GKf | 0% | 0 | ||
| Parameter 1 | Change | Parameter 2 | Change | Discrepancy reduction (%) | |
| Best fits obtained by changing pairs of parameters | |||||
| 1 | GKs | −38% | GKf | +32% | 61.7 |
| 2 | GKs | −37.5% | PNap | −25% | 48.5 |
| 3 | GKs | −37.5% | PNa | −16% | 45.7 |
| 4 | GKs | −5% | Er | −0.3 mV | 38.0 |
| 5 | GLk | +21% | Er | −1.1 mV | 36.2 |
‘Discrepancy’ is scored as the weighted sum of the error terms: [(xm−xn)/sn]2, where xm is the threshold of the model, xn the mean and sn the standard deviation of the threshold for the real nerves.
Er = resting potential; GKs = slow potassium conductances (nodal and internodal); GKf = fast potassium conductances (nodal and internodal); GBB = ‘Barrett–Barrett’ conductance across myelin sheath; GLk = leak conductances (nodal and internodal); PNa = sodium permeability (nodal); PNap = persistent sodium conductance (as % of PNa).
Discussion
This study documents for the first time changes in peripheral nerve, specifically reflecting loss of slow K+ channel function in asymptomatic KCNQ2 mutation carriers. Although seizures had remitted years ago in these adults and patients had no clinical features of peripheral nerve hyperexcitability, the functional effects of the mutations could still be demonstrated in vivo.
Changes related to decreased slow K+ channel function
The ‘M’ current was first identified in frog sympathetic neurons (Brown and Adams, 1980), and a similar slowly activating outwardly rectifying K+ current was described in myelinated frog axons by Dubois (1981) and later in rat spinal roots (Baker et al., 1987), where blockade by tetraethylammonium or barium results in an increase in excitability to depolarization (see also Kocsis et al., 1983). Subsequent voltage clamp analysis of human nodal currents has revealed that the cumulative activation of this conductance limits repetitive activity (Reid et al., 1999). The M current is now known to be mediated by heterotetrameric K+ channels containing subunits of the Kv7 family, including Kv7.2 and Kv7.3 (Wang et al., 1998; Selyanko et al., 2000).
Mutations in the KCNQ2 gene encoding Kv7.2 were subsequently identified in benign familial neonatal epilepsy (Biervert et al., 1998; Singh et al., 1998). This was the first human genetic epilepsy to be attributed to a gene encoding a voltage-gated ion channel. XE991 and linopirdine are relatively specific blockers of the Kv7.2-containing channels, and these agents, as well as the non-specific blocker tetraethylammonium, reduced accommodation to 100 ms subthreshold depolarizing currents and reduced late sub-excitability (Schwarz et al., 2006).
The in vivo changes in peripheral nerve excitability described in this article in subjects with benign familial neonatal epilepsy reproduce some of the changes reported with pharmacological blockade of Kv7.2 and may therefore be reasonably ascribed to the effect of KCNQ2 mutations on the slow K+ channel, i.e. reduction in accommodation to depolarizing conditioning stimuli and a lesser threshold undershoot when the depolarizing current ends. The increase in superexcitability and reduction in relative refractory period are also consistent with the effects of XE991 on rat nerve (Schwarz et al., 2006), although these changes were only evident in rat after multiple conditioning stimuli. Interestingly, late sub-excitability was not significantly different in mutation carriers. This may seem surprising, because there is good evidence that late sub-excitability is due to GKs. However, it is clear from the electrotonus and recovery cycle recordings that there was only a partial reduction of GKs in the benign familial neonatal epilepsy patients. A partial reduction of GKs in the human motor axon model produces a membrane depolarization that causes a greater proportion of the maximum conductance to be activated by a nerve impulse so that late sub-excitability is maintained.
The homogeneity of the recordings from benign familial neonatal epilepsy patients, which were not significantly more variable than those from controls, contrasts with the much greater variability found previously in patients with episodic ataxia type 1 and KCNA1 mutations (Tomlinson et al., 2010) and also with the heterogeneous, mutation-dependent recordings obtained from mutant Kv7.2 channels in vitro. This homogeneity may reflect the limited range of mutations amongst our patients. It would be surprising, for example, if the KCNQ2 mutation described by Dedek et al. (2001), which causes myokymia as well as neonatal convulsions, did not result in different changes in peripheral nerve excitability.
