Abstract
An exaggerated response to emotional stimuli is one of several symptoms widely reported by veterans of the 1991 Persian Gulf War. Many have attributed these symptoms to post-war stress; others have attributed the symptoms to deployment-related exposures and associated damage to cholinergic, dopaminergic, and white matter systems. We collected event-related potential (ERP) data from 20 veterans meeting Haley criteria for Gulf War Syndromes 1–3 and from 8 matched Gulf War veteran controls, who were deployed but not symptomatic, while they performed an auditory three-condition oddball task with gunshot and lion roar sounds as the distractor stimuli. Reports of hyperarousal from the ill veterans were significantly greater than those from the control veterans; different ERP profiles emerged to account for their hyperarousability. Syndromes 2 and 3, who have previously shown brainstem abnormalities, show significantly stronger auditory P1 amplitudes, purported to indicate compromised cholinergic inhibitory gating in the reticular activating system. Syndromes 1 and 2, who have previously shown basal ganglia dysfunction, show significantly weaker P3a response to distractor stimuli, purported to indicate dysfunction of the dopaminergic contribution to their ability to inhibit distraction by irrelevant stimuli. All three syndrome groups showed an attenuated P3b to target stimuli, which could be secondary to both cholinergic and dopaminergic contributions or disruption of white matter integrity.
Keywords: Gulf War Illness, hyperarousal, ERPs, P1, P3a, P3b, cholinergic, dopaminergic
1. Introduction
Many veterans returned from the 1991 Persian Gulf War with multiple symptoms, including cognitive impairments, emotional disturbances, fatigue, loss of balance/dizziness, tremors/shaking, headaches, joint pain, rashes, and sleep disturbance (Fukuda et al., 1998; Haley, 1997; Levine et al., 2006). These symptom complexes have been attributed to postwar stress syndromes such as posttraumatic stress disorder (PTSD; Dlugosz et al., 1999; Gifford et al., 2006; Gray et al., 1999; Lincoln et al., 2006; Stimpson et al., 2003), which can manifest with many of the reported symptoms. However, the contention that PTSD per se underlies these cognitive, psychological, and somatic symptoms has been challenged (Golier et al., 2007; Haley, 1997). Other studies (e.g., Research Advisory Committee on Gulf War Veterans’ Illnesses, 2008) have attributed the psychological, somatic, and cognitive symptoms to the results of deployment-related exposures. Both the studies that attribute the unexplained symptoms to PTSD and those that attribute them to organic/physiological origins agree that deployed veterans report more nonspecific symptoms than do those not deployed.
Hyperarousal is one of the symptoms widely reported by Gulf War (GW) veterans (Thompson et al., 2004). An exaggerated response to emotional stimuli is not only a principal marker of PTSD but is also observed among individuals with other anxiety disorders (Erwin et al., 2006; Pillay et al., 2006; Ruscio et al., 2004; Sachs et al., 2004), schizophrenia (Nakamura et al., 2003), and traumatic brain injury (Rapoport et al., 2002).
It is possible to assess hyperarousal by measuring objective, electrophysiological responses to threatening stimuli by recording event-related potentials (ERPs) derived from scalp-recorded electroencephalographic (EEG) data. A heightened ERP response to emotional, threatening stimuli has been deemed indicative of a hyperarousal response (Stanford et al., 2001). This type of response can be elicited by using threatening stimuli in an oddball paradigm for eliciting the P3a ERP component (Courchesne et al., 1975). In a three-condition oddball task, the target stimuli are presented approximately 20% of the time and require a specific response from the subjects each time they detect a target stimulus. The ERP component elicited by response to the target is a positive deflection occurring around, or after, 300 ms after the onset of the stimulus and has been variably termed the target P3, the P300b, or P3b. The standard nontarget stimuli are presented approximately 60% of the time and require a standard response or no response. The nontarget distractors are novel stimuli that are presented the remaining 20% of the time. The subject gives the same response to these stimuli as to the standard nontarget stimuli. ERP responses to the nontarget distractor show a positive deflection with a peak latency shorter than that of P3b, and is called a P300a or P3a. P3b has been proposed to reflect the intentional allocation of attentional resources to a target to which a subject responds, whereas P3a indexes an involuntary capture of attention (Friedman et al., 2001). A P3a response to trauma-related distractors that is greater than the P3b response to target stimuli has been considered a marker of a hyperaroused response or biased attention toward those stimuli in PTSD (Karl et al., 2006).
In addition to the P300 responses, ERP components that index pre-attentional processes are of value in evaluating hyperarousal. The auditory P1, or P50, component, a positive deflection occurring 40–80 ms after sound onset, can be an indicator of hyperarousal, as it has shown a heightened response in states of stress (Ermutlu et al., 2005), and has been associated with the attentional difficulties and anxiety experienced in Huntington’s disease (Uc et al., 2003), irritable bowel syndrome (Berman et al., 2002; Blomhoff et al., 2001), and PTSD (Gillette et al., 1997; Skinner et al., 1999). The heightened P1 has been attributed to compromised inhibitory gating in the very early stages of auditory processing.
Previous neuroimaging studies of veterans meeting criteria for Gulf War Illness have found differences in brain systems that are contributors to the three ERP components described above. Reduced neuronal integrity in basal ganglia associated with higher dopaminergic activity has been indicated in Haley Syndromes 1 and 2 (Haley et al., 2000a, 2000b; Meyeroff et al., 2001). Additionally, Syndrome 1 showed significantly lower choline-to-creatine (Cho/Cr) ratio in basal ganglia (Haley et al., 2000b). The P3b to target stimuli has been shown to have a large contribution from basal ganglia (Rektor et al., 2005) and to exhibit an inverted-U relationship with dopamine level wherein both low and high dopaminergic systems are associated with blunted P3b amplitudes (Ergen et al., 2008; Galvan and Wichmann, 2008; Li et al., 2003). The dopamine system is also considered a major contributor to the generation of the P3a component (Polich, 2007). Increased P3a amplitudes have been associated with increased arousal such as in panic disorder (Clark et al., 1996), which may be due to a dysregulated dopamine system (Nikolaus et al., 2010). However, atypical dopaminergic systems such as Parkinson’s disease and restless leg syndrome have also be associated with reduced P3a amplitudes (Poceta et al., 2006).
The cholinergic system has been shown to contribute to the early P1 and the later P3b. Both the brainstem peduculopontine nucleus (PPN)-to-thalamus contribution to the P1 and the hippocampal suppression of the P1 to repeated stimuli are mediated by cholinergic projections (Adler et al., 1998; Reese et al., 1995). Acetylcholine (ACh) receptor agonist administration has resulted in increased P3b amplitude (Münte et al., 1988) and ACh receptor antagonist administration has resulted in decreased P3b amplitude (Hammond et al., 1987; Meador et al., 1989). Disrupted cholinergic systems have been strongly associated with GW Illness symptomology. Syndromes 2 and 3 tended to show exaggerated reactions to the reversible cholinesterase inhibitor pyridostigmine bromide, which was used as an anti–nerve-gas agent during the 1991 Gulf War (Haley et al., 1999). In response to the cholinesterase inhibitor physostigmine, Haley Syndromes 2 and 3 showed changes in regional cerebral blood flow (rCBF) in hippocampus that were significantly different from that of controls and Syndrome 1 (Li et al., 2011) and showed reduced neuronal integrity in brainstem as measured by N-acetylaspartate-to creatine (NAA/Cr) ratio (Haley et al., 2000b).
The Research Advisory Committee on Gulf War Veterans’ Illnesses (2008) stated that studies of GW veterans have indicated a PTSD rate of 3–6%, but whether the hyperarousal reported by ill GW veterans is similar to PTSD or is secondary to the etiology of other GW-related symptoms is less clear. In order to assess the hyperarousal symptom of GW illness, we analyzed ERP data of 28 GW veterans during their performance of an auditory three-condition oddball task where a lion roar and a gunshot were the threatening distractors. In addition, each veteran received Clinician Administered PTSD Scale (CAPS) and Structured Clinical Interview for DSM-IV (SCID) evaluations to assess for the presence of PTSD as a contributing factor to the ERP differences.
Given the association of a heightened P3a to hyperarousal, the dopaminergic contribution to this component, and the dopamine system dysregulation observed in the syndrome groups, we hypothesized that the P3a amplitude to threatening distractors would be higher in the ill GW veterans than in the controls. Given that the P3b receives contribution from dopaminergic and cholinergic systems, both of which have shown dysfunction in GW Illness, we hypothesized that the P3b to target stimuli would be lower in all syndrome groups, especially among the veterans meeting criteria for Haley Syndromes 1 and 2. We also hypothesized that the early P1 component would be higher especially in the veterans meeting criteria for Haley Syndromes 2 and 3, given the brainstem, hippocampal, and cholinergic differences observed in these groups. We used analyses of variance followed by three non-orthogonal contrasts to inform these hypotheses: Controls compared to Syndromes 1, 2, and 3; Controls and Syndrome 3 compared to Syndromes 1 and 2; and Controls and Syndrome 1 compared to Syndromes 2 and 3.
