Abstract
Background
Sleep-disordered breathing, a common condition in obese children, is a frequent indication for tonsillectomy.
Objective
The purpose of this study was to examine the association between obesity and perioperative complications in children undergoing tonsillectomy.
Methods/Materials
A sample of 100 severely obese children (body mass index for age, BMIA, ≥98th percentile) between ages 2 to 18 years who underwent tonsillectomy at Mayo Clinic Rochester was randomly selected. Each severely obese child was age (±2 years) and sex matched to two normal weight children (body mass index for age between 25th-75th percentiles) undergoing tonsillectomy in the same calendar year, and their medical records were reviewed.
Results
Severely obese children had a significantly higher incidence of comorbid conditions including respiratory disorders and severe systemic disorders or syndromes. Severely obese children had a higher frequency of perioperative airway complications (15.0% vs. 2.0%). From post-hoc analyses, severe obesity remained a significant risk factor for perioperative adverse events after adjusting for the presence of severe systemic disorders or syndromes (OR 8.8; 95% CI 2.8-27.5, P<0.001) and also after adjusting for preoperative respiratory disorders (OR 7.7; 95% CI 2.5-24.3, P<0.001). When children with planned admissions were excluded from the analysis, severe obesity was associated with an increased rate of unplanned hospital admission (OR 3.80, 95% CI 1.83-7.87, P<0.001).
Conclusions
Severe obesity in children undergoing tonsillectomy is independently associated with an increased risk of perioperative complications. It appears that both severe obesity and systemic comorbid condition contribute to higher proportions of inpatient tonsillectomies performed in our institution.
Keywords: Obesity, tonsillectomy, pediatrics, perioperative complications
INTRODUCTION
Tonsillectomy is one of the most common surgical procedures performed in children (1, 2). Sleep disordered breathing (SDB) has replaced frequent infections as the most frequent indication for tonsillectomy (3). Children with SDB who undergo tonsillectomy have a higher rate of perioperative complications (4-8). Because SDB is frequently associated with obesity (4, 6), an increasing proportion of children presenting for tonsillectomy are overweight or obese. In 2007-2008, in the United States, more than 31.7% of children and adolescents ages 2 through 19 years were overweight (body mass index for age [BMIA] ≥85th percentile), 16.9% are obese (BMIA ≥95th percentile), and 11.9% are above the 97th percentile of the BMI-for-age growth charts (9, 10). Prior work, both in children undergoing non-cardiac surgery in general and tonsillectomy in particular (4, 11, 12), suggests that obesity is associated with increased perioperative risk. However, the high rate of concurrence between SDB and obesity can complicate efforts to attribute risk separately to each factor.
If obesity independently affects the risk for complications after tonsillectomy, we speculated that this risk would be greatest in severely obese children (defined in this paper as having a BMIA ≥98th percentile). However, these children frequently have comorbid diseases that themselves may increase risk. The purpose of this retrospective, observational, matched cohort study of children undergoing tonsillectomy was to test the hypothesis that severe obesity is an independent risk factor for the occurrence of perioperative events. We also describe the absolute incidence of such events, their clinical significance, and associations with admission status. This represents the largest reported experience with severely obese children undergoing tonsillectomy.
MATERIALS AND METHODS
Study design and patients
This study was approved by the Mayo Clinic Institutional Review Board (Rochester, Minnesota, USA). The medical records of all children between age 2 and 18 years who underwent tonsillectomy with or without adenoidectomy at Mayo Clinic from January 1, 1996 to December 31, 2005 were reviewed to identify severely obese children, defined as BMI ≥98th percentile for age and gender (13). Review of the electronic medical records revealed a total of 104 children with BMIA ≥98th percentile who had their weight and height recorded on two separate occasions within six months of tonsillectomy. After reviewing these charts, four cases were eliminated due to suspicious inconsistencies in body weight measurement or because patient underwent multiple procedures at the time of scheduled adenotonsillectomy. Each of the remaining severely obese children (n = 100) were matched to two normal weight (BMIA 25th–75th percentile) controls of the same sex, similar age (within two years) and the same year of surgery, who underwent identical procedures.
