Abstract
Various types of stem cells have been shown to have beneficial effects on cardiac function. It is still debated whether fusion of injected stem cells with local resident cardiomyocytes is one of the mechanisms. To better understand the role of fusion in stem cell-based myocardial regeneration, the present study was designed to investigate the fate of human adipose tissue-derived stem cells (hASCs) fused with neonatal rat cardiomyocytes in vitro. hASCs labeled with the green fluorescent probe Vybrant DiO were cocultured with neonatal rat cardiomyocytes labeled with the red fluorescent probe Vybrant DiI and then treated with fusion-inducing hemagglutinating virus of Japan (HVJ). Cells that incorporated both red and green fluorescent signals were considered to be hASCs that had fused with rat cardiomyocytes. Fusion efficiency was 19.86 ± 4.84% at 5 d after treatment with HVJ. Most fused cells displayed cardiomyocyte-like morphology and exhibited spontaneous rhythmic contraction. Both immunofluorescence staining and lentiviral vector labeling showed that fused cells contained separate rat cardiomyocyte and hASC nuclei. Immunofluorescence staining assays demonstrated that human nuclei in fused cells still expressed the proliferation marker Ki67. In addition, hASCs fused with rat cardiomyocytes were positive for troponin I. Whole-cell voltage-clamp analysis demonstrated action potentials in beating fused cells. RT-PCR analysis using rat- or human-specific myosin heavy chain primers revealed that the myosin heavy-chain expression in fused cells was derived from rat cardiomyocytes. Real-time PCR identified expression of human troponin T in fused cells and the presence of rat cardiomyocytes induced a cardiomyogenic protein expression of troponin T in human ASCs. This study illustrates that hASCs exhibit both stem cell (proliferation) and cardiomyocyte properties (action potential and spontaneous rhythmic beating) after fusion with rat cardiomyocytes, supporting the theory that fusion, even if artificially induced in our study, could indeed be a mechanism for cardiomyocyte renewal in the heart.—Metzele, R., Alt, C., Bai, X., Yan, Y., Zhang, Z., Pan, Z., Coleman, M., Vykoukal, J., Song, Y.-H., Alt, E. Human adipose tissue-derived stem cells exhibit proliferation potential and spontaneous rhythmic contraction after fusion with neonatal rat cardiomyocytes.
Keywords: differentiation, action potential
Previous studies from our group and others have shown that adult stem cells isolated from bone marrow (1), skeletal muscle (2), and adipose tissue (3) exhibit beneficial effects on cardiac function following myocardial infarction. However, the underlying mechanisms responsible for stem cell-based myocardial regeneration are not yet fully understood. Several groups have attributed this potential to the transdifferentiation capacity of stem cells (4). Others, however, have demonstrated that the cardiac differentiation of injected stem cells resulted from spontaneous fusion of donor stem cells with recipient cardiomyocytes. Injected bone marrow-derived stem cells spontaneously fused with native cardiomyocytes and formed multinucleated cells in the heart (5–7). The fate of stem cells after fusion with local resident cardiomyocytes, however, remains unclear.
Human adipose tissue-derived stem cells (hASCs) are a promising cell source for potential stem cell-based clinical therapies, since a large number of hASCs can be relatively easily harvested from patients via minimally invasive liposuction and transplanted in an autologous manner without expansion in vitro (8). In addition, ASCs have the potential for proliferation and differentiation into multiple cell lineages, including cardiomyocytes, endothelial cells, and smooth muscle cells while exposed to an appropriate microenvironment in vitro or in vivo (3, 9, 10). Several studies have shown that transplantation of ASCs improved cardiac function after experimental myocardial infarction (3, 11). However, controversy remains regarding the mechanisms by which ASCs contribute to tissue repair. In particular, the role of fusion in stem cell-based myocardial regeneration is debated.
Several in vitro cell-fusion techniques are in use. The fusion efficacy induced by hemagglutinating virus of Japan (HVJ) reportedly is higher than that induced by polyethylene glycol (12). Accordingly, the present study was designed to investigate the fate of HVJ-induced hASC fusion with neonatal rat cardiomyocytes.
MATERIALS AND METHODS
Isolation and culture of hASCs
hASCs were provided by InGeneron (Houston, TX, USA) under a material transfer agreement. Adipose tissue was acquired from donors undergoing elective lipoplasty with informed consent under a tissue acquisition protocol approved by the M. D. Anderson Cancer Center Review Board. hASCs were isolated from the adipose tissue, as described previously (13, 14). Adipose tissue was minced and then incubated with agitation for 90 min at 37°C with Liberase Blendzyme 3 (Roche, Mannheim, Germany) at a concentration of 4 U/g adipose tissue in PBS. The digested tissue was sequentially filtered through 100- and 40-μm filters (Fisher Scientific, Waltham, MA, USA) and centrifuged at 450 g for 10 min. The resulting supernatant containing adipocytes and debris was discarded, and the pelleted cells were washed twice with Hanks' balanced salt solution (Cellgro; Mediatech, Inc., Manassas, VA, USA) and resuspended in a growth medium. The growth medium contained α modification of Eagle's medium (Cellgro), 20% FBS (Atlanta Biologicals, Lawrenceville, GA, USA), 2 mM glutamine (Cellgro), and 100 U/ml penicillin with 100 μg/ml streptomycin (Cellgro). Plastic-adherent cells were designated as hASCs and grown in Nunclon culture vials (Nunc, Roskilde, Denmark) at 37°C in a humidified atmosphere containing 5% CO2, followed by daily washes to remove red blood cells and nonattached cells. After confluence of hASCs in culture vials (passage 0), cells were digested and seeded at a density of 3000 cells/cm2 (passage 1).