2. Method
2.1. Participants
The participants were 28 male veterans who had been deployed during the 1991 Persian Gulf War. Twenty of these met the Haley et al. (1997; 2000a) criteria for one of the syndromes of GW Illness. Six met criteria for GW Syndrome 1, which is associated with impaired cognition, marked by memory problems, confused thought, distractibility, and fatigue. Eight were identified as Syndrome 2, which is associated with more debilitating neurocognitive issues—confusion, word-finding and reasoning difficulties, emotional lability—and balance problems such as frequent stumbling and vertigo. Six were identified as Syndrome 3, which is generally associated with somatic complaints such as fatigue, joint and muscle pain, weakness, and numb or tingling extremities. The remaining eight veterans who remained well served as controls. The control group ranged in age from 51 to 76 years (M=61.6, SD=7.58), Syndrome 1 from 47 to 60 years (M=53.17, SD=5.38), Syndrome 2 from 56 to 73 years (M=63.75, SD=7.05), and Syndrome 3 from 47 to 63 years (M=53.833, SD=6.85). All participants had served in the same construction battalion of the United States Naval Reserve during the 1991 Persian Gulf War and had participated in prior studies of GW syndrome (Haley et al., 1997, 2000a, 2000b; Iannacchione et al., 2011). The subjects were housed and monitored at The University of Texas Southwestern Medical Center’s Clinical and Translational Research Center in 2008 and 2009, and underwent a week-long multi-modal neuropsychological, neuroimaging, and biomarker study. An audiometric examination was performed on each veteran. An analysis of variance showed that there were neither main effects of syndrome group or ear nor an interaction on pure-tone average thresholds, p>.31. All subjects gave written informed consent according to a protocol approved by the university’s institutional review board.
2.2. Hyperarousal Ratings
We evaluated hyperarousal using a subset of items from the Mississippi Scale for Combat-Related PTSD (Keane et al., 1988), administered to each veteran as part of their psychological evaluation during their week-long participation in this study. Five doctoral-level clinicians agreed on seven items (item nos. 16, 20, 21, 25, 30, 31, and 34) that were most representative of hyperarousal. Internal consistency reliability for the seven-item subset was high (Cronbach’s α = .88). Only 4 of the 20 ill veterans (two from the Syndrome 1 group, one from Syndrome 2, and one from Syndrome 3), and none of the controls, had been diagnosed with PTSD by psychiatrist’s or psychologist’s clinical interview following a structured interview technique (SCID).
2.3. Task Stimuli
The stimuli for the auditory task consisted of four 500-ms sounds sampled at a rate of 22050 Hz with 16-bit amplitude resolution. A 1000-Hz square wave tone served as the frequent nontarget stimulus and represented 54% of the 224 trials. A 250-Hz square wave tone served as the target stimulus, which was presented for 18% of the trials. The sound of a gunshot (14% of the trials) and the sound of a mountain lion roar (14% of trials) served as the threatening distractor stimuli. The root-mean-squared amplitudes of all four sounds were digitally equated.
The stimuli were presented every 2 seconds from two speakers positioned approximately 1 meter in front of the subject. Each participant was assigned to one of six randomized sequences of the 224 stimulus presentations. Intensity was adjusted to a level that was reported as audible and comfortable for each subject. Each subject sat in a comfortable chair in a soundproof booth and was told to keep his eyes open during the task.
2.4. Procedure
After the participants were fitted with the electrode cap and prior to the beginning of the task, they were shown and read the written instructions and were allowed to have their questions answered. The participants heard examples of the target low (250-Hz) tone and frequent high (1000-Hz) tone and were instructed to press the response button under their right middle finger for the low tone and to press the response button under their right index finger for all other sounds. The response buttons interfaced with Stim2 (Compumedics Neuroscan) software, which recorded the accuracy of the responses and their reaction times. A time-locked mark of each stimulus onset and response was recorded on the continuous EEG.
The subject was not informed that any of the stimuli would represent threatening circumstances. At the beginning of the task, the first stimulus was an aural repetition of the instructions the subject had learned prior to the beginning of the task.
2.5. EEG Acquisition
EEG activity was recorded via a 64-electrode array mounted within an elastic cap that was placed on the participant’s head. Electrodes placed at the superior and inferior orbital margins monitored blinks and vertical eye movements. The reference electrode was located near the vertex and the APZ electrode served as the ground electrode. Impedance for each electrode did not exceed 10 kΩ as measured before the beginning of the task.
The EEG was recorded using a Neuroscan Synamps2 amplifier at a 1000-Hz sampling rate. Data from the continuous EEG were high-pass filtered at .15 Hz and were re-referenced to the global mean amplitude. Blink artifacts were filtered from the continuous EEG file by using a spatial filter process in the Scan 4.4 Edit (Compumedics Neuroscan) software. From each participant’s continuous EEG, 224 1400-ms slices, or epochs, consisting of 200 ms prior to the presentation of each stimulus through 1200 ms after the onset of each stimulus, were used to create four averages: an average of the responses to the target tone, an average of responses to the nontarget tone, and the same for the responses to the lion roar and to the gunshot. Each average consisted of epochs that had been baseline-corrected based on the 200-ms prestimulus data and low-pass filtered at 20 Hz.
3. Results
3.1. Hyperarousal Scores
An analysis of variance (ANOVA) where the hyperarousal subscore from the Mississippi Scale for Combat-Related PTSD (Keane et al., 1988) was the dependent variable and GW Illness syndrome group (control, Syndrome1, Syndrome2, Syndrome3; Haley et al., 1997) was the between-subjects factor indicated a significant effect of GW Illness group on hyperarousal scores, F(3, 24) = 10.601, MSerror= 26.217, p = .0001, η2=.570. Pairwise comparisons showed that the control group’s hyperarousal scores were significantly lower than scores from each of the ill veteran groups, p<.0007 (p< .0042, Bonferroni-corrected). See Figure 1.
Figure 1.
Mean hyperarousal scores of each of the ill veterans (Syndromes 1–3) were significantly higher than the scores of the control veterans (p< .0007). Error bars indicate standard deviation.
3.2. Event-related potentials
3.2.1. P1 Component
The P1 component was defined as the most positive point in the event-related potential average from the frontal midline electrode (FZ) in the interval between 30 and 75 ms after the onset of the auditory stimulus. The amplitude and latency measures were used as the dependent variables in two mixed ANOVAs where GW Illness syndrome group was the between-subjects factor and condition (target tone, nontarget tone, gunshot, lion roar) was the within-subjects factor. Three non-orthogonal contrasts were also computed to inform our research questions regarding the contributions of the overall effect of GW Illness (Contrast 1: Controls vs. Syndromes 1, 2, and 3), the basal ganglia/dopaminergic dysfunction in Syndromes 1 and 2 (Contrast 2: Controls and Syndrome 3 vs. Syndromes 1 and 2), and the brainstem, cholinergic, and hippocampal dysfunction observed in Syndromes 2 and 3 (Contrast 3: Controls and Syndrome 1 vs. Syndromes 2 and 3). An alpha level of .0166 was used to determine the significance of these contrasts. Age predicted P1 amplitude (β =.264, p = .005) and latency (β =.204, p = .031) and was used as a covariate in the analyses. There was an effect of GW Illness syndrome group on age (F(3, 24) = 4.239, MSerror= 47.043, p = .0154, η2=.346) but this effect was not significant in any of the contrasts (p > .12).
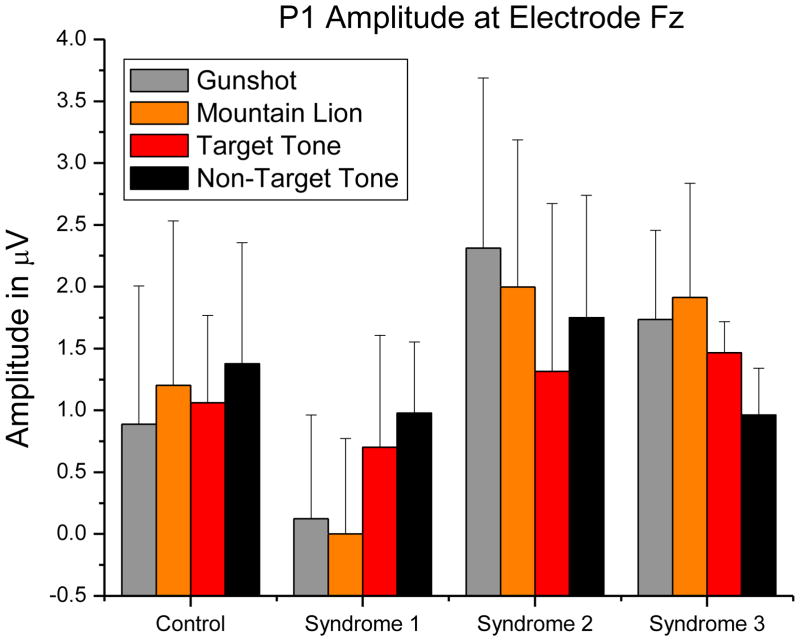
There was a significant main effect of GW Illness syndrome group on P1 amplitude, F(3, 23) = 3.509, MSerror= 2.021, p = .031, η2=.171. Only Contrast 3, comparing Syndromes 2 and 3 to Controls and Syndrome 1, was significant, F(1, 23) = 9.915, p = .004. There was no main effect of condition on P1 amplitude, F(3, 69) = 1.630, MSerror= .617, p = .19. A very strong trend toward an interaction between group and condition was indicated, F(9, 69) = 3.509, MSerror= .617, p = .050, η2=.09. We used three orthogonal single-df interaction contrasts to test whether the groups differed in their mean change between distractor stimuli (gunshot and lion roar) and task-relevant stimuli (target and nontarget tones): (a) comparing controls to Syndromes 1, 2, and 3; (b) comparing Syndromes 2 and 3 to Syndrome 1; and (c) comparing Syndrome 2 to Syndrome 3. Only the second contrast was significant, p = .0006 (p=.0018, Bonferroni-corrected). The mean increase in P1 amplitude from distractor to tone stimuli for the Syndrome 1 group was significantly different from the mean decrease in P1 amplitude observed in Syndromes 2 and 3. This pattern of P1 mean amplitudes is shown in Figure 2.
