Abstract
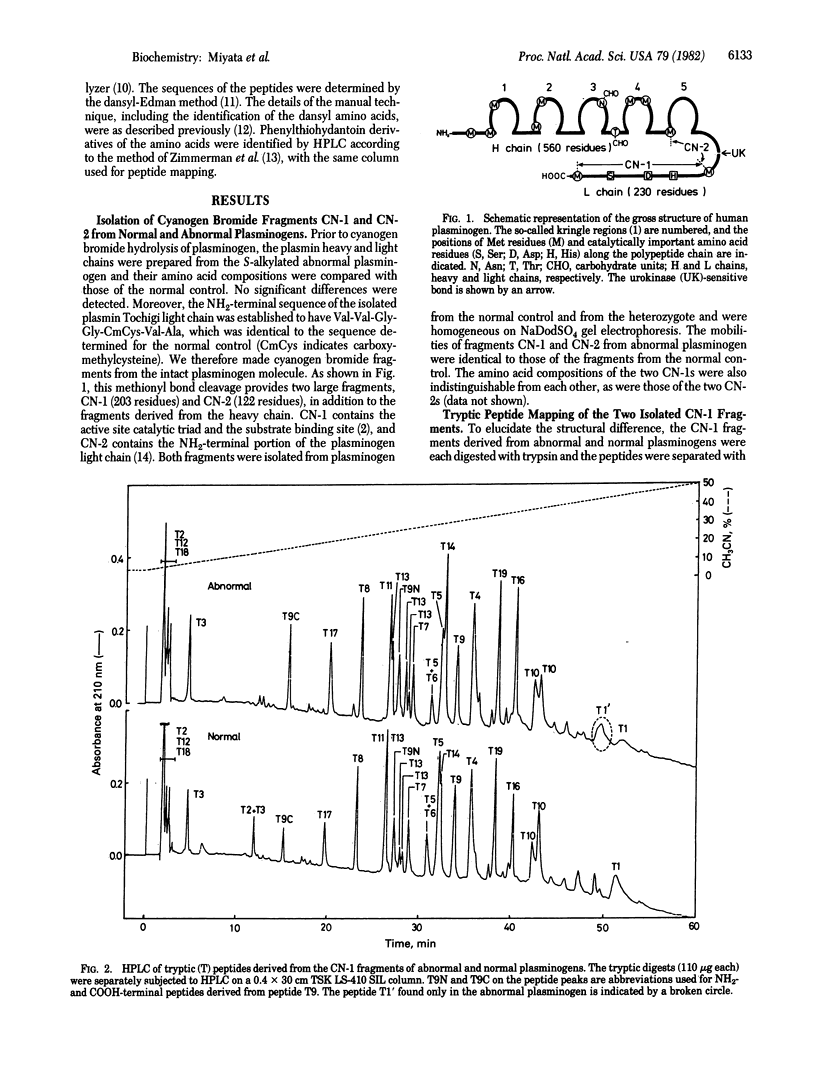
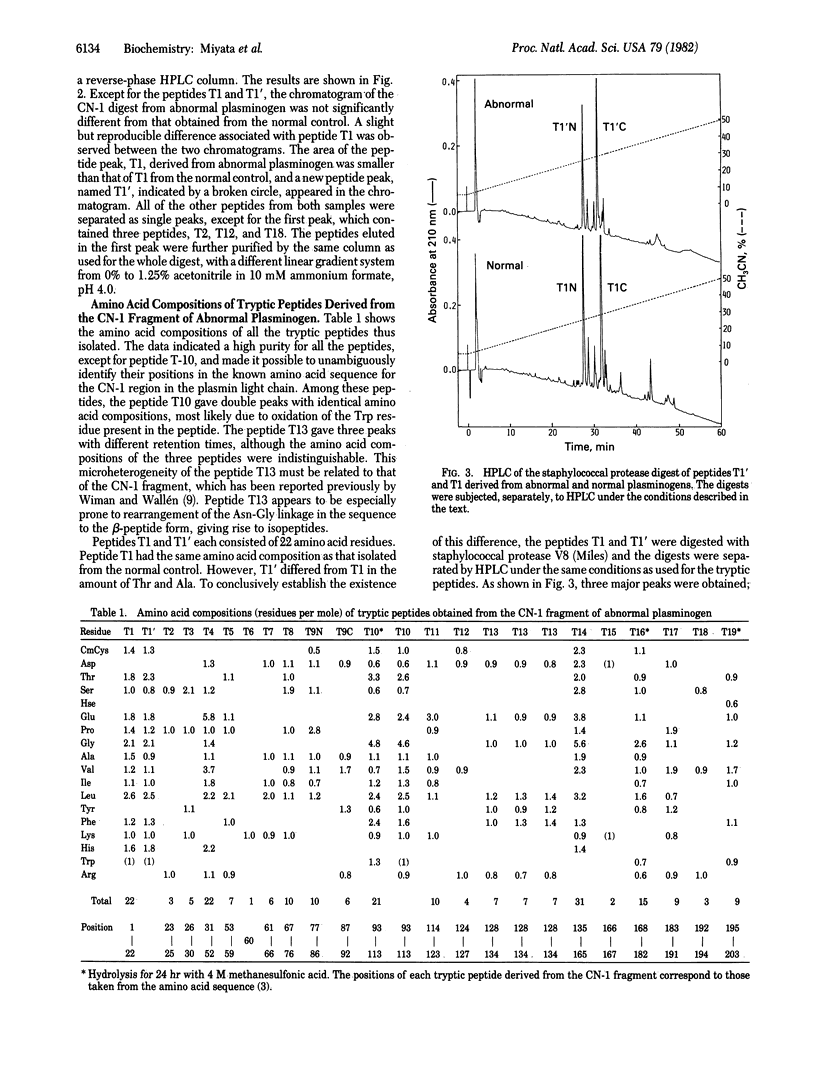
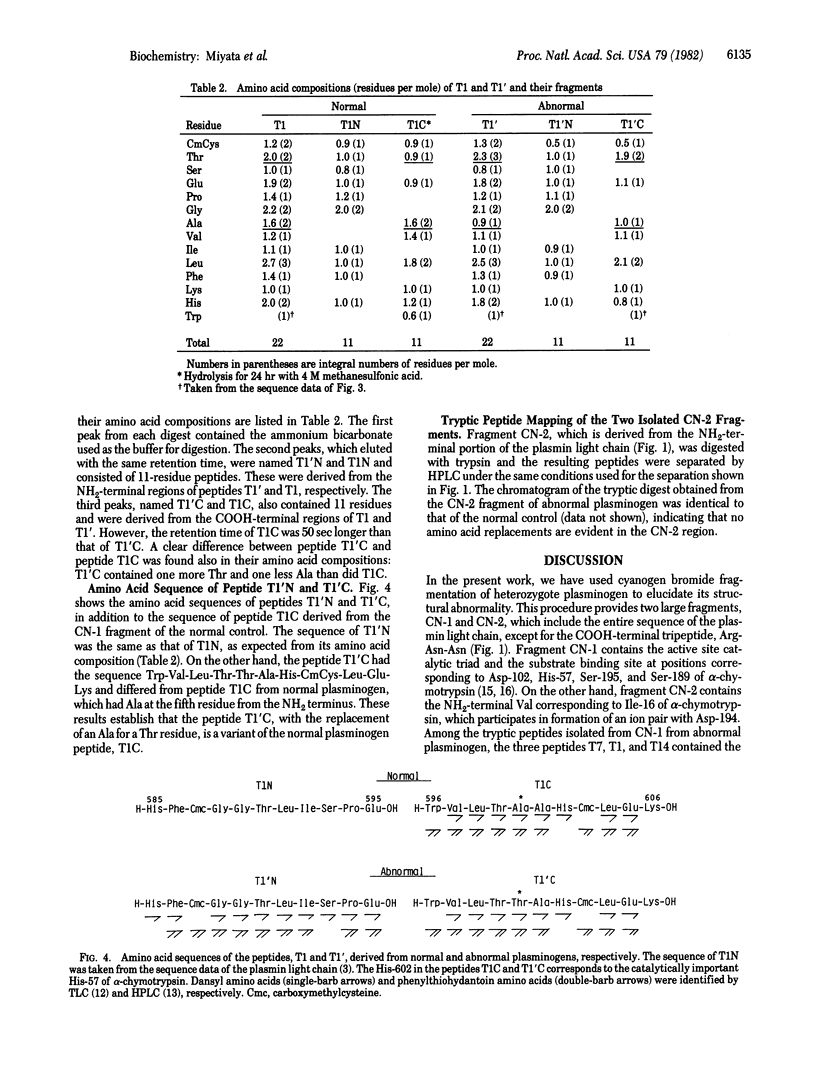
Structural studies on a hereditarily abnormal plasminogen, plasminogen Tochigi, have been performed to identify the difference responsible for its lack of proteolytic activity. The plasminogen sample used was from a heterozygote and thus consisted of apparently equal amounts of normal and defective plasminogen molecules. Amino acid sequence analysis of a tryptic peptide isolated from the abnormal plasminogen indicated that Ala-600 (equivalent to Ala-55 in the chymotrypsin numbering system) had been replaced by Thr. No other substitutions in the active-site residues--namely, His-57, Asp-102, and Ser-195--were found. Molecular models for chymotrypsin and the bovine trypsin-pancreatic trypsin inhibitor complex indicate that Ala-55 is very near the active-site His. The Thr at position 55 in plasminogen (plasmin) Tochigi may perturb His-57 such that the proton transfers associated with the normal catalytic process cannot occur in the abnormal plasmin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki N., Moroi M., Sakata Y., Yoshida N., Matsuda M. Abnormal plasminogen. A hereditary molecular abnormality found in a patient with recurrent thrombosis. J Clin Invest. 1978 May;61(5):1186–1195. doi: 10.1172/JCI109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birktoft J. J., Blow D. M. Structure of crystalline -chymotrypsin. V. The atomic structure of tosyl- -chymotrypsin at 2 A resolution. J Mol Biol. 1972 Jul 21;68(2):187–240. doi: 10.1016/0022-2836(72)90210-0. [DOI] [PubMed] [Google Scholar]