Cell differentiation
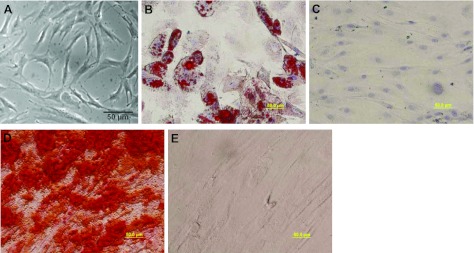
To establish the intrinsic differentiation potential of hASCs used in this study, the adipogenic differentiation potential of hASCs was analyzed as described previously (15). Osteogenic differentiation of hASCs was performed according to Oedayrajsingh-Varma et al. (16). The cells were then observed under a phase-contrast microscope (Fig. 1).
Figure 1.
Characterization of hASCs. A) Phase-contrast photomicrograph of hASCs. hASCs exhibited a spindle- or triangle-like morphology at passage 3. B–E) Differentiation potential of hASCs. Cells were cultured in an adipogenic medium (B), adipogenic control medium (C), osteogenic medium (D), or osteogenic control medium (E) for 3 wk. Adipogenesis and osteogenesis of hASCs were confirmed by the presence of lipid drops stained red with Oil Red O (B) and calcium deposits stained red with Alizarin Red S (D) in hASCs cultured in induction medium, respectively. Scale bars = 50 μm.
Isolation and culture of neonatal rat cardiomyocytes
Laboratory animal experiments were performed with requisite approval and appropriate M. D. Anderson Cancer Center Animal Care and Use Committee oversight. Cardiomyocytes were isolated from 2-d-old Sprague-Dawley rats using a commercial kit (Worthington, Lakewood, NJ, USA) according to the manufacturer's protocol. Cardiomyocytes cultured in a Leibovitz L-15 medium supplemented with 10% FBS were used for subsequent experiments. Cardiomyocyte culture contained cardiomyocytes and cardiac fibroblasts. To increase the fusion efficiency of hASCs and rat cardiomyocytes, the proliferation of cardiac fibroblasts was inhibited by treatment of the cells isolated from rat hearts with mitomycin C at a concentration of 3 μg/ml for 2 h on d 2 after the start of cell culture. The cardiomyocytes were identified by their expression of troponin I, using immunofluorescence staining analysis. Cell nuclei were stained blue with 4′-6-diamidino-2-phenylindole (DAPI). For analysis of the proliferation of cardiac fibroblasts and the purity of cardiomyocytes in culture with or without treatment with mitomycin C, cardiomyocyte cultures in 24-well plates were imaged using a charge-coupled device (CCD) camera under a fluorescence microscope (Carl Zeiss, Oberkochen, Germany) at different time points after the start of cell culture. The cell images were taken in 3 random fields in each well for a total of 3 wells for each treatment per time point. Troponin I-positive cardiomyocytes and DAPI-stained cells were counted, and the ratio of the number of troponin I-positive red blood cells to DAPI-positive blue cells was used to determine the relative proportion of cardiomyocytes in the culture.
Cell labeling
Vybrant DiI and DiO fluorescent tracers
To track the fusion formation between hASCs and rat cardiomyocytes, hASCs at passage 3 and neonatal cardiomyocytes on d 3 after the start of the culture were labeled with two different fluorescent dyes, Vybrant DiO (a fluorochrome emitting green light) and Vybrant DiI (a fluorochrome emitting red light), according to the protocol provided by Invitrogen (Carlsbad, CA, USA). The DiO-labeled hASCs were fused with cultured cardiomyocytes labeled with DiI at a ratio of 1:10, as described below. Cells with both red and green fluorescent signals were considered to be fused or hybridized cells. Cells were then stained with DAPI. The numbers of green and red double-positive hASCs fused with cardiomyocytes and DAPI-positive total cells were counted in 3 random fields in each well (24-well plate) for a total of 3 wells on d 1 and 5 after treatment of HVJ. The fusion efficiency was analyzed by calculation of the ratio between the number of red and green double-positive fused cells and the number of DAPI-positive cells in wells at different time points after treatment with HVJ, thus giving us the percentage of rat cardiomyocytes that were correctly fused with hASCs.
Lentiviral vector construction and hASC labeling with lentiviral vector
pCAG-SH-GH was generously provided by the laboratory of Dr. Richard R. Behringer (M. D. Anderson Cancer Center). To generate a lentiviral construct (pLVX-Puro-HS-GR), H2B-EGFP-2A-mCherry-GPI (HS-GR) was cut from vector pCAG-SH-GH and then subcloned into lentiviral vector pLVX-Puro (Clontech, Mountain View, CA, USA) between the XhoI and XbaI sites by digestion with specific restriction enzyme and ligation with DNA ligase. This subcloned lentiviral vector (pLVX-Puro-HS-GR) contained both a green fluorescence protein (GFP) reporter gene expressed in the nuclei and a red fluorescence protein (mCherry) reporter gene expressed in the membrane of transduced cells. The lentivirus production and transduction procedures were described previously (10). To track whether HVJ induced cellular cytoplasm fusion or nuclear incorporation, we transduced hASCs with the dual-fluorescent protein reporter gene contained within the lentiviral vector (pLVX-Puro-HS-GR). Transduced hASCs were maintained in the culture medium containing puromycin at a concentration of 5 μg/ml for 1 wk. hASCs with green fluorescence in the nuclei and red fluorescence in the cell membrane were then cocultured with rat cardiomyocytes and treated with HVJ as described above. At 5 d after treatment with HVJ, the cells in coculture were stained with DAPI and observed under a fluorescence microscope to analyze the distributions and origins of nuclei in hASCs fused with rat cardiomyocytes.