Figure 2.
P1 amplitudes showed a main effect of syndrome group, p=.0154. Syndromes 2 and 3 P1 amplitudes were significantly higher, p=.004 (p = .012, Bonferroni-corrected). A strong trend toward an interaction between syndrome group and stimulus condition (p=.050) was due to Syndromes 2 and 3 showing a stronger response to the threatening stimuli whereas Syndrome 1 showed a stronger response to the task-relevant stimuli (p=.035, Bonferroni-corrected). Error bars indicate standard deviation.
There was also a main effect of syndrome group on P1 latency, F(3, 23) = 7.416, MSerror= 115.830, p = .001, η2=.246. Only Contrast 3, comparing Syndromes 2 and 3 to Controls and Syndrome 1, was significant, F(1, 23) = 22.025, p = .0001. Syndromes 2 and 3 showed longer mean P1 latencies. This pattern of P1 mean latencies is shown in Figure 3. There was no effect of condition on P1 latency, F(3, 69) = 1.467, MSerror= 69.327, p = .231, nor was there an interaction, F(9, 69) = 7.416, MSerror= 115.830, p = .988.
Figure 3.
P1 peak latencies showed an effect of syndrome group, p = .001. Syndromes 2 and 3 were significantly longer than those of controls and Syndrome 1, p= .0001 (p =.0003, Bonferroni-corrected). Error bars indicate standard deviation.
3.2.2. P3 Components
The gunshot and lion roar distractors were both included in the task in order to evaluate whether the hyperarousal (measured with the P3a) to war-related (gun) stimuli was higher than hyperarousal to less war-related (lion) distractor stimuli, as would be expected in PTSD patients. There was, however, no difference between the P3a responses to the gun and lion stimuli nor was there an interaction between distractor stimulus type and group, p>.273. Thus both distractors were collapsed into one level. The auditory task ERP data showed the P3a response to the threatening distractor stimuli (response to the lion and gun stimuli) to be maximal at right frontal electrode F4, and the P3b to the target tone stimuli maximal at the right centroparietal electrode CP2. This predominantly right distribution is typical in nonverbal sound tasks (e.g., Kayser et al., 1998; Tillman, 2010). The P3a peak amplitude chosen from each participant’s ERP average of their responses to the distractor stimuli was defined as the most positive point in the ERP average from right frontal electrode F4 in the interval between 245 and 400 ms after the onset of the distractor stimulus. The P3b peak amplitude was defined as the most positive point in the ERP average to the target tone from right centroparietal electrode CP2 in the interval between 350 and 800 ms after the onset of the target stimulus.
Amplitude and latency measures for the P3a to distractor stimuli and the P3b to target stimuli were used in four separate analyses of variance where syndrome group was the between subjects variable. The same three contrasts that were computed to define differences in P1 amplitude and latency were used in the analysis of the P3a and P3b components. An alpha level of .0166 was used to determine the significance of these contrasts. Age showed a trend toward a linear relationship with the amplitude of the P3a (β =.343, p = .074) and of the P3b (β =.332, p = .084), consistent with previous studies (e.g., Goodin et al., 1978; Mueller et al., 2008); thus, age was used as a covariate in the analyses.
3.2.2.1. P3a
Mean P3a amplitudes from each syndrome group are shown in Figure 4. The one-way between-subjects ANOVA showed an effect of syndrome group on P3a amplitude, F(3, 23) = 4.700, MSerror= 1.188, p = .011, η2=.060. Only Contrast 2, comparing Syndromes 1 and 2 to Controls and Syndrome 3, was significant, F(1, 23) = 11.172, p = .003.
Figure 4.
P3a mean amplitudes to distractor stimuli showed an effect of syndrome group, p = .011. Mean amplitudes of Syndromes 1 and 2 were significantly lower than those of controls and Syndrome 3, p = .003 (p = .009, Bonferroni-corrected). Error bars indicate standard deviation.
There was no omnibus effect of GW Illness syndrome group on P3a latency, F(3, 23) = 1.775, MSerror= 441.372, p = .18, nor were any of the contrasts significant.
3.2.2.2. P3b
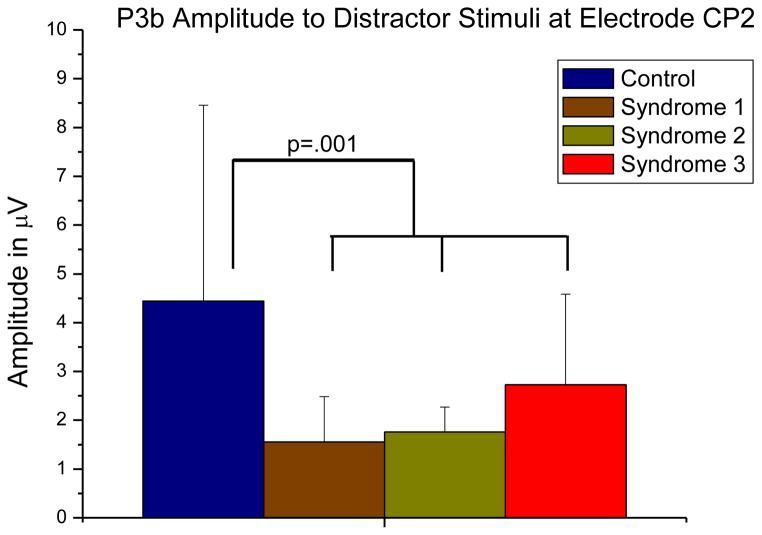
An ANOVA revealed an effect of GW Illness syndrome group on P3b amplitudes, F(3, 23) = 5.287, MSerror= 4.020, p = .006, η2=.169. Only Contrast 1 was significant (F(1,23) = 14.316, p = .001), indicating an overall effect of GW Illness (Figure 5).
Figure 5.
P3b mean amplitudes to target stimuli showed an effect of syndrome group, p = .006. Mean amplitudes of the ill veteran groups were significantly lower than those of controls, p = .001 (p = .003, Bonferroni-corrected). Error bars indicate standard deviation.
There was no omnibus effect of GW Illness syndrome group on P3b latency, F(3, 23) = 1.959, MSerror= 15016.08, p = .148, nor were any of the contrasts significant.
3.3. Behavioral Data
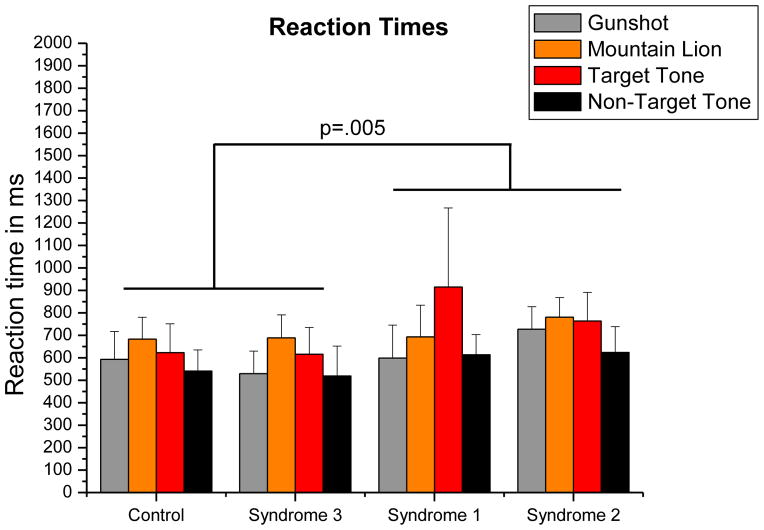
Percent correct and reaction times were used as dependent variables in two separate ANOVAs where GW Illness syndrome group was the between-subjects factor and condition was the within-subjects factor. The mean reaction times are depicted in Figure 6. There was a main effect of syndrome group on reaction time, F(3, 24) =3.323, MSerror=38407.416, p=.0367, η2=.237. Only Contrast 2 was significant (F(1, 23) = 9.509, p =.005), indicating that mean reaction times of Syndromes 1 and 2 were significantly longer. There was also a significant main effect of condition on reaction time, F(3, 72) =12.609, MSerror=12352.248, p<.0001, η2=.2896. Correct responses both to the gun shot and to the nontarget tone were significantly faster than responses both to the lion roar and to the target tone, p<.003 (p= .018, Bonferroni-corrected). The test for interaction between GW Illness syndrome group and condition was also significant, F(9, 72) =2.310, MSerror=12352.248, p=.0242, η2=.159. We examined the nature of this interaction using the same three orthogonal single-df interaction contrasts as were used to examine the interaction effect on P1 amplitudes. The second contrast was significant, p=.011 (p=.034, Bonferroni-corrected). The mean reaction times for distractor stimuli (gunshot, mountain lion) are shorter than for task-relevant stimuli (tones) in the Syndrome 1 group, but the opposite is true for Syndromes 2 and 3, whose mean reaction times for distractor stimuli are longer than for task-relevant stimuli. When an outlier from the Syndrome 1 group was removed from the analysis, the nature and significance of the interaction remained.