Data collection
Individual medical records of severely obese and normal weight children were reviewed for patient characteristics, comorbid conditions, perioperative data, complications, and discharge status (outpatient, planned inpatient surgery, unplanned admission, discharge and readmission). Patient height and weight recorded within the six months of surgery were utilized for calculation of BMI (kg/m2) and BMI percentile for age. Preoperative comorbid conditions were recorded and classified as respiratory disorders (reactive airway disease [daily use of asthma controlling medications/inhalers], current upper respiratory symptoms [acute cough and rhinorrhea symptoms within one week noted in the medical records], and sleep disordered breathing [either by clinical diagnosis or diagnosed by polysomnography]) or severe systemic disease or syndrome.
We recorded the duration of anesthesia and the time of day (before [morning] or after [afternoon] 12:00 PM) when surgery was completed. Adverse respiratory events during anesthetic induction, maintenance, and emergence were defined as follows: difficulties in tracheal intubation evidenced by the use or techniques other than direct laryngoscopy, lowest intraoperative oxyhemoglobin saturation as assessed by pulse oximetry (categorized as mild [≤90%] or severe [≤70%] oxyhemoglobin desaturation), episodes of bronchospasm (as documented or requiring treatment with bronchodilator), stridor/laryngospasm (as documented or requiring treatment with positive pressure, racemic epinephrine or corticosteroids), airway obstruction (as documented as an event that required at least one of the following: oral or nasal airway insertion, chin lift maneuver, or placement of laryngeal mask airway (LMA) or endotracheal tube), and clinically-evident aspiration (as documented or evidenced from chest radiogram).
Postanesthesia care unit (PACU) events included bronchospasm, airway obstruction, aspiration, hypoxemia (SpO2 ≤90% on room air or requiring continuous supplemental oxygen to maintain SpO2 >90% at time of PACU discharge), postoperative bleeding, requirement for tracheal reintubation, and hemodynamic instability requiring pharmacologic treatment. Immediate postoperative nausea and vomiting and treatment were also recorded.
Postoperative disposition was defined as follows. Planned admissions reflected specific preoperative documentation of this intention. Admissions (ward or intensive care unit [ICU]) occurring in the absence of such documentation, or when same-day surgery was specified preoperatively, were defined as unplanned. For those children admitted to the hospital, admission indications were categorized as related to 1) airway management (e.g., need for airway monitoring, airway obstruction, need for ventilatory support, aspiration), 2) inadequate hemostasis (bleeding), or; 3) miscellaneous causes (e.g., poor pain control, nausea/vomiting, inadequate oral intake).
Statistical analysis
Data are summarized using mean ± SD for continuous variables and proportions for nominal variables. Baseline patient characteristics and postoperative adverse events were compared between severely obese children and controls using two-sample t-test for continuous variables and chi square or Fisher’s exact test for categorical variables. Multivariable logistic regression was used for a post-hoc analysis to assess whether severe obesity was associated with perioperative complications after adjusting for the presence of a severe systemic disorder/syndrome. An additional post-hoc analysis was also performed to assess the association between severe obesity and severe perioperative complication after adjusting for preoperative airway or breathing disorders.
Among severely obese patients scheduled for outpatient surgery, characteristics were compared between those with unplanned admissions vs. not using two-sample t-test for continuous variables and chi square or Fisher’s exact test for categorical variables.
Two-tailed P-values ≤0.05 were considered statistically significant. Analyses were performed using SAS statistical software (Version 9.2, SAS Institute, Inc, Cary, NC).
RESULTS
Preoperative characteristics and comorbidities
Demographics, preoperative comorbidities and characteristics of anesthetic management for normal weight and severely obese children are shown in Table 1. By design, the sex distribution is identical between groups. Groups were also matched on age. The median (25th, 75th percentile) absolute difference in age between severely obese children and their matched controls was 1.1 (0.4, 3.1) months, with 89% matched within ± 6 months of age. The burden of comorbidities was greater in severely obese children as reflected by higher rates of reactive airway disease (P<0.001), sleep disordered breathing (P=0.003), and presence of severe systemic disorders/syndromes (P=0.001). In all children, normal weight and severely obese, tracheal intubation was successfully performed using direct laryngoscopy. Anesthetic induction was performed using inhalational agents in 151 (75.5%) of normal weight children, and in 79 (79.0%) severely obese children. In these patients, the most frequently used inhalational agent was sevoflurane. Of patients receiving intravenous induction, the most frequently used agent was propofol (47/49 normal weight, and 17/21 severely obese patients). Severely obese children had a slightly longer duration of anesthesia when compared to normal weight children (mean 1.4 ± 0.6 hours vs. 1.3 ± 0.4 hours, respectively, P=0.004).