Virus-mediated fusion formation between hASCs and neonatal rat cardiomyocytes
Fusion of hASCs and rat cardiomyocytes was induced by treatment with HVJ (GenomONETM-CF; HVJ envelope cell fusion kit; Ishihara Sangyo Kaisha, Osaka, Japan), following the manufacturer's recommendations. The virus in the HVJ-E kit is inactivated by UV irradiation. Using this preparation, we found that the ability of the virus to replicate was lost completely, but its fusion activity was retained. Following the cell-labeling procedure, the hASCs were digested with trypsin/EDTA, washed, and combined with HVJ-E. These cells were left to stand on ice for 5 min to allow absorption of HVJ-E into the cell membrane, centrifuged at 2000 rpm for 5 min, and then resuspended in ice-cooled fusion buffer. These hASCs were then added to cultured cardiomyocytes at a ratio of 1:10. The mixed cells were centrifuged at 1000 rpm for 5 min and incubated at 37°C in a 5% CO2 environment for 15 min.
Immunofluorescence staining
Cells cultured in the 24-well plates were washed 3 times with PBS and then fixed with 4% paraformaldehyde for 10 min at room temperature. Cells were then washed 3 times with PBS containing 0.3% Triton X-100 (Sigma) and then blocked with 10% serum for 30 min at room temperature. Next, cells were incubated with primary antibodies, including a mouse anti-human nuclear antibody (Chemicon, Temecula, CA, USA), rabbit anti-human and anti-rat (or specific anti-human) troponin I antibody, or rabbit anti-Ki67 antibody for 1 h at 37°C. An anti-human nuclear antibody and anti-troponin I antibody were used to track hASCs and cardiomyocytes, respectively, in the coculture of hASCs and rat cardiomyocytes. An anti-Ki67 antibody was used to track proliferation of cells. After 3 washes, the cells were incubated with appropriate fluorophore-conjugated secondary antibodies for 1 h at room temperature. The nuclei were counterstained with DAPI. The cells were then examined under a fluorescence microscope. The efficiency of Ki67-positive fused cells was calculated by the ratio of Ki67-positive hASCs fused with cardiomyocytes against total fused cells from 3 fields/well for a total of 3 wells (24-well plate) 5 d after treatment with HVJ.
RT-PCR analysis of mRNA expression in hASCs fused with rat cardiomyocytes
Total RNA was extracted from isolated fused hASC-cardiomyocytes using an RNAqueous-Micro kit (Ambion, Austin, TX, USA). cDNA was synthesized from 1 μg of total RNA using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) in 20 μl of reaction volume according to the manufacturer's instructions. cDNA samples were subjected to PCR amplification using AccuPrime SuperMix I (Invitrogen) with specific primers. The primers and their sequences were as follows: myosin heavy chain (MHC)-specific primers for human cardiomyocytes, forward 5′-AGAAGCAGAGGAACTTCGAC-3′ and reverse 5′-CCTTGATCTGGTTGAACTCC-3′; MHC-specific primers for rat cardiomyocytes, forward 5′-GGAGACCTTCAAGCGGGAGA-3′ and reverse 5′-AGGGCTGACTGCAGCTCCA-3′. Negative control reactions consisted of the PCR amplification mix described above with primers but no cDNA template. Commercially available human heart cDNAs (Ambion) were used as positive controls. PCR was performed using an Eppendorf Mastercycler gradient (Eppendorf, Westbury, NY, USA). The cycles were programmed as follows: 94°C for 10 min, 35 cycles of 30 s of denaturation at 94°C, 30 s at an annealing temperature of 57°C (for a human-specific MHC primer) or 61°C (for a rat-specific MHC primer), a 45-s extension step at 72°C, and a final extension step at 72°C for 5 min. Expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as an internal control. The PCR product size was confirmed by loading 10 μl of PCR reaction mixtures to 1.5% agarose gel electrophoresis. Real-time PCR was performed using a light cycler from Bio-Rad. Program: 95°C (10 s) and 58°C(45 s) for 50 cycles. Human troponin type 2: forward 5′-GAC CTG CAG GAG AAG TTC AA-3′, reverse 5′-GAG GAG CAG ATC TTT GGT GA-3′. Human GAPDH: forward 5′GAGTCAACGGATTTGGTCGT3′, reverse 5′TTGATTTTGGAGGGATCTC3′.
Images of beating fused cells
Images of beating hASCs fused with rat cardiomyocytes (with green and red fluorescent signals) were recorded using a CCD camera with a phase-contrast microscope (Zeiss) 5 d after treatment of coculture (hASCs and rat cardiomyocytes) with HVJ.
Whole-cell voltage-clamp recordings
hASCs (passage 3), rat cardiomyocytes (8 d after culture), or hASCs cocultured with rat cardiomyocytes (5 d after treatment with HVJ) cultured on glass coverslips in 24-well plates were used for whole-cell voltage-clamp analysis. For recording of action potential of cultured cells, one of the coverslips was transferred to a chamber perfused with modified Tyrode's solution at a rate of 1.5 ml/min. The perfusing solution was equilibrated in 5% CO2 and 95% O2 and maintained at room temperature (21–22°C). The contents of the perfusing solution was as follows: 136 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 0.33 mM NaH2PO4, 10 mM glucose, and 10 mM HEPES; the pH was adjusted to 7.3 with NaOH, and the osmolarity was 320–330 mosmol/L. Visualized whole-cell voltage-clamp recordings were obtained using a glass pipette (resistance, 2–3 MΩ) filled with a solution containing: 140 mM potassium gluconate, 10 mM NaCl, 2 mM MgCl2, 2 mM EGTA, 5 mM HEPES; the pH was adjusted to 7.3 with KOH, and the osmolarity was 300–310 mosmol/L. An AxoPatch 1D amplifier and the AxoGraph software program (Axon Instruments, Union City, CA, USA) were used for data acquisition and online and offline data analyses. A seal resistance ≥ 2 GΩ and an access resistance ≤ 10 MΩ were considered acceptable. Series resistance was optimally compensated for 80%, and it was decreased to 60% when current oscillation was detected. The access resistance was monitored throughout the experiment. The cells were held at −80 mV. Voltage steps of 300 ms from −60 to +60 mV in 10-mV increments were used to induce action potential. Data were digitized at 20 kHz and filtered at 2 kHz. The experiments were conducted at room temperature (∼22°C).