Figure 6.
Mean reaction times grouped by syndrome group. There was a significant main effect of syndrome group on reaction time, p = .0367. Syndromes 1 and 2 mean reaction time was longer than those of controls and Syndrome 3, p = .005 (p = .015, Bonferroni-corrected). Stimulus condition also had a main effect on reaction times, p < .0001. Responses to both the gun shot and the nontarget tone were faster than responses to mountain lion and target tone stimuli, p < .003 (p<.018, Bonferroni-corrected). An interaction between syndrome group and stimulus condition was also indicated, p = .0242. Mean reaction times for distractor stimuli (gunshot, mountain lion) are longer than those for task-related tones for Syndromes 2 and 3, whereas the opposite is seen in Syndrome 1, p=.011 (p=.034, Bonferroni-corrected). Error bars indicate standard deviation.
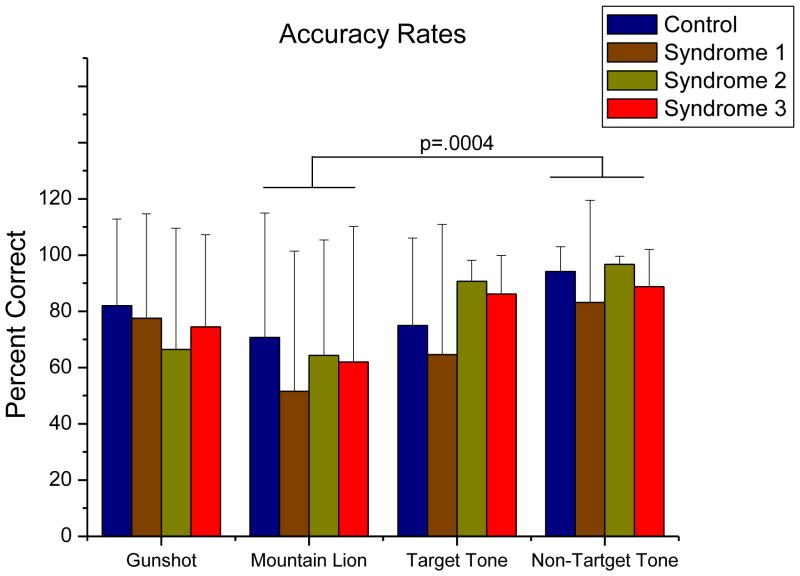
There was an effect of condition on accuracy, F(3, 72) =4.736, MSerror=.081, p=.0045, η2=.0649. Post hoc comparisons showed that this was due solely to a significantly lower correct response rate to the lion roar stimulus than to the nontarget tone stimulus, p=.0004. This is depicted in Figure 7. There were no other main effects or interactions.
Figure 7.
Accuracy rates grouped by stimulus condition. There was a significant effect of stimulus condition on accuracy (p = .0045) due solely to the difference between the percent correct responses to the lion stimulus and percent correct response to the nontarget stimulus, p = .0004 (p = .0012, Bonferroni-corrected). Error bars indicate standard deviation.
4. Discussion
We found that those participants meeting the criteria for Haley syndromes 1–3 (Haley et al., 1997), reported significantly more symptoms of hyperarousal than deployed controls. Our hypotheses were only partially supported. The early P1 component amplitude was significantly higher in those meeting criteria for Syndromes 2 and 3, especially in response to the threatening distractor stimuli, but the Syndrome 1 group showed the opposite response. Contrary to our hypothesis, the amplitude of the P3a to threatening stimuli in the veterans meeting criteria for Syndromes 1 and 2, who reported high hyperarousal symptoms, was significantly lower than the P3a amplitude of the veterans who served as controls. The P3a amplitude of those meeting criteria for Syndrome 3 was similar to that of controls, although Syndrome 3 veterans reported significant hyperarousal symptoms as well. The P3b to target stimuli was significantly lower among all the ill veterans, rather than lower in only Syndromes 1 and 2. Taken together, these data are consistent with there being at least two possible sources of hyperarousal in GW Illness: dysfunction in the early sensory response system indicative of inadequate filtering or habituation, seen more in Syndromes 2 and 3; and dysfunction in later attentional control processes, seen more in Syndromes 1 and 2.
Although many questions remain addressing the nature of GW Illnesses and the nature of hyperarousal as a strongly represented symptom of those illnesses, the ERP component patterns observed among the syndrome groups here are quite compatible with previous findings in imaging and self-report studies of GW veterans, especially with regard to previously reported observations of dysfunction of the cholinergic system, damage to the basal ganglia and dopaminergic system, and disruption of white matter integrity.
4.1. Cholinergic System
Many of the symptoms associated with GW Illness have been linked to agents that act on cholinergic systems. Exaggerated reactions to the anti–nerve-gas agent pyridostigmine bromide, a reversible cholinesterase inhibitor, were shown to be strongly associated with Haley syndromes 2 and 3 (Haley et al., 1999). GW veterans with an R allele of the PON1 gene—who are less able to hydrolyze the cholinesterase inhibitors sarin, soman, and diazinon—were more likely to have suffered severe pyridostigmine reaction and were more likely to experience the neurological symptoms that mark GW Illness (Haley et al., 1999). Additionally, Li et al. (2011) found abnormal hippocampal blood flow in all three syndrome groups at baseline, but that Syndromes 2 and 3 showed an increase in rCBF in response to physostigmine, whereas Syndrome 1 and controls showed a decrease. Pesticides, which included cholinesterase-inhibiting organophosphate chemicals, were widely used by GW troops to manage the pervasive insect problem. The insect repellent N,N-diethyl-m-toluamide (DEET) and the insecticide permethrin were also widely used (Institute of Medicine, 1995). It has been reported that in adult rats even low exposure to pyridostigmine bromide, DEET, and permethrin, combined with stress, was associated with blood-brain barrier disruption, neuronal death, decreased acetylcholine esterase activity, and decreased acetylcholine receptor binding (Abdel-Rahman et al., 2004; Abou-Donia et al., 1996, 2004).
Importantly, target P3b amplitude, which was significantly reduced in the ill veterans in this study, has been shown to be sensitive to changes in cholinergic activity. Decreased P3b amplitude has accompanied impaired performance on memory tasks after the administration of the muscarinic antagonist scopolamine (Hammond et al., 1987; Meador et al., 1989), whereas the muscarinic receptor agonist WEB1881 FU (Nebracetam) has resulted in increased P3b amplitudes (Münte et al., 1988). Irrespective of location of damage, dysfunction in the acetylcholine system can contribute to the attenuated P3b amplitudes seen in these GW veterans.
The cholinergic system also contributes to the generation of the P1 potential (Buchwald et al., 1991; Dickerson and Buchwald, 1991; Reese et al., 1995; Uc et al., 2003). Evidence has accrued to support the significant contribution of cholinergic input from the pedunculopontine nucleus (PPN) of the reticular activating system (RAS) to P1 amplitude (see Reese et al., 1995). The overarching function of the brainstem RAS is to regulate arousal; thus, Buchwald et al. (1991) concluded that the P1 reflects an aspect of mental alertness. Many of the cholinergic projections from the PPN project to muscarinic receptor populations (Dickerson and Buchwald, 1991) in many areas of thalamus (Reese et al., 1995), including the intralaminar nuclei. The posterior intralaminar nucleus projects to amygdalae (Ledoux et al., 1991), as part of the noncortical pathway for relay of coarse but possibly survival-salient auditory information. The P1 is known to habituate to rapidly repeated auditory stimuli in healthy subjects (Erwin and Buchwald, 1986), but this auditory sensory gating has been found to be significantly attenuated in subjects with PTSD (Skinner et al., 1999), schizophrenia (Siegel et al., 1984), Huntington’s disease (Uc et al., 2003), and traumatic brain injury (Arciniegas et al., 200), all of which are associated with anxiety and attentional difficulties. The suppression of the P1 to repeated auditory stimuli is mediated by cholinergic projections to nicotinic receptors in hippocampus (Adler et al., 1998; Bickford et al., 1993; Freedman et al., 1995; Luntz-Leybman et al., 1992), in which pathology has been indicated in Syndromes 2 and 3 (Li et al., 2011). Syndromes 2 and 3, whose P1 amplitude and latencies to especially the distractor stimuli showed higher values, also showed reduced neuronal integrity in brainstem as measured by N-acetylaspartate-to creatine (NAA/Cr) ratio (Haley et al., 2000b). Although significant brainstem dysfunction was not indicated in the Syndrome 1 group (Haley et al., 2000b), report of deployment exposure to agents that act on cholinergic systems and lower blood flow to hippocampus (Li et al., 2011) in Syndrome 1 may indicate small physiological changes that are more easily detected using electrophysiology. Thus the high-amplitude P1, as seen in the Syndromes 2 and 3 groups, or low-amplitude P1 of Syndrome 1, may indicate dysfunction of cholinergic PPN neurons, thalamic muscarinic receptor sites, or hippocampal nicotinic-receptor–mediated cholinergic mechanisms. Dysregulation of cholinergic activity in this brainstem area may also play a role in the central pain (Haley et al., 2001) that has been used to identify Syndrome 3. Antinociceptive effects are induced by acetylcholine binding to nicotinic receptors of the PPN (Iwamoto, 1991; Iwamoto and Marion, 1993), but are known to be modulated by several adrenergic, serotonergic, and muscarinic sites in spinal cord.