The most striking finding is that the abnormalities remain demonstrable in peripheral nerve as a lasting signature of the mutation responsible for the epilepsy, even in subjects who have not suffered seizures for decades and have no symptoms attributable to peripheral nerve hyperexcitability. The lack of neuromyotonia in otherwise healthy subjects carrying the benign familial neonatal epilepsy mutation contrasts with its almost invariant occurrence in episodic ataxia type 1, due to fast K+ channel mutations (Tomlinson et al., 2009, 2010), but this is not surprising given that in benign familial neonatal epilepsy the excitability changes are more subtle, possibly due in part to an adaptive increase in fast K+ channel expression (see below).
Possible adaptive changes in other channels
The modelling suggests that there were also some adaptive changes in the axons in addition to the loss of slow potassium conductance. Reduction of GKs in the model by more than a limited amount caused the axon to fire during depolarizing electrotonus and produced a greater increase in superexcitability than observed in the subjects with benign familial neonatal epilepsy. The modelling further suggested that this hyperexcitability and superexcitability produced by loss of GKs may have been compensated by upregulation of GKf, the fast potassium conductance largely mediated by Kv1.1 (Tomlinson et al., 2010). The recordings were best matched by a 38% decrease in GKs coupled with a 32% increase in GKf (Table 3). Such an adaptive change could provide a simple explanation for the absence of symptoms of peripheral nerve hyperexcitability in these subjects, and if a similar adaptive change occurs in the central nervous system, it would provide an explanation for the remission of seizures despite the continued expression of mutant Kv7.2 channels. An alternative explanation for the remission of seizures in subjects despite detectable peripheral nerve changes may be that local cell responses to mutant channels (such as post-translational modification, differential splicing, altered regulation of channel expression or channel turnover) may differ between different neuronal cell types.
Clinical value
The finding of channel-specific changes in this cohort of asymptomatic individuals implies that nerve excitability studies may have clinical utility in screening patients with suspected but genetically undetermined abnormalities of ion channels in familial epilepsy syndromes. This would complement their established value in peripheral neuromuscular disorders (Krishnan et al., 2009; Krishnan, 2010). However, because such studies are not yet routine in most clinical neurophysiology units, this can be advocated only in centres where appropriate expertise is available.
Sequencing of KCNQ2 is not readily available clinically and may only yield a result in 60% of typical cases. Furthermore, it is clear that the phenotype of KCNQ2 mutations is broader than first thought; mutations are described in patients with epileptic encephalopathies as well as in cases with isolated peripheral nerve hyperexcitability syndromes (Steinlein et al., 2007; Weckhuysen et al., 2012). Nerve excitability testing can provide channel-specific information not only for the slow potassium channel but also for other Na+ and K+ channelopathies (Kiernan et al., 2005b; Tomlinson et al. 2010). The present findings confirm that excitability measurements can identify dysfunction of axonally expressed ion channels and auxillary proteins, even in the absence of peripheral nerve symptoms.
Funding
The authors gratefully acknowledge funding for this work from the following organisations: UK Charities’ Aid Foundation Patrick Berthoud Fellowship, British Medical Association Vera Down Fellowship, Brain Research Trust (UK), Medical Research Council (UK), Brain Foundation (Australia), Sydney Foundation for Medical Research, National Health and Medical Research Council (Australia), Wellcome Trust (UK), Action Medical Research and the European Research Council. M.G.H. is supported by an MRC Centre Grant. I.E.S. and S.F.B. are supported by an NHMRC Program Grant.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors thank the families for participating in this research.