Table 1.
Patient demographics, comorbidities and anesthetic characteristics in normal weight and severely obese children undergoing tonsillectomy
| Characteristic | Normal weight (n=200) |
Severely Obese (n=100)‡ |
P |
|---|---|---|---|
| Age (years) | 9.1 ± 3.9 | 9.2 ± 4.0 | 0.780 |
| Sex | 1.000 | ||
| Male | 100 (50.0) | 50 (50.0) | |
| Female | 100 (50.0) | 50 (50.0) | |
| Body mass index (kg/m2) | 17.0 ± 2.0 | 28.4 ± 7.5 | <0.001 |
| BMIA (percentile) | 51.7 ± 14.5 | 99.0 ± 0.8 | <0.001 |
| Any respiratory/breathing disorders | 150 (75.0) | 92 (92.0) | <0.001 |
| Reactive airway disease | 22 (11.0) | 26 (26.0) | 0.001 |
| Current upper respiratory infection | 2 (1.0) | 3 (3.0) | 0.200 |
| Sleep disordered breathing | 143 (71.5) | 87 (87.0) | 0.003 |
| Other major comorbidities | 14 (14.0) | 0.001 | |
| Trisomy 21 | 1 | 7 | |
| Lowe Syndrome | 1 | 0 | |
| Ring chromosome #15 syndrome | 0 | 1 | |
| Bardet Biedl Syndrome | 0 | 1 | |
| Hypochondrogenesis | 0 | 1 | |
| Liver transplant recipient | 1 | 0 | |
| Inborn disorder of metabolism | 1 | 2 | |
| Hypotonia with developmental delay | 1 | 1 | |
| Insulin dependent diabetes mellitus | 1 | 1 | |
| Pfeiffer’s syndrome | 1 | 0 | |
| Airway management (endotracheal intubation) | 200 (100) | 100 (100) | --- |
| Need for fiberoptic intubation | --- | ||
| Type of induction | 0.499 | ||
| Inhalation | 151 (75.5) | 79 (79.0) | |
| Intravenous | 49 (24.5) | 21 (21.0) | |
| Use of nitrous oxide | 147 (73.5) | 72 (72.0) | 0.783 |
| Intraoperative use of opioids | 195 (97.5) | 99 (99.0) | 0.382 |
| Duration of anesthesia (hours) | 1.3 ±0.4 | 1.4 ± 0.6 | 0.004 |
The data are presented as n (%) or mean ± standard deviation as appropriate.
P-values are from chi square tests for categorical variables and two-sample t-test for continuous variables.
Excludes obesity and systemic conditions exacerbated by obesity.
Abbreviations: BMIA: Body mass index for age.
Intraoperative and PACU events
Mild intraoperative oxyhemoglobin desaturations (SpO2 <90%) were present in 19 (9.5%) normal weight patients and 19 (19.0%) severely obese patients (P=0.044). The occurrence of any severe adverse perioperative event (shown in Table 2) was significantly (P<0.001) more frequent in severely obese children (15.0%, 95% CI 8.7% to 23.5%) compared to normal weight children (2.0%, 95% CI 0.6% to 5.0%). The majority of severe adverse perioperative events were airway and breathing complications (Table 2). There were no cardiovascular complications in either group. In the PACU, one normal weight patient developed tonsillar hemorrhage requiring surgical re-exploration, and one severely obese child developed bronchospasm, and both required emergent tracheal reintubation. The percentage of patients treated for postoperative nausea and vomiting was similar between groups (18 [9.0%] vs. 6 [6.0%], P=0.499, in normal weight and severely obese groups, respectively). The majority of patients received intraoperative antiemetic prophylaxis (ondansetron and/or dexamethasone), however, 19 control patients (9.5%) and 10 (10.0%) severely obese did not receive any antiemetic prophylaxis. Both therapies were simultaneously given in 109 (55.5%) control patients, and in 66 (66.0%) severely obese children.