4.2. Basal Ganglia and Dopamine System
Basal ganglia and associated dopamine dysregulation may also be reflected in the ERP patterns found in ill GW veterans. Acetylcholine is known to play a role in striatal function (Calabresi et al., 2000; Bonsi et al., 2011) and in modulating dopaminergic activity (Exley and Cragg, 2008), which in turn modulates acetylcholine activity (Aosaki et al., 2010; Deboer et al., 1996). Thus, dysfunction in either system can result in dysfunction in the other. Magnetic resonance spectroscopy (MRS) studies measuring N-acetylaspartate-to-creatine (NAA/Cr) ratio showed evidence of reduced neuronal integrity in basal ganglia in Haley GW Syndromes 1 and 2), and that lower NAA/Cr ratio in left basal ganglia was closely associated with higher dopaminergic activity (Haley et al., 2000a, 2000b; Meyeroff et al., 2001). The basal ganglia choline-to-creatine (Cho/Cr) ratio was significantly lower in the Syndrome 1 group (Haley et al., 2000b). In an investigation of neural contributors to the target P3 (Rektor et al., 2005), intracranial recordings from motor cortices and the basal ganglia, made while subjects performed auditory and visual oddball tasks, demonstrated significantly higher target P3b amplitude in basal ganglia than in cortical areas, indicating a prominent role for these deep gray matter structures in the generation of the target P3b. Dopamine has been implicated in P300 amplitude variance, in that systems with both low dopaminergic activity, such as in Parkinson patients (Galvan and Wichmann, 2008), and high activity, such as schizophrenia (Howes and Kapur, 2009), show decreased P3b amplitudes (Ergen et al., 2008; Li et al., 2003).
Polich (2007) concluded that the dopamine system is the most important contributor to P3a generation. Reduced P3a amplitudes have been associated with atypical dopaminergic systems (Bonsi et al., 2011; Connor et al., 2009; Sagvolden et al., 2005; Toda and Abi-Dargham, 2007), such as those of persons with schizophrenia (Merrin and Floyd, 1994), restless leg syndrome or Parkinson’s disease (Poceta et al., 2006), attention-deficit/hyperactivity disorder (Kemner et al., 1996), and the met/met allelic variant of the catechol-O-methyltransferase (COMT) gene (Marco-Pallarés et al., 2010). Each of these syndromes is also marked by anxiety and/or poor emotional regulation (Anastopoulos et al., 2010; Drabant et al., 2006; Laviolette, 2007; Sevim et al., 2004; Stocchi and Brusa, 2000), generally interpreted to be due to inefficient inhibitory mechanisms.
That a reduced P3a is attributable to inefficient inhibition is consistent with many interpretations of the nature of the P3 response to novel or distractor stimuli. The P3a has been assumed to represent an involuntary capture of attention to or processing of deviant stimuli, but the complex nature of the P3a component is still being assessed (Friedman et al., 2001; Goldstein et al., 2002; Polich, 2007). Goldstein et al. (2002) posited that detection of rare or deviant stimuli by preconscious mechanisms may initiate several processes—as may be indicated by the multiple contributors to P3a generation (Alho et al., 1998; Baudena et al., 1995; Dien et al., 2003; Elting et al., 2008; Knight, 1984, 1996)—whose purpose is to generate the appropriate response to the deviant stimulus. At any point in this cascade of initiated processes, correct identification of the stimulus and task relevance information requires the active inhibition of further processing, no matter the emotional salience of the deviant stimulus. Polich (2007) described and distinguished the P3a, novelty P3, and NoGo P3 but concluded that the three components are most likely variations of the same component, and that inhibition may be the underlying process. A reduced NoGo P3 amplitude is associated with impaired inhibition, or high false-alarm rate, on a Go-NoGo task. Children with ADHD (Jonkman et al., 2003; Spronk et al., 2008), patients with schizophrenia (Weisbrod et al., 2000), and symptomatic GW veterans (Tillman et al., 2010) have all shown high false-alarm rates and low NoGo P3 amplitudes.
Whereas the enhanced P3a in PTSD presents a scenario where considerable effort—as indicated by overactivity in the ventrolateral prefrontal cortex (PFC; Fassbender et al., 2004; Morey et al., 2009)—is exerted in the need to inhibit further processing of emotional but task-irrelevant stimuli, individuals with ADHD or schizophrenia show less ventrolateral PFC activation (Kaladjian et al., 2011; Stevens et al., 2007) and a blunted P3a (Jonkman et al., 2003; Merrin and Floyd, 1994; Spronk et al., 2008), but considerable distraction by novel stimuli and poor behavioral inhibition. We suggest that GW veterans with the reduced P3a amplitude are more similar to patients with syndromes marked by anomalous dopamine systems than to those with PTSD in that the GW veterans’ heightened distraction by novel stimuli is due less to a greater need to inhibit and more to a compromised ability to inhibit due to dysfunction in dopaminergic systems. The robust P3a seen in the ERPs of the deployed controls, who have significantly lower hyperarousal scores, may indicate the successful inhibition of the further processing of alerting but task-irrelevant stimuli.
Although the PPN both receives and sends input from dopaminergic areas of basal ganglia (see Reese et al., 1995, for a review), few studies have reported a specific dopaminergic contribution to P1 generation. Thus, as revealed by the current auditory oddball task, veterans meeting criteria for Syndromes 1 and 2 seem to be more affected by basal ganglia/dopamine dysregulation than those meeting criteria for Syndrome 3. This is consistent with the findings of greater dysregulation in basal ganglia and altered dopaminergic activity among Syndromes 1 and 2, whereas Syndrome 3 demonstrated greater brainstem dysfunction (Haley et al., 2000a, 2000b; Meyerhoff et al., 2001).
4.3. White Matter Integrity
Another plausible etiology of the P3b findings is disruption of white matter integrity. Reductions in white matter volume in GW veterans have been shown to be significantly correlated with the amount of sarin exposure (Heaton et al., 2007). Using data from MRI and ERPs recorded during a visual three-stimulus oddball task, Cardenas et al. (2005) found that P3a latency variability was not related to brain structural integrity; however, P3b latency variability was related chiefly to white matter volume and not to gray matter factors. These results imply that the connectivity between generators rather than solely the generators themselves influences the latency variability of the P3b. Latency variability, which can occur with compromised white matter integrity, can account for differences in amplitude and is especially prevalent in endogenous components such as the target P3b (Spencer, 2005). All syndrome groups in the current study showed significantly reduced P3b amplitude relative to controls.
4.4. Limitations
Limitations of this study include the possible confound of age, the unknown role of depression, and too few subjects in each syndrome group limiting the power to perform all pertinent post hoc comparisons. Additionally, although the findings from this study correspond well to findings from previous non-ERP studies of ill GW veterans, the findings need to be replicated in a more representative sample of the GW veteran population. Although the mean ages of the controls and Syndrome 2 group were higher than the mean ages of the Syndrome 1 and Syndrome 3 group, this difference was not significant in any of the contrasts that were used to examine the omnibus effects. In addition we used age as a covariate in all the analyses. Depression is considered a major symptom in the clusters identified by Haley et al. (1997, 2001) and Fukuda et al. (1998). In this study there was a significant difference in the distribution of depression diagnoses across group ((χ2=16.156, p=.0011, Cramer’s V = .76), in that only one of the controls was SCID-diagnosed as depressed and only two of the 20 ill veterans were not diagnosed as depressed, a disparity that precluded using depression diagnosis as a factor in the analyses. Studies examining the effects of depression on the amplitude and latency of ERP components show conflicting results, which have be attributed to depression type, task paradigm, and task difficulty variances (Bruder et al., 2012). In general, both the P3a and P3b tend to be attenuated in depressed patients and tend to show longer latencies in those with melancholic or bipolar depression. Both components were attenuated in the Syndrome 1 and Syndrome 2 groups and the P3b was attenuated in the Syndrome 3 group in this study, but the latencies were not different from those of controls. Assessing how depression contributed to the lower amplitudes, toxic exposure contributed to the depression and to the P3 attenuations, or both will require further study.
4.5. Conclusion
In summary, Gulf War veterans meeting criteria for Haley Syndromes 1–3 indicated significantly higher hyperarousal rates than controls. The heightened early P1 amplitudes observed in Syndromes 2 and 3 suggest that the hyperarousal symptoms in these two syndrome groups may be at least partially due to an early over-response to or an under-inhibition of incoming auditory stimuli, likely indicative of brainstem cholinergic dysfunction. This is consistent with the findings of lower NAA/Cr ratios in brainstem, the report of more negative reactions to pyridostigmine bromide (Haley et al., 2000a), and abnormal increase in hippocampal rCBF in Syndromes 2 and 3 (Li et al., 2011). The attenuated P3a amplitudes observed in Syndromes 1 and 2 suggest that dysfunction of the dopaminergic contribution to later inhibitory mechanisms may be a greater contributor to their hyperarousal symptoms. This is consistent with the lower NAA/Cr and Cho/Cr ratios in basal ganglia and associated dopamine system dysfunction in Syndromes 1 and 2 (Haley et al., 2000a, 2000b). The P3b response, which has dopaminergic, cholinergic, and white matter contributions, was attenuated in all three syndrome groups.