References
- Baker M, Bostock H. Depolarization changes the mechanism of accommodation in rat and human motor axons. J Physiol. 1989;411:545–61. doi: 10.1113/jphysiol.1989.sp017589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Bostock H, Grafe P, Martius P. Function and distribution of three types of rectifying channel in rat spinal root myelinated axons. J Physiol. 1987;383:45–67. doi: 10.1113/jphysiol.1987.sp016395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini G, Miceli F, Soldovieri MV, Miraglia del Giudice E, Pascotto A, Taglialatela M. Benign Familial neonatal seizures. In: Pagon RA, Bird TD, Dolan CR, Stephens K, editors. GeneReviews. Seattle, WA: University of Washington, Seattle; April 27, 1993–2010. Advance Access published on August 4, 2011. [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–85. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P, Jentsch TJ, et al. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279:403–6. doi: 10.1126/science.279.5349.403. [DOI] [PubMed] [Google Scholar]
- Biervert C, Steinlein OK. Structural and mutational analysis of KCNQ2, the major gene locus for benign familial neonatal convulsions. Hum Genet. 1999;104:234–40. doi: 10.1007/pl00008713. [DOI] [PubMed] [Google Scholar]
- Bostock H. MEMFIT: a computer program to aid interpretation of multiple excitability measurements on human motor axons. Clin Neurophysiol. 2006;117:S85. [Google Scholar]
- Bostock H, Baker M. Evidence for two types of potassium channel in human motor axons in vivo. Brain Res. 1988;462:354–8. doi: 10.1016/0006-8993(88)90564-1. [DOI] [PubMed] [Google Scholar]
- Bostock H, Rothwell JC. Latent addition in motor and sensory fibres of human peripheral nerve. J Physiol. 1997;498:277–94. doi: 10.1113/jphysiol.1997.sp021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–6. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Charlier C, Singh NA, Ryan SG, Lewis TB, Reus BE, Leach RJ, et al. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat Genet. 1998;18:53–5. doi: 10.1038/ng0198-53. [DOI] [PubMed] [Google Scholar]
- Dedek K, Fusco L, Teloy N, Steinlein OK. Neonatal convulsions and epileptic encephalopathy in an Italian family with a missense mutation in the fifth transmembrane region of KCNQ2. Epilepsy Res. 2003;54:21–4. doi: 10.1016/s0920-1211(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Dedek K, Kunath B, Kananura C, Reuner U, Jentsch TJ, Steinlein OK. Myokymia and neonatal epilepsy caused by a mutation in the voltage sensor of the KCNQ2 K+ channel. Proc Natl Acad Sci USA. 2001;98:12272–7. doi: 10.1073/pnas.211431298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois JM. Evidence for the existence of three types of potassium channels in the frog Ranvier node membrane. J Physiol. 1981;318:297–316. doi: 10.1113/jphysiol.1981.sp013865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng DL, Gordon TR, Kocsis JD, Waxman SG. Development of 4-AP and TEA sensitivities in mammalian myelinated nerve fibers. J Neurophysiol. 1988;60:2168–79. doi: 10.1152/jn.1988.60.6.2168. [DOI] [PubMed] [Google Scholar]
- Farrar MA, Vucic S, Lin CS-Y, Park SB, Johnston HM, du Sart D, et al. Dysfunction of axonal membrane conductances in adolescents and young adults with spinal muscular atrophy. Brain. 2011;134:3185–97. doi: 10.1093/brain/awr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron SE, Cox K, Grinton BE, Zuberi SM, Kivity S, Afawi Z, et al. Deletions or duplications in KCNQ2 can cause benign familial neonatal seizures. J Med Genet. 2007;44:791–6. doi: 10.1136/jmg.2007.051938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells J, Trevillion L, Bostock H, Burke D. The voltage dependence of Ih in human myelinated axons. J Physiol. 2012;590:1625–40. doi: 10.1113/jphysiol.2011.225573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan MC, Burke D, Andersen KV, Bostock H. Multiple measures of axonal excitability: a new approach in clinical testing. Muscle Nerve. 2000;23:399–409. doi: 10.1002/(sici)1097-4598(200003)23:3<399::aid-mus12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Isbister GK, Lin CS-Y, Burke D, Bostock H. Acute tetrodotoxin-induced neurotoxicity after ingestion of puffer fish. Ann Neurol. 2005a;57:339–48. doi: 10.1002/ana.20395. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Krishnan AV, Lin CS-Y, Burke D, Berkovic SF. Mutation in the Na+ channel subunit SCN1B produces paradoxical changes in peripheral nerve excitability. Brain. 2005b;128:1841–6. doi: 10.1093/brain/awh520. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Ruiz JA, Waxman SG. Maturation of mammalian myelinated fibers: changes in action-potential characteristics following 4-aminopyridine application. J Neurophysiol. 1983;50:449–63. doi: 10.1152/jn.1983.50.2.449. [DOI] [PubMed] [Google Scholar]