Data analysis
The reported data are expressed as means ± sd. The statistical significance of the differences between groups was determined using the Student's t test. A level of P ≤ 0.05 was considered statistically significant.
RESULTS
hASCs exhibit differentiation potential
hASCs isolated from human adipose tissue exhibited fibroblast or spindle-like morphology (Fig. 1A). To evaluate the differentiation capacity of hASCs, hASCs were cultured in adipogenic and osteogenic medium for 3 wk. hASCs in an adipogenic induction medium displayed characteristic multiple intracellular bright white oil droplets. These droplets exhibited red vesicles when stained with Oil Red O solution (Fig. 1B). No lipid droplets were observed in cells cultured in control medium (Fig. 1C). Cells cultured in osteogenic medium showed black regions within the monolayer, which indicated calcification deposits from differentiated osteoblasts. Calcification of the extracellular matrix (Fig. 1D) appeared red after induced hASCs were stained with Alizarin Red S dye. However, red staining was not observed in cells cultured in a control medium (Fig. 1E).
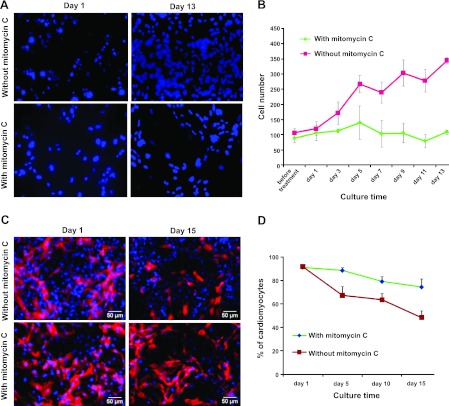
Treatment with mitomycin C inhibits the proliferation of cardiac fibroblasts and increases the percentage of cardiomyocytes in culture
Freshly isolated cells from neonatal rat hearts contained both cardiomyocytes and fibroblasts in cardiomyocyte culture medium. Cell proliferation was measured by counting nuclei stained blue with DAPI. The number of cells isolated from rat hearts increased by >3-fold within 13 d of culture, mainly because of the proliferation of cardiac fibroblasts (Fig. 2A, B). Cardiomyocytes were identified by their expression of troponin I, using immunofluorescent staining analysis with a rat/human reactive antibody. Because of the limited proliferation potential of cardiomyocytes and the strong proliferation potential of fibroblasts, the relative proportion of cardiomyocytes in culture decreased by more than half within 15 d after culture (Fig. 2C, D). To increase the proportion of cardiomyocytes and thereby maximize the fusion efficiency of hASCs with rat cardiomyocytes, the cultured cells were treated with mitomycin C to inhibit the proliferation of cardiac fibroblasts before treatment with HVJ. As shown in Fig. 2C, D, this treatment inhibited the proliferation of fibroblasts markedly and increased the proportion of cardiomyocytes in the culture.
Figure 2.
Treatment with mitomycin C inhibits cardiac fibroblast proliferation (A, B) and increases the percentage of cardiomyocytes in rat cardiomyocyte culture (C, D). A) Fluorescent images of a rat cardiomyocyte culture stained with DAPI on d 1 and 13 after seeding. B) Growth rate of cells was measured by counting DAPI-positive cells in cardiomyocyte culture at different time points. C) Immunofluorescent staining analysis of troponin I expression in rat cardiomyocyte culture on d 1 and 15 after treatment with or without mitomycin C. Blue staining indicates nuclear signals; red staining indicates troponin I signals. Scale bars = 50 μm. D) Percentage of troponin I-positive cardiomyocytes in rat cardiomyocyte culture over time after treatment with or without mitomycin C. Data are expressed as means ± sd (n=3).
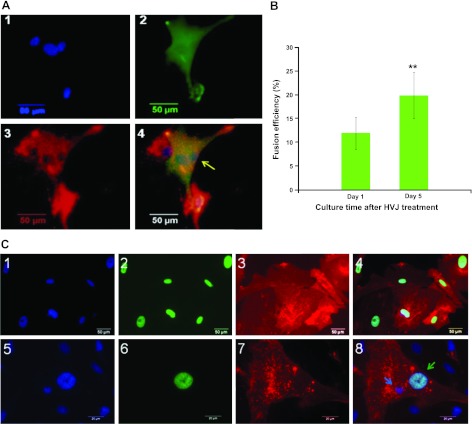
HVJ induces the fusion formation between hASCs and neonatal rat cardiomyocytes
hASCs (DiO green labeled) were fused with rat cardiomyocytes (DiI red labeled) after treatment with HVJ. Fused cells showed both green and red fluorescence signals and multiple nuclei under a fluorescence microscope (Fig. 3A). Most fused cells exhibited spontaneous rhythmic contraction on d 2 after HVJ treatment. Fusion efficiency increased with culture time; fusion efficiency was 12.48 ± 4.36% after 1 d and 19.86 ± 4.84% 5 d after treatment with HVJ (Fig. 3B, P<0.01). To further investigate whether HVJ induced cellular cytoplasm fusion or nuclear incorporation, we transduced hASCs with the lentiviral vector. Transduced hASCs displayed green nuclei and red cell membranes (Fig. 3C1–4). After treatment with HVJ, fused hASC-cardiomyocytes contained both hASC nuclei and rat cardiomyocyte nuclei (Fig. 3C5–8). These results indicate that HVJ induced cytoplasmic fusion between hASCs and rat cardiomyocytes but did not induce cell nuclear fusion.