Dysfunction in the basal ganglia or white matter, or in the dopaminergic or cholinergic neurotransmitter systems have most likely contributed to the anomalous P1 amplitudes, insufficient P3a responses to threatening stimuli, and even more reduced P3bs to target stimuli in ill GW veterans. The dysfunction associated with these etiologies is consistent with neurotoxic exposure in these subjects resulting in the neurobiologic disruptions. Each of these plausible dysfunctions has neurotoxicological etiologies that have been linked to specific agents these veterans were exposed to during the GW.
Highlights.
Hyperarousal is more prominent in ill veterans than in control veterans.
Syndromes 2 and 3 show stronger auditory P1 amplitudes, indicating inhibitory gating issues.
Syndromes 1 and 2 show weaker P3a, suggesting dysfunction in inhibition to distraction.
Ill veterans show reduced P3b, possibly secondary to cholinergic, dopaminergic, and/or white matter damage.
Each dysfunction has etiologies that can be linked to neurotoxic exposure during the Gulf War.
Acknowledgments
This study was supported by IDIQ contract VA549-P-0027, awarded and administered by the Department of Veterans Affairs Medical Center, Dallas, TX; U.S. Army Medical Research and Materiel Command grant number DAMD17-01-1-0741; and Grant Number UL1RR024982, titled North and Central Texas Clinical and Translational Science Initiative (Milton Packer, M.D., PI), from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. The content does not necessarily reflect the position or the policy of the Federal government or the sponsoring agencies, and no official endorsement should be inferred. The corresponding author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Rahman A, Dechkovskaia AM, Goldstein LB, Bullman SH, Khan W, El-Masry EM, Abou-Donia MB. Neurological deficits induced by malathion, DEET, and permethrin, alone or in combination in adult rats. J Toxicol Environ Health, Part A. 2004;67:331–56. doi: 10.1080/15287390490273569. [DOI] [PubMed] [Google Scholar]
- Abou-Donia MB, Dechkovskaia AM, Goldstein LB, Abdel-Rahman A, Bullman SL, Khan WA. Co-exposure to pyridostigmine bromide, DEET, and/or permethrin causes sensorimotor deficit and alterations in brain acetylcholinesterase activity. Pharmacol Biochem Behav. 2004;77:253–62. doi: 10.1016/j.pbb.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Abou-Donia MB, Wilmarth KR, Jensen KF, Oehme FW, Kurt TL. Neurotoxicity resulting from coexposure to pyridostigmine bromide, DEET, and permethrin: implications of Gulf War chemical exposures. J Toxicol Environ Health. 1996;48:35–56. doi: 10.1080/009841096161456. [DOI] [PubMed] [Google Scholar]
- Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998;24:189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- Alho K, Winnkler I, Escera C, Huotilainen M, Virtanen J, Jaaskelainen IP, et al. Processing of novel sounds and frequency changes in the human auditory cortex: magnetoencephalographic recordings. Psychophysiol. 1998;35:211–24. [PubMed] [Google Scholar]
- Anastopoulos AD, Smith TF, Garrett ME, Morrissey-Kane E, Schatz NK, Sommer JL, et al. Self-regulation of emotion, functional impairment, and comorbidity among children with ADHD. J Atten Disord. 2010 doi: 10.1177/1087054710370567. [Epub 2010 Aug 4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aosaki T, Miura M, Suzuki T, Nishimura K, Masuda M. Acetylcholine-dopamine balance hypothesis in the striatum: an update. Geriatr Gerontol Int. 2010;10 (Suppl 1):S148–57. doi: 10.1111/j.1447-0594.2010.00588.x. [DOI] [PubMed] [Google Scholar]
- Arciniegas D, Olincy A, Topkoff J, McRae K, Cawthra E, Filley CM, et al. Impaired auditory gating and P50 nonsuppression following traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2000;12:77–85. doi: 10.1176/jnp.12.1.77. [DOI] [PubMed] [Google Scholar]
- Baudena P, Halgen E, Heit G, Clarke JM. Intracerebral potentials to rare target and distractor auditory and visual stimuli. III. Frontal cortex. Electroencephal Clin Neurophysiol. 1995;94:251–64. doi: 10.1016/0013-4694(95)98476-o. [DOI] [PubMed] [Google Scholar]
- Berman SM, Naliboff BD, Chang L, FitzGerald L, Antolin T, Camplone A, Mayer EA. Enhanced preattentive central nervous system reactivity in irritable bowel syndrome. The Am J Gasteroenterol. 2002;97:2791–97. doi: 10.1111/j.1572-0241.2002.07024.x. [DOI] [PubMed] [Google Scholar]
- Bickford PC, Luntz-Leybman V, Freedman R. Auditory sensory gating in the rat hippocampus: Modulation by brainstem activity. Brain Res. 1993;607:33–38. doi: 10.1016/0006-8993(93)91486-c. [DOI] [PubMed] [Google Scholar]
- Blomhoff S, Spetalen S, Jacobsen MB, Malt UF. Phobic anxiety changes the function of brain-gut axis in irritable bowel syndrome. Psychosom Med. 2001;63:959–65. doi: 10.1097/00006842-200111000-00015. [DOI] [PubMed] [Google Scholar]
- Bonsi P, Cuomo D, Martella G, Madeo G, Schirinzi T, Puglisi F, et al. Centrality of striatal cholinergic transmission in Basal Ganglia function. Front Neuroanat. 2011;5:6. doi: 10.3389/fnana.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Kayser J, Tenke CE. Event-related brain potentials in depression: Clinical, cognitive and neurophysiologic implications. In: Luck SJ, Kappenman ES, editors. The Oxford Handbook of Event-Related Potential Components. Vol. 2012. New York: Oxford University Press; 2012. pp. 563–592. [Google Scholar]
- Buchwald JS, Rubinstein EH, Schwafel J, Strandburg RJ. Midlatency auditory evoked responses: differential effects of a cholinergic agonist and antagonist. Electroencephal Clin Neurophysiol. 1991;80:303–9. doi: 10.1016/0168-5597(91)90114-d. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 2000;23(3):120–6. doi: 10.1016/s0166-2236(99)01501-5. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Chao LL, Blumenfeld R, Song E, Meyerhoff DJ, Weiner MW, Studholme C. Using automated morphometry to detect associations between ERP latency and structural brain MRI in normal adults. Hum Brain Mapp. 2005;25:317–27. doi: 10.1002/hbm.20103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CR, McFarlane AC, Weber DL, Battersby M. Enlarged frontal P300 to stimulus change in panic disorder. Biol Psychiatry. 1996;39(10):845–56. doi: 10.1016/0006-3223(95)00288-x. [DOI] [PubMed] [Google Scholar]
- Connor JR, Wang XS, Allen RP, Beard JL, Wiesinger JA, Felt BT, Early CJ. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain. 2009;132:2403–12. doi: 10.1093/brain/awp125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the evoked potential in man. Electroencephal Clin Neurophysiol. 1975;39:131–43. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Deboer P, Heeringa MJ, Abercrombie ED. Spontaneous release of acetylcholine in striatum is preferentially regulated by inhibitory dopamine D2 receptors. Eur J Pharmacol. 1996;317(2–3):257–62. doi: 10.1016/s0014-2999(96)00761-3. [DOI] [PubMed] [Google Scholar]
- Dickerson LW, Buchwald JS. Midlatency auditory-evoked responses: effect of scopolamine in the cat and implications for brain stem cholinergic mechanisms. Exp Neurol. 1991;112:223–39. doi: 10.1016/0014-4886(91)90074-m. [DOI] [PubMed] [Google Scholar]
- Dien J, Spencer KM, Donchin E. Localization of the event-related potential novelty response as defined by principal components analysis. Brain Res Cogn Brain Res. 2003;17(3):637–50. doi: 10.1016/s0926-6410(03)00188-5. [DOI] [PubMed] [Google Scholar]
- Dlugosz LJ, Hocter WJ, Kaiser KS, Knoke JD, Heller JM, Hamid NA, et al. Risk factors for mental disorder hospitalization after the Persian Gulf War: US armed forces, June 1, 1991-September 30. J Clin Epidemiol. 1999;52:1267–78. doi: 10.1016/s0895-4356(99)00131-6. [DOI] [PubMed] [Google Scholar]
- Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS, et al. Catechol O-methyltransferase Val158Met genotype and neural mechanisms related to affective arousal and regulation. Arch Gen Psychiatry. 2006;63:1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- Elting JW, Maurits N, van Weerden T, Spikeman J, deKeyser J, vanderNaalt J. P300 analysis techniques in cognitive impairment after brain injury: Comparison with neurophsychological and imaging data. Brain Injury. 2008;22:870–81. doi: 10.1080/02699050802403581. [DOI] [PubMed] [Google Scholar]
- Ergen M, Marbach S, Brand A, Başar-Eroğlu C, Demiralp T. P3 and delta band responses in visual oddball paradigm in schizophrenia. Neurosci Lett. 2008;440(3):304–8. doi: 10.1016/j.neulet.2008.05.054. [DOI] [PubMed] [Google Scholar]
- Ermutlu MN, Karamürsel S, Ugur EH, Senturk L, Gokhan N. Effects of cold stress on early and late stimulus gating. Psychiatry Res. 2005;136:201–209. doi: 10.1016/j.psychres.2003.03.002. [DOI] [PubMed] [Google Scholar]