- Krishnan AV. The excitement about excitability. Clin Neurophysiol. 2010;121:805–6. doi: 10.1016/j.clinph.2010.01.023. [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Lin CS-Y, Park SB, Kiernan MC. Axonal ion channels from bench to bedside: a translational neuroscience perspective. Prog Neurobiol. 2009;89:288–313. doi: 10.1016/j.pneurobio.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Maljevic S, Wuttke TV, Lerche H. Nervous system Kv7 disorders: breakdown of a subthreshold brake. J Physiol. 2008;586:1791–801. doi: 10.1113/jphysiol.2008.150656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogyoros I, Lin CS-Y, Kuwabara S, Cappelen-Smith C, Burke D. Strength-duration properties and their voltage dependence as measures of a threshold conductance at the node of Ranvier of single motor axons. Muscle Nerve. 2000;23:1719–26. doi: 10.1002/1097-4598(200011)23:11<1719::aid-mus8>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Plouin P, Anderson VE. Benign familial and non-familial neonatal seizures. In: Roger J, Bureau M, Dravet C, Genton P, Tassinari CA, Wolf P, editors. Epileptic syndromes in infancy, childhood and adolescence. 4th edn. Eastleigh: John Libbey & Co.; 2005. pp. 3–15. [Google Scholar]
- Reid CA, Berkovic SF, Petrou S. Mechanisms of human inherited epilepsies. Prog Neurobiol. 2009;87:41–57. doi: 10.1016/j.pneurobio.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Reid G, Scholz A, Bostock H, Vogel W. Human axons contain at least five types of voltage-dependent potassium channel. J Physiol. 1999;518:681–696. doi: 10.1111/j.1469-7793.1999.0681p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards MC, Heron SE, Spendlove HE, Scheffer IE, Grinton B, Berkovic SF, et al. Novel mutations in the KCNQ2 gene link epilepsy to a dysfunction of the KCNQ2-calmodulin interaction. J Med Genet. 2004;41:e35. doi: 10.1136/jmg.2003.013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JR, Glassmeier G, Cooper EC, Kao TC, Nodera H, Tabuena D, et al. KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier. J Physiol. 2006;573:17–34. doi: 10.1113/jphysiol.2006.106815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JR, Reid G, Bostock H. Action potentials and membrane currents in the human node of Ranvier. Pflügers Arch. 1995;430:283–92. doi: 10.1007/BF00374660. [DOI] [PubMed] [Google Scholar]
- Selyanko AA, Hadley JK, Brown DA. Properties of single M-type KCNQ2/KCNQ3 potassium channels expressed in mammalian cells. J Physiol. 2000;534:15–24. doi: 10.1111/j.1469-7793.2001.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NA, Charlier C, Stauffer D, DuPont BR, Leach RJ, Melis R, et al. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat Genet. 1998;18:25–9. doi: 10.1038/ng0198-25. [DOI] [PubMed] [Google Scholar]
- Singh NA, Westenskow P, Charlier C, Pappas C, Leslie J, Dillon J, et al. BFNC Physician Consortium. KCNQ2 and KCNQ3 potassium channel genes in benign familial neonatal convulsions: expansion of the functional and mutation spectrum. Brain. 2003;126:2726–37. doi: 10.1093/brain/awg286. [DOI] [PubMed] [Google Scholar]
- Steinlein OK, Conrad C, Weidner B. Benign familial neonatal convulsions: always benign? Epilepsy Res. 2007;73:245–9. doi: 10.1016/j.eplepsyres.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Tomlinson SE, Hanna MG, Kullmann DM, Tan SV, Burke D. Clinical neurophysiology of the episodic ataxias: insights into ion channel dysfunction in vivo. Clin Neurophysiol. 2009;120:1768–76. doi: 10.1016/j.clinph.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Tomlinson SE, Tan SV, Kullmann DM, Griggs RC, Hanna MG, Burke D, et al. Nerve excitability studies characterize Kv1.1 fast potassium channel dysfunction in patients with episodic ataxia type 1. Brain. 2010;133:3530–40. doi: 10.1093/brain/awq318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevillion L, Howells J, Burke D. Outwardly rectifying deflections in threshold electrotonus due to K+ conductances. J Physiol. 2007;580:685–96. doi: 10.1113/jphysiol.2006.126003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, et al. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–3. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- Weckhuysen S, Mandelstam S, Suls A, Audenaert D, Deconinck T, Claes LRF, et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71:15–25. doi: 10.1002/ana.22644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.