- Blow D. M., Birktoft J. J., Hartley B. S. Role of a buried acid group in the mechanism of action of chymotrypsin. Nature. 1969 Jan 25;221(5178):337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Huber R., Kukla D., Bode W., Schwager P., Bartels K., Deisenhofer J., Steigemann W. Structure of the complex formed by bovine trypsin and bovine pancreatic trypsin inhibitor. II. Crystallographic refinement at 1.9 A resolution. J Mol Biol. 1974 Oct 15;89(1):73–101. doi: 10.1016/0022-2836(74)90163-6. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Iwanaga S., Suzuki T. On the activation of bovine plasma factor XIII. Amino acid sequence of the peptide released by thrombin and the terminal residues of the subunit polypeptides. J Biochem. 1975 Dec;78(6):1247–1266. doi: 10.1093/oxfordjournals.jbchem.a131023. [DOI] [PubMed] [Google Scholar]
- Robbins K. C., Summaria L., Hsieh B., Shah R. J. The peptide chains of human plasmin. Mechanism of activation of human plasminogen to plasmin. J Biol Chem. 1967 May 25;242(10):2333–2342. [PubMed] [Google Scholar]
- Sakata Y., Aoki N. Molecular abnormality of plasminogen. J Biol Chem. 1980 Jun 10;255(11):5442–5447. [PubMed] [Google Scholar]
- Stroud R. M., Kay L. M., Dickerson R. E. The structure of bovine trypsin: electron density maps of the inhibited enzyme at 5 Angstrom and at 2-7 Angstron resolution. J Mol Biol. 1974 Feb 25;83(2):185–208. doi: 10.1016/0022-2836(74)90387-8. [DOI] [PubMed] [Google Scholar]