Figure 3.
HVJ induces fusion formation between hASCs and neonatal rat cardiomyocytes. A) Fluorescent images of hASCs cocultured with rat cardiomyocytes 5 d after treatment with HVJ. 1) Nuclei were stained with DAPI. 2) hASCs were stained green with DiO. 3) Cardiomyocytes were stained red with DiI. 4) Overlay of images 1–3. Fused hASC-cardiomyocytes displayed both green and red fluorescent signals and showed yellow staining (arrow). B) Fusion efficiency of hASCs with cardiomyocytes was analyzed by calculation of the ratio between number of Dil and DiO double-positive cells and number of DAPI-positive cells at each time point (on d 1 and 5) after treatment of HVJ. Data are expressed as means ± sd (n=3). C) HVJ induces cellular cytoplasm fusion between hASCs and rat neonatal cardiomyocytes but does not induce nuclear fusion. hASCs were transduced with a lentiviral vector pLVX-Puro-HS-GR. 1–4) Transduced hASCs were green in nuclei (panel 2) and red in cell membrane (panel 3). Panel 1 shows fluorescent image of hASCs stained blue with DAPI. Panel 4 shows overlay of images 1–3. 5–8) At 5 d after HVJ-mediated fusion, fused hASCs contained separate green hASC nuclei and rat cardiomyocyte nuclei (green and blue arrows, respectively; panel 8). Staining as in 1–4. Scale bars = 50 μm (A, C1–4); 20 μm (C5–8).
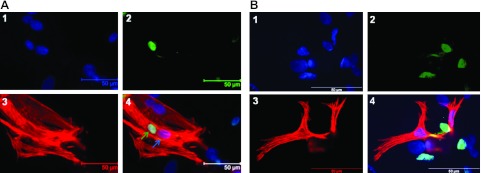
hASCs fused with rat cardiomyocytes express cardiomyocyte markers encoded by rat cardiomyocytes
We performed immunofluorescent staining to trace the fate of hASCs after fusion with rat cardiomyocytes. Figure 4A shows hASCs fused with cardiomyocytes, as identified by the existence of both green fluorescent signals in nuclei labeled with a FITC-conjugated anti-human nuclear antibody and red fluorescent signals from a rat/human reactive antibody directed against cardiac-specific marker troponin I. In addition, fused cells contained both human nuclei and rat cardiomyocyte nuclei. These results suggest that fused hASC-cardiomyocytes exhibit a cardiomyocyte phenotype and further indicates that fusion occurred by cellular cytoplasm incorporation between hASCs and cardiomyocytes. To confirm that fusion and the cardiac phenotype displayed in hASCs after 5 d of coculture with rat cardiomyocytes resulted from HVJ treatment, we also performed coculture experiments without treatment with HVJ. Neither fusion nor cardiac phenotype (Fig. 4B) and beating were observed in hASCs 5 d after coculture. Cardiac-specific marker troponin I expression was observed in hASCs 3 wk after coculture with cardiomyocytes (data not shown) without the use of HVJ fusion mediator. However, we did not observe spontaneous contraction of hASCs in coculture.
Figure 4.
hASCs fused with cardiomyocytes express cardiomyocyte markers 5 d after treatment with HVJ. A) Immunofluorescent staining analysis showed that hASCs fused with rat cardiomyocytes were positive for troponin I after treatment with HVJ. 1) Nuclei were stained blue with DAPI. 2) Human cells were stained green with an anti-human nuclear antibody. 3) Cardiomyocytes were stained red with an antibody against troponin I. 4) Overlay of images 1–3. Green arrow indicates the presence of human nuclei independent from rat nuclei (blue arrow) within the same cell. B) hASCs did not fuse with rat cardiomyocytes without treatment of HVJ, as evidenced by the negative expression of troponin I (red signals) in hASCs with green nuclei stained by an anti-human nuclear antibody (panel 4). 1) Nuclei were stained blue with DAPI. 2) Human cells were stained green with an anti-human nuclear antibody. 3) Cardiomyocytes were stained red with an antibody against troponin I. 4) Overlay of images 1–3. Scale bars = 50 μm.
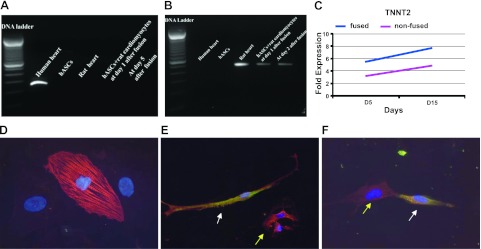
Next, we investigated whether cardiac gene expression in fused hASC-cardiomyocytes would be seen in hASCs or from rat cardiomyocytes. We performed RT-PCR with human- and rat-specific MHC primers to analyze MHC expression in fused cells. Electrophoretic analysis of the PCR product show that fused hASC-cardiomyocytes only expressed rat-specific MHC and did not express human-specific MHC, which is a structural cardiac protein expressed late in differentiation (Fig. 5A, B). However, real-time PCR analysis of human-specific troponin T type 2 (TNNT2) shows that the expression level of TNNT2 increased in both fused and confused cocultured cells between d 5 and 15 when compared to the mean value of human ASCs (Fig. 5C). Furthermore, the expression of TNNT2 was consistently higher in fused cells compared to confused cells (Fig. 5C). We also found that some ASCs formed close contact with rat neonatal cardiomyocytes and expressed troponin T protein induced by the presence of the rat cardiomyocytes (Fig. 5E, F).