Table 2.
Severe intraoperative and immediate postoperative adverse events among severely obese and normal weight children undergoing tonsillectomy
| Adverse Events | Normal Weight (n=200) |
Severely Obese (n=100) |
P* |
|---|---|---|---|
| Any intraoperative or emergence events† | 3 (1.5) | 14 (14.0) | <0.001 |
| Bronchospasm | 1 | 2 | 0.259 |
| Airway obstruction | 0 | 7 | <0.001 |
| Stridor/laryngospasm | 1 | 4 | 0.044 |
| Aspiration | 0 | 0 | --- |
| Severe hypoxemia, SpO2 ≤70% | 1 | 5 | 0.017 |
| Any recovery room events† | 1 (0.5) | 3 (3.0) | 0.074 |
| Bronchospasm | 0 | 1 | 1.000 |
| Airway obstruction | 0 | 3 | 0.036 |
| Aspiration | 0 | 0 | --- |
| Reoperation for tonsillar bleeding | 1 | 0 | 1.000 |
| Tracheal reintubation‡ | 1 | 1 | 1.000 |
| Any Perioperative events† | 4 (2.0) | 15 (15.0) | <0.001 |
The data are presented as n (%).
P-value from Fisher’s exact test.
The number of children experiencing any adverse event is less than the sum of the individual events because some children experienced multiple complications.
Indication for tracheal intubation: in normal weight child, bleeding; in severely obese child, bronchospasm.
Abbreviations: SpO2: Oxyhemoglobin saturation
Since severely obese patients had a significantly higher prevalence of severe systemic disorders/syndromes, we performed a post-hoc analysis to assess whether this contributed to the increased rate of complications in this group. Of the 19 (four normal weight, 15 severely obese) patients who experienced severe perioperative events, only two had a severe systemic disorder/syndrome, and both were severely obese. From a multivariable logistic regression analysis (adjusted for the presence of severe systemic disorder or syndrome), severe obesity remained a significant independent risk factor for the development of perioperative severe adverse events (OR 8.8; 95% CI 2.8-27.5, P<0.001). Severe obesity was also found to be a significant risk factor for the development of perioperative severe adverse events in an analysis that adjusted for any preoperative respiratory disorder (OR 7.7; 95% CI 2.5-24.3, P<0.001).
Hospital admission status
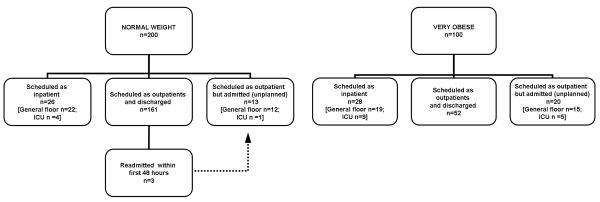
The proportion of tonsillectomies scheduled as planned admissions was significantly (P=0.001) greater in severely obese children (28.0%) vs. normal weight children (13.0%) (Figure 1). When children with planned admissions were excluded from the analysis, severe obesity was associated with an increased rate of unplanned hospital admission (Figure 1, OR 3.80; 95% CI 1.83-7.87, P<0.001). The most frequent indication for unplanned admission was concern regarding breathing, (5/16 of normal weight children and 8/20 of severely obese children), including one severely obese child who required reintubation and mechanical ventilation for airway obstruction. Another severely obese child was admitted for observation of a bleeding tonsillar bed. The other children were admitted for miscellaneous other reasons such as pain, nausea, vomiting, and poor oral intake. Of the 20 (20.0%) severely obese children who required an unplanned admission, five were admitted to the intensive care unit (ICU), compared with 16 (8.0%) of normal weight patients, of which one was admitted to the ICU. However, three of the normal weight children were readmitted to the hospital within 48 hour for poor fluid intake, dehydration, and pain management issues; none of the severely obese children were readmitted.
Figure 1.
Flow chart showing admission status of normal weight and severely obese children after tonsillectomy.
Abbreviations: ICU: Intensive care unit; n, number of patients.