- Summaria L., Hsieh B., Groskopf W. R., Robbins K. C. The isolation and characterization of the S-carboxymethyl beta (light) chain derivative of human plasmin. The localization of the active site on the beta (light) chain. J Biol Chem. 1967 Nov 10;242(21):5046–5052. [PubMed] [Google Scholar]
- Wiman B. Primary structure of the B-chain of human plasmin. Eur J Biochem. 1977 Jun 1;76(1):129–137. doi: 10.1111/j.1432-1033.1977.tb11578.x. [DOI] [PubMed] [Google Scholar]
- Wiman B., Wallén P. Activation of human plasminogen by an insoluble derivative of urokinase. Structural changes of plasminogen in the course of activation to plasmin and demonstration of a possible intermediate compound. Eur J Biochem. 1973 Jul 2;36(1):25–31. doi: 10.1111/j.1432-1033.1973.tb02880.x. [DOI] [PubMed] [Google Scholar]
- Wiman B., Wallén P. Amino-acid sequence of the cyanogen-bromide fragment from human plasminogen that forms the linkage between the plasmin chains. Eur J Biochem. 1975 Oct 15;58(2):539–547. doi: 10.1111/j.1432-1033.1975.tb02403.x. [DOI] [PubMed] [Google Scholar]
- Wiman B., Wallén P. On the primary structure of human plasminogen and plasmin. Purification and characterization of cyanogen-bromide fragments. Eur J Biochem. 1975 Sep 15;57(2):387–394. doi: 10.1111/j.1432-1033.1975.tb02312.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. L., Appella E., Pisano J. J. Rapid analysis of amino acid phenylthiohydantoins by high-performance liquid chromatography. Anal Biochem. 1977 Feb;77(2):569–573. doi: 10.1016/0003-2697(77)90276-7. [DOI] [PubMed] [Google Scholar]