Figure 5.
Analysis of cardiac genes and protein expression. A, B) MHC as a late marker of cardiomyocyte gene expression in PCR products of cells using a human-specific MHC primer (A) and a rat-specific MHC primer (B). Samples loaded in lanes (left to right): 100-bp DNA ladder, PCR products from human heart cDNA, rat heart cDNA, and cDNA of hASCs fused with rat cardiomyocytes 5 d after fusion. C) Real-time PCR analysis of human troponin T type 2 (TNNT2) in fused and nonfused cells. Fold expression relative to mean values of human ASCs cultured at d 5, 10, and 15. D) Rat neonatal cardiomyocyte expression troponin T. E) Human ASCs (passage 3, GFP labeled) express troponin T (red) when cocultured for 2 wk with rat neonatal cardiomyocytes. F) Red rat cardiomyocyte and yellow human ASCs form a junction. White arrows: GFP-labeled human ASCs express GFP (green) and troponin T (red), resulting in a yellow cytoplasm. Yellow arrows: rat neonatal cardiomyocyte stained with troponin T (red).
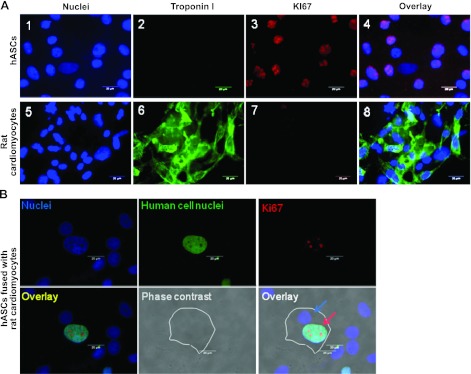
We also sought to determine whether hASCs fused with cardiomyocytes maintain their proliferative potential. Immunofluorescence staining assays showed that hASCs expressed Ki67, a marker of proliferating cells, both before (Fig. 6A1–4) and after (Fig. 6B) fusion with rat cardiomyocytes. The percentage of Ki67-positive fused cells among total fused cells was 7.87 ± 1% on d 5 after treatment of HVJ. However, rat cardiomyocytes were negative for Ki67 (Fig. 6A5–8).
Figure 6.
hASCs maintain self-renewal potential, as evidenced by expression of proliferating marker Ki67. A) 1–4) hASCs cultured alone and stained as indicated express Ki67 but do not express troponin I. 5–6) Troponin I-positive rat cadiomyocytes were negative for Ki67. B) Rat cardiomyocytes fused with hASCs contained both rat cardiomyocyte nuclei (blue arrow) and hASC nuclei (labeled green using antibody against human nuclei) positive for Ki67 (red arrow). White outline traces the fused cell. Scale bars = 20 μm.
hASCs fused with cardiomyocytes exhibit action potential
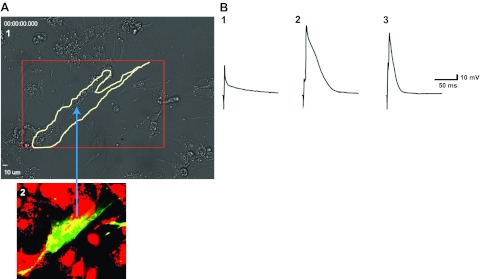
We performed whole-cell voltage-clamp action potential analysis under a microscope in hASCs, rat cardiomyocytes, and hASCs fused with rat cardiomyocytes. Figure 7A demonstrates images taken from the same field in the culture as in Supplemental Video S1. Fused hASC-cardiomyocytes (tracked by a white line in Fig. 7A) contained both green and red fluorescence signals and showed spontaneous contraction (Supplemental Video S1). Fused cells with both red and green fluorescence signals were able to generate action potentials, as demonstrated in 8 recorded beating fused cells. However, unfused hASCs did not beat or generate any action potentials (Fig. 7B).
Figure 7.
hASCs fused with rat cardiomyocytes show spontaneous contraction and exhibit action potential 5 d after treatment with HVJ. A) 1) Fluorescence image taken from same field in the culture as in Supplemental Video S1. Area outlined in white line is fused cells that beat spontaneously, shown in Supplemental Video S1. 2) White-outlined cells in panel 1 containing both red and green fluorescent signals displayed yellow, indicating fusion formation between hASCs and rat cardiomyocytes. B) Representative voltage responses to the depolarizing voltage steps from an hASC (trace 1), a neonatal rat cardiomyocyte (trace 2), and an hASC fused with a rat neonatal cardiomyocyte (trace 3) under current clamp. Only rat cardiomyocytes and hASCs fused with rat cardiomyocytes displayed action potentials.
DISCUSSION
This is the first study to examine the fate of hASCs fused with rat cardiomyocytes after HVJ treatment. We demonstrate that cells of fused hASC-cardiomyocytes display a cardiomyocyte phenotype and spontaneous rhythmic contraction and generate an action potential in vitro. While late cardiac structural proteins such as myosin heavy chain exhibited in fused hASC-cardiomyocytes were primarily derived from rat cardiomyocytes, genes encoding human-specific toponin T were up-regulated in fused ASCs compared nonfused cocultured cells.