- Erwin BA, Heimberg RG, Marx BP, Franklin ME. Traumatic and socially stressful life events among persons with social anxiety disorder. J Anxiety Disord. 2006;18(5):629–46. doi: 10.1016/j.janxdis.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Erwin RJ, Buchwald JS. Midlatency auditory evoked responses: Differential recovery cycle characteristics. Electroencephal Clin Neurophysiol. 1986;64:417–23. doi: 10.1016/0013-4694(86)90075-1. [DOI] [PubMed] [Google Scholar]
- Exley R, Cragg SJ. Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol. 2008;153 (Suppl 1):S283–97. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Murphy K, Foxe JJ, Wylie GR, Javitt DC, Robertson IH, et al. A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Cogn Brain Res. 2004;20:132–43. doi: 10.1016/j.cogbrainres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neurosci Biobehav Rev. 2001;25:355–73. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Nisenbaum R, Steward G, Thompson WW, Robin L, Washko RM, et al. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA. 1998;280:981–88. doi: 10.1001/jama.280.11.981. [DOI] [PubMed] [Google Scholar]
- Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol. 2008;119:1459–74. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford RK, Ursano RJ, Stuart JA, Engel CC. Stress and stressors of the early phases of the Persian Gulf War. Phil Trans R Soc B. 2006;361:585–91. doi: 10.1098/rstb.2006.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette GM, Skinner RD, Rasco LM, Fielstein EM, Davis DH, Pawelak JE, et al. Combat Veterans with posttraumatic stress disorder exhibit decreased habituation of the P1 amplitude midlatency auditory evoked potential. Life Sci. 1997;64:1421–34. doi: 10.1016/s0024-3205(97)00688-7. [DOI] [PubMed] [Google Scholar]
- Goldstein A, Spencer KM, Donchin E. The influence of stimulus deviance and novelty on the P300 and Novelty P3. Psychophysiology. 2002;39:781–90. [PubMed] [Google Scholar]
- Golier JA, Schmeidler J, Legge J, Yehuda R. Twenty-four hour plasma cortisol and adrenocorticotropic hormone in Gulf War veterans: relationships to posttraumatic stress disorder and health symptoms. Biol Psychiatry. 2007;62:1175–78. doi: 10.1016/j.biopsych.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Goodin DS, Squires KC, Henderson BH, Starr A. Age-related variations in evoked potentials to auditory stimuli in normal human subjects. Electroencephalogr Clin Neurophysiol. 1978;44(4):447–58. doi: 10.1016/0013-4694(78)90029-9. [DOI] [PubMed] [Google Scholar]
- Gray GC, Kaiser KS, Hawksworth AW, Hall FW, Barrett-Connor E. Increased postwar symptoms and psychological morbidity among U.S. Navy Gulf War veterans. Am J Trop Med Hyg. 1999;60:758–66. doi: 10.4269/ajtmh.1999.60.758. [DOI] [PubMed] [Google Scholar]
- Haley RW. Is Gulf War Syndrome due to stress? The evidence reexamined Am J Epidemiol. 1997;146:695–703. doi: 10.1093/oxfordjournals.aje.a009343. [DOI] [PubMed] [Google Scholar]
- Haley RW, Billecke S, LaDu BN. Association of low PON1 type Q (type A) arylesterase activity with neurologic symptom complexes in Gulf War veterans. Toxicol Appl Pharmacol. 1999;157:227–33. doi: 10.1006/taap.1999.8703. [DOI] [PubMed] [Google Scholar]
- Haley RW, Fleckenstein JL, Marshall WW, McDonald GG, Kramer GL, Petty F. Effect of basal ganglia injury on central dopamine activity in Gulf War syndrome: correlation of proton magnetic resonance spectroscopy and plasma homovanillic acid levels. Arch Neurol. 2000a;57:1280–85. doi: 10.1001/archneur.57.9.1280. [DOI] [PubMed] [Google Scholar]
- Haley RW, Kurt TL, Hom J. Is there a Gulf War Syndrome? Searching for syndromes by factor analysis of symptoms. JAMA. 1997;277:215–22. [PubMed] [Google Scholar]
- Haley RW, Luk GD, Petty F. Use of structural equation modeling to test the construct validity of a case definition of Gulf War syndrome: invariance over developmental and validation samples, service branches and publicity. Psychiatry Res. 2001;102:175–200. doi: 10.1016/s0165-1781(01)00241-4. [DOI] [PubMed] [Google Scholar]
- Haley RW, Marshall WW, McDonald GG, Daugherty MA, Petty F, Fleckenstein JL. Brain abnormalities in Gulf War Syndrome: evaluation with 1H MR Spectroscopy. Radiology. 2000b;215:807–17. doi: 10.1148/radiology.215.3.r00jn48807. [DOI] [PubMed] [Google Scholar]
- Hammond EJ, Meador KJ, Aung-Din R, Wilder BJ. Cholinergic modulation of human P3 event-related potentials. Neurol. 1987;37:346–50. doi: 10.1212/wnl.37.2.346. [DOI] [PubMed] [Google Scholar]
- Heaton KJ, Palumbo CL, Proctor SP, Killiany RJ, Yurgelun-Todd DA, White RF. Quantitative magnetic resonance brain imaging in US army veterans of the 1991 Gulf War potentially exposed to sarin and cyclosarin. Neurotoxicology. 2007;28:761–769. doi: 10.1016/j.neuro.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: Version III--the final common pathway. Schizophr Bull. 2009;35:549–62. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannacchione VG, Dever JA, Bann CM, Considine KA, Creel D, Best H, Carson CP, Haley RW. Validation of a research case definition of Gulf War illness in the 1991 U.S. military population. Neuroepidemiology. 2011 doi: 10.1159/000331478. (in press) [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Health consequences of service during the Persian Gulf War: initial findings and recommendation for immediate action. Washington, DC: National Academy Press; 1995. [PubMed] [Google Scholar]
- Iwamoto E. Characterization of the antinociception induced by nicotine in the pedunculopontine tegmental nuceus and the nucleus raphe magnus. J Pharmacol Exp Ther. 1991;257:120–33. [PubMed] [Google Scholar]
- Iwamoto ET, Marion L. Adrenergic, serotonergic and cholinergic components of nicotinic antinociception in rats. J Pharmacol Exp Ther. 1993;265:777–789. [PubMed] [Google Scholar]
- Jonkman LM, Lansbergen M, Stauder JEA. Developmental differences in behavioral and event-related brain responses associated with response preparation and inhibition in a go/nogo task. Psychophysiology. 2003;40:752–61. doi: 10.1111/1469-8986.00075. [DOI] [PubMed] [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin J-M, Anton J-L, Mazzola-Pomietto P. Impulsivity and neural correlates of response inhibition in schizophrenia. Psychol Med. 2011;41:291–299. doi: 10.1017/S0033291710000796. [DOI] [PubMed] [Google Scholar]
- Karl A, Malta LS, Maercker A. Meta-analytic review of event-related potential studies in post-traumatic stress disorder. Biol Psychol. 2006;71:123–147. doi: 10.1016/j.biopsycho.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Bruder GE. Dissociation of brain ERP topographies for tonal and phonetic oddball tasks. Psychophysiol. 1998;35:576–90. doi: 10.1017/s0048577298970214. [DOI] [PubMed] [Google Scholar]
- Keane TM, Caddell JM, Taylor KL. Mississippi Scale for Combat-Related Posttraumatic Stress Disorder: three studies in reliability and validity. J Consult Clin Psychol. 1988;56:85–90. doi: 10.1037//0022-006x.56.1.85. [DOI] [PubMed] [Google Scholar]
- Kemner C, Verbaten MN, Koelega HS, Buitelaar JK, van der Gaag RJ, Camfferman G, van Engeland H. Event-related brain potentials in children with attention-deficit and hyperactivity disorder: effects of stimulus deviancy and task relevance in visual and auditory modality. Biol Psychiatry. 1996;15:522–34. doi: 10.1016/0006-3223(95)00429-7. [DOI] [PubMed] [Google Scholar]
- Knight RT. Decreased response to novel stimuli after prefrontal lesions in man. Electroencephal Clin Neurophysiol. 1984;59:9–20. doi: 10.1016/0168-5597(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Knight RT. Contribution of human hippocampal region to novelty detection. Nature. 1996;383:256–9. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Laviolette SR. Dopamine modulation of emotional processing in cortical and subcortical neural circuits: evidence for a final common pathway in schizophrenia? Schizophr Bull. 2007;33(4):971–81. doi: 10.1093/schbul/sbm048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux JE, Farb CR, Romanksi LM. Overlapping projections to the amygdala and striatum from auditory processing areas of the thalamus and cortex. Neurosci Lett. 1991;16:139–44. doi: 10.1016/0304-3940(91)90526-y. [DOI] [PubMed] [Google Scholar]
- Levine PH, Richardson PK, Zolfaghari L, Cleary SD, Geist CE, Potolicchio S, et al. A study of Gulf War veterans with a possible deployment-related syndrome. Arch Environ Occup Health. 2006;61(6):271–8. doi: 10.3200/AEOH.61.6.271-278. [DOI] [PubMed] [Google Scholar]
- Li M, Kuroiwa Y, Wang L, Kamitani T, Takahashi T, Suzuki Y, Omoto S. Early sensory in formation processes are enhanced on visual oddball and S1-S2 tasks in Parkinson’s disease: a visual event-related potential study. Parkinsonism Relat Disord. 2003;9(6):329–40. doi: 10.1016/s1353-8020(02)00094-9. [DOI] [PubMed] [Google Scholar]