Both experimental and clinical studies have demonstrated that transplanted adult stem cells could enhance cardiac function after myocardial infarction (3, 17). Furthermore, several previous reports have shown the fusion formation between transplanted donor stem cells and host cardiomyocytes. Although the fusion frequency was low, fusion was considered to be one of the mechanisms underlying the beneficial effects of adult stem cells on cardiac function (18, 19). In this study, in order to obtain a sufficient number of fused cells to study their fate in vitro, we administered HVJ as a treatment mode to artificially induce the fusion formation between DiO-labeled hASCs and DiI-labeled rat cardiomyocytes.
The percentage of cardiomyocytes in culture increased to more than 80% on d 15 after the start of the culture. Therefore, the fusion efficiency of hASCs and mitomycin C-treated rat cardiomyocytes arrived at 19.86 ± 4.84% on d 5 after treatment with HVJ. However, we did not observe fusion in the culture without treatment with HVJ, although we found ASCs that formed close contact with cardiomyocytes and induced the expression cardiac troponin T in human ASCs. The reason for this may have been that the fusion efficiency in vitro without HVJ is low, as described previously (20); and the fusion formation might occur in a special niche after tissue injury.
In this study, we used different approaches to monitor the cardiac features of hASCs after coculture with rat cardiomyocytes and treatment with HVJ. The experimental data showed that hASCs exhibited action potential and spontaneous rhythmic contraction 2 d after fusion with rat cardiomyocytes. We did not observe spontaneous contraction of hASCs in the 21-d culture without treatment with HVJ. A relatively low percentage of troponin I-positive hASCs were observed in the 3-wk coculture without HVJ treatment. This result indicates that the rat cardiomyocyte environment alone has a low induction on hASCs to differentiate into functional cardiomyocytes, and that the cardiac functional properties of hybridized hASCs resulted from fusion with rat cardiomyocytes but not from transdifferentiation of stem cells. This finding is consistent with previous reports. For example, Ishikawa et al. (21) demonstrated that purified human hematopoietic stem cells contributed to the generation of cardiomyocytes via cell fusion. Zhang et al. (20) demonstrated that hypoxia and cytokines such as IL-6 and TNF-α released after myocardial infarction promote the fusion of transplanted CD34-positive cells with the host cardiomyocytes. Also, C2C12 myoblasts have been shown to form hybrid skeletal-cardiac cells both in vivo and in vitro by Reinecke et al. (22). Whether the mechanisms of HVJ-mediated fusion formation in vitro are similar to those of fusion occurring in injured tissue environments in vivo, and whether hASCs fused with cardiomyocytes have the same electrical properties as native cardiomyocytes remain to be further investigated. Nevertheless, our present study demonstrated that fusion between ASC and cardiomyocytes indeed occurred and can be enhanced by HVJ virus. This is an initial, but important, step in establishing fusion as a potential mechanism for ASC-based therapy. In follow-up studies, we will investigate how fusion occurs naturally and explore approaches that are more amenable to clinical use and explore the role of hypoxia and cytokines such as IL-6 and TNF-α to determine whether these conditions or other inflammatory cytokines could eventually replace the HVJ virus to induce spontaneous cell fusion.
Both our immunofluorescence staining and RT-PCR analyses show that cardiac features of fused cells were derived primarily from rat cardiomyocytes. Fused cells contained multiple separate hASC- and rat cardiomyocyte-derived nuclei (Figs. 4–6) but did not contain fused nuclei. These results suggest that fusion of hASCs with cardiomyocytes was able to induce the expression of endogenous cardiac-specific genes in hASCs but not of the respective proteins within a short period of time. Lapidos et al. (23) described a similar experimental phenomenon in which fusion of donor bone marrow-derived, wild-type side population stem cells with striated muscle failed to express sarcoglycan following transplantation into mice lacking δ-sarcoglycan expression (a model of cardiomyopathy and muscular dystrophy).
Fused hASC-cardiomyocytes continue to display their functional cardiac properties but also demonstrate stem cell self-renewal capacity, as confirmed by the Ki67 expression in fused cells (Fig. 7B). Ki67 is expressed in all phases of the cell cycle except the G0 phase. This result suggests that cardiomyocytes could enter the cell cycle and at the same time maintain a cardiac phenotype after fusion with stem cells. In this way, fusion of stem cells with resident cardiomyocytes might act as a rapid, intermediate repair mechanism that enables salvage of the damaged heart en route to more durable regeneration of the myocardium.
This study demonstrates that rat cardiomyocytes fused with ASCs display both stem cell proliferating capacity and functional cardiomyocyte properties (action potential, and spontaneous rhythmic beating) within 5 d after fusion. Our in vitro data indicate that stem cells are capable and have the theoretical capacity to compensate for a sudden loss of cardiomyocytes immediately following myocardial infarction via fusion with cardiomyocytes, supporting the concept that fusion, even if artificially induced in our study, is a potential mechanism for cardiomyocyte renewal in the heart.
Supplementary Material
Acknowledgments
The authors thank Sebastian Gehmert, Sanga Gehmert, Grace Wu, Martha French, and Cynthia Austin for their excellent technical assistance.