- Li X, Spence JS, Bruhner DM, Hart J, Jr, Cullum CM, Biggs MM, Hester AL, Odegard TN, Carmack PS, Briggs RW, Haley RW. Hippocampal dysfunction in Gulf War Veterans: investigation with ASL perfusion MR imaging and physostigmine challenge. Radiology. 2011;261(1):218–25. doi: 10.1148/radiol.11101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln AE, Helmer DA, Schneiderman A, Li M, Copeland HL, Prisco MK, et al. The war-related illness and injury study centers: a resource for deployment-related health concerns. Mil Med. 2006;171(7):577–85. doi: 10.7205/milmed.171.7.577. [DOI] [PubMed] [Google Scholar]
- Luntz-Leybman V, Bickford PC, Freedman R. Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Res. 1992;587:130–6. doi: 10.1016/0006-8993(92)91437-j. [DOI] [PubMed] [Google Scholar]
- Marco-Pallarés J, Nager W, Krämer UM, Cunillera T, Cámara E, Cucurell D, et al. Neurophysiological markers of novelty processing are modulated by COMT and DRD4 genotypes. NeuroImage. 2010;53(3):962–9. doi: 10.1016/j.neuroimage.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Loring DW, Davis HC, Sethi KD, Patel BR, Adams RJ, Hammond EJ. Cholinergic and serotonergic effects on the P3 potential and recent memory. J Clin Exp Neurophychol. 1989;11:252–60. doi: 10.1080/01688638908400887. [DOI] [PubMed] [Google Scholar]
- Merrin EL, Floyd TC. P300 Responses to novel auditory stimuli in hospitalized schizophrenic patients. Biol Psychiatry. 1994;36:527–42. doi: 10.1016/0006-3223(94)90617-3. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Lindgren J, Hardin D, Griffis JM, Weiner MW. Metabolic abnormalities in the brain of subjects with Gulf War Illness [Abstract] Proc Int Soc Magn Reson Med. 2001;9:994. [Google Scholar]
- Morey RA, Dolcos F, Petty CM, et al. The role of trauma-related distractors on neural systems for working memory and emotion processing in posttraumatic stress disorder. J Psychiatr Res. 2009;43:809–817. doi: 10.1016/j.jpsychires.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller V, Brehmer Y, von Oertzen T, Li SC, Lindenberger U. Electrophysiological correlates of selective attention: a lifespan comparison. BMC Neurosci. 2008;9:18. doi: 10.1186/1471–2202–9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münte TF, Heinze HJ, Scholz M, Künkel H. Effects of a cholinergic nootropic (WEB 1881 FU) on event-related potentials recorded in incidental and intentional memory tasks. Neuropsychobiology. 1988;19:158–68. doi: 10.1159/000118453. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Matsushima E, Ohta K, Ando K, Kojima T. Relationship between attention and arousal level in schizophrenia. Psychiatry Clin Neurosci. 2003;57:472–77. doi: 10.1046/j.1440-1819.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- Nikolaus S, Antke C, Beu M, Müller HW. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders—results from in vivo imaging studies. Rev Neurosci. 2010;21(2):119–39. doi: 10.1515/revneuro.2010.21.2.119. [DOI] [PubMed] [Google Scholar]
- Pillay SS, Gruber SA, Rogowska J, Simpson N, Yurgelun-Todd DA. fMRI of fearful facial affect recognition in panic disorder: the cingulate gyrus-amygdala connection. J Affect Disorder. 2006;94:173–81. doi: 10.1016/j.jad.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Poceta SJ, Houser M, Polich J. Event-related potentials in restless leg syndrome and Parkinson’s disease [Abstract] Sleep. 2006;28:A274. [Google Scholar]
- Polich J. Updating P300: an intergrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–48. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport M, McCauley S, Levin H, Song J, Feinstein A. The role of injury severity in neurobehavioral outcome 3 months after traumatic brain injury. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15:123–132. [PubMed] [Google Scholar]
- Reese NB, Garcia-Rill E, Skinner RD. The pedunculopontine nucleus—auditory input, arousal and pathophysiology. Prog Neurobiol. 1995;42:105–33. doi: 10.1016/0301-0082(95)00023-o. [DOI] [PubMed] [Google Scholar]
- Rektor I, Bares M, Brázdil M, Kanovsky P, Rektorova I, Sochurkova D, et al. Cognitive- and movement-related potentials recorded in the human basal ganglia. Mov Disord. 2005;20:562–68. doi: 10.1002/mds.20368. [DOI] [PubMed] [Google Scholar]
- Research Advisory Committee on Gulf War Veterans’ Illnesses. Gulf War Illness and the Health of Gulf War Veterans: Scientific Findings and Recommendations. Washington, D. C: U. S. Government Printing Office; 2008. [Google Scholar]
- Ruscio AM, Borkovec TD. Experience and appraisal of worry among high worriers with and without generalized anxiety disorder. Behav Res Ther. 2004;42:1469–82. doi: 10.1016/j.brat.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Sachs G, Anderer P, Dantendorfer K, Saletu B. EEG mapping in patients with social phobia. Psychiatry Res. 2004;131:237–47. doi: 10.1016/j.pscychresns.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Johnasen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci. 2005;28:397–419. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- Sevim S, Dogu O, Kaleagasi H, Aral M, Metin O, Camdeviren H. Correlation of anxiety and depression symptoms in patients with restless legs syndrome: a population based survey. J Neurol Neurosurg Psychiatry. 2004;72:226–30. [PMC free article] [PubMed] [Google Scholar]
- Siegel C, Waldo M, Mizner G, Adler LE, Freedman R. Deficits in sensory gating in schizophrenic patients and their relatives: evidence obtained with auditory evoked responses. Archives of General Psychiatry. 1984;41:607–12. doi: 10.1001/archpsyc.1984.01790170081009. [DOI] [PubMed] [Google Scholar]
- Skinner RD, Rasco LM, Fitzgerald J, Karson CN, Matthew M, Williams DK, Garcia-Rill E. Reduced sensory gating of the P1 potential in rape victims and combat veterans with posttraumatic stress disorder. Depress Anxiety. 1999;9:122–30. doi: 10.1002/(sici)1520-6394(1999)9:3<122::aid-da4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Spencer KM. Averaging, detection, and classification of single-trial ERPs. In: Handy TC, editor. Event-related potentials: a method handbook. Cambridge, MA: MIT Press; 2005. pp. 209–27. [Google Scholar]
- Spronk M, Jonkman LM, Kemner C. Response inhibition and attention processing in 5- to 7-year-old children with and without symptoms of ADHD: An ERP study. Clin Neurophysiol. 2008;119:2738–52. doi: 10.1016/j.clinph.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Stanford MS, Vasterling JJ, Mathias CW, Constans JI, Houston RJ. Impact of threat relevance on P3 event-related potentials in combat-related post-traumatic stress disorder. Psychiatry Res. 2001;102:125–37. doi: 10.1016/s0165-1781(01)00236-0. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Pearlson GD, Kiehl KA. An fMRI auditory oddball study of combined-subtype attention deficit hyperactivity disorder. Am J Psychiatry. 2007;164:1737–49. doi: 10.1176/appi.ajp.2007.06050876. [DOI] [PubMed] [Google Scholar]
- Stimpson NJ, Thomas HV, Weightman AL, Dunstan F, Lewis G. Psychiatric disorder in veterans of the Persian Gulf War of 1991: Systematic review. Brit J Psychiatry. 2003;182:391–403. [PubMed] [Google Scholar]
- Stocchi F, Brusa L. Cognition and emotion in different stages and subtypes of Parkinson’s disease. J Neurol. 2000;247(Suppl 2II):114–21. doi: 10.1007/pl00022912. [DOI] [PubMed] [Google Scholar]
- Thompson KE, Vasterling JJ, Benotsch EG, Brailey K, Constans J, Uddo M, Sutker PB. Early symptom predictors of chronic distress in Gulf War veterans. J Nerv Ment Dis. 2004;192:146–52. doi: 10.1097/01.nmd.0000110286.10445.ab. [DOI] [PubMed] [Google Scholar]
- Tillman GD. Estradiol levels during the menstrual cycle differentially affect latencies to right and left hemispheres during dichotic listening: an ERP study. Psychoneuroendocrinology. 2010;35:249–61. doi: 10.1016/j.psyneuen.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Tillman GD, Green TA, Ferree TC, Calley CS, Maguire MJ, Briggs R, et al. Impaired response inhibition in ill Gulf War veterans. J Neurol Sci. 2010;297:1–5. doi: 10.1016/j.jns.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Toda M, Abi-Dargham A. Dopamine hypothesis of schizophrenia: making sense of it all. Current Psychiatry Reports. 2007;9:329–36. doi: 10.1007/s11920-007-0041-7. [DOI] [PubMed] [Google Scholar]
- Uc EY, Skinner RD, Rodnitzky RL, Garcia-Rill E. The midlantency auditory evoked potential P50 is abnormal in Huntington’s disease. J Neurol Sci. 2003;212:1–5. doi: 10.1016/s0022-510x(03)00082-0. [DOI] [PubMed] [Google Scholar]
- Weisbrod M, Kiefer M, Marzinzik F, Spitzer M. Executive control in disturbed in schizophrenia: evidence from event-related potentials in a go/nogo task. Biol Psychiatry. 2000;47:51–60. doi: 10.1016/s0006-3223(99)00218-8. [DOI] [PubMed] [Google Scholar]