This work was supported by the Alliance of Cardiovascular Researchers, grant 543102 (to E.A.).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Dawn B., Tiwari S., Kucia M. J., Zuba-Surma E. K., Guo Y., Sanganalmath S. K., Abdel-Latif A., Hunt G., Vincent R. J., Taher H., Reed N. J., Ratajczak M. Z., Bolli R. (2008) Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells 26, 1646–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Farahmand P., Lai T. Y., Weisel R. D., Fazel S., Yau T., Menasche P., Li R. K. (2008) Skeletal myoblasts preserve remote matrix architecture and global function when implanted early or late after coronary ligation into infarcted or remote myocardium. Circulation 118, S130–137 [DOI] [PubMed] [Google Scholar]
- 3. Valina C., Pinkernell K., Song Y. H., Bai X., Sadat S., Campeau R. J., Le Jemtel T. H., Alt E. (2007) Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur. Heart. J. 28, 2667–2677 [DOI] [PubMed] [Google Scholar]
- 4. Deb A., Wang S., Skelding K. A., Miller D., Simper D., Caplice N. M. (2003) Bone marrow-derived cardiomyocytes are present in adult human heart: A study of gender-mismatched bone marrow transplantation patients. Circulation 107, 1247–1249 [DOI] [PubMed] [Google Scholar]
- 5. Noiseux N., Gnecchi M., Lopez-Ilasaca M., Zhang L., Solomon S. D., Deb A., Dzau V. J., Pratt R. E. (2006) Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol. Ther. 14, 840–850 [DOI] [PubMed] [Google Scholar]
- 6. Alvarez-Dolado M., Pardal R., Garcia-Verdugo J. M., Fike J. R., Lee H. O., Pfeffer K., Lois C., Morrison S. J., Alvarez-Buylla A. (2003) Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 425, 968–973 [DOI] [PubMed] [Google Scholar]
- 7. Rota M., Kajstura J., Hosoda T., Bearzi C., Vitale S., Esposito G., Iaffaldano G., Padin-Iruegas M. E., Gonzalez A., Rizzi R., Small N., Muraski J., Alvarez R., Chen X., Urbanek K., Bolli R., Houser S. R., Leri A., Sussman M. A., Anversa P. (2007) Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc. Natl. Acad. Sci. U. S. A. 104, 17783–17788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Strem B. M., Hedrick M. H. (2005) The growing importance of fat in regenerative medicine. Trends Biotechnol. 23, 64–66 [DOI] [PubMed] [Google Scholar]
- 9. Planat-Benard V., Menard C., Andre M., Puceat M., Perez A., Garcia-Verdugo J. M., Penicaud L., Casteilla L. (2004) Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ. Res. 94, 223–229 [DOI] [PubMed] [Google Scholar]
- 10. Mochizuki H., Schwartz J. P., Tanaka K., Brady R. O., Reiser J. (1998) High-titer human immunodeficiency virus type 1-based vector systems for gene delivery into nondividing cells. J. Virol. 72, 8873–8883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cai L., Johnstone B. H., Cook T. G., Tan J., Fishbein M. C., Chen P. S., March K. L. (2009) IFATS series: human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells 27, 230–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim J., Okada Y. (1987) Difference in capacities for virion-to-virion fusion of young and aged HVJ (Sendai virus): a model of membrane fusion. J. Membr. Biol. 97, 241–249 [DOI] [PubMed] [Google Scholar]
- 13. Gimble J., Guilak F. (2003) Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy 5, 362–369 [DOI] [PubMed] [Google Scholar]
- 14. Bai X., Ma J., Pan Z., Song Y. H., Freyberg S., Yan Y., Vykoukal D., Alt E. (2007) Electrophysiological properties of human adipose tissue-derived stem cells. Am. J. Physiol. Cell Physiol. 293, C1539–C1550 [DOI] [PubMed] [Google Scholar]
- 15. Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., Mosca J. D., Moorman M. A., Simonetti D. W., Craig S., Marshak D. R. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 [DOI] [PubMed] [Google Scholar]
- 16. Oedayrajsingh-Varma M. J., van Ham S. M., Knippenberg M., Helder M. N., Klein-Nulend J., Schouten T. E., Ritt M. J., van Milligen F. J. (2006) Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy 8, 166–177 [DOI] [PubMed] [Google Scholar]
- 17. Stamm C., Kleine H. D., Westphal B., Petzsch M., Kittner C., Nienaber C. A., Freund M., Steinhoff G. (2004) CABG and bone marrow stem cell transplantation after myocardial infarction. Thorac. Cardiovasc. Surg. 52, 152–158 [DOI] [PubMed] [Google Scholar]
- 18. Nygren J. M., Jovinge S., Breitbach M., Sawen P., Roll W., Hescheler J., Taneera J., Fleischmann B. K., Jacobsen S. E. (2004) Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat. Med. 10, 494–501 [DOI] [PubMed] [Google Scholar]
- 19. Oh H., Bradfute S. B., Gallardo T. D., Nakamura T., Gaussin V., Mishina Y., Pocius J., Michael L. H., Behringer R. R., Garry D. J., Entman M. L., Schneider M. D. (2003) Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc. Natl. Acad. Sci. U. S. A. 100, 12313–12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang S., Shpall E., Willerson J. T., Yeh E. T. (2007) Fusion of human hematopoietic progenitor cells and murine cardiomyocytes is mediated by alpha 4 beta 1 integrin/vascular cell adhesion molecule-1 interaction. Circ. Res. 100, 693–702 [DOI] [PubMed] [Google Scholar]
- 21. Ishikawa F., Shimazu H., Shultz L. D., Fukata M., Nakamura R., Lyons B., Shimoda K., Shimoda S., Kanemaru T., Nakamura K., Ito H., Kaji Y., Perry A. C., Harada M. (2006) Purified human hematopoietic stem cells contribute to the generation of cardiomyocytes through cell fusion. FASEB J. 20, 950–952 [DOI] [PubMed] [Google Scholar]
- 22. Reinecke H., Minami E., Poppa V., Murry C. E. (2004) Evidence for fusion between cardiac and skeletal muscle cells. Circ. Res. 94, e56–e60 [DOI] [PubMed] [Google Scholar]
- 23. Lapidos K. A., Chen Y. E., Earley J. U., Heydemann A., Huber J. M., Chien M., Ma A., McNally E. M. (2004) Transplanted hematopoietic stem cells demonstrate impaired sarcoglycan expression after engraftment into cardiac and skeletal muscle. J. Clin. Invest. 114, 1577–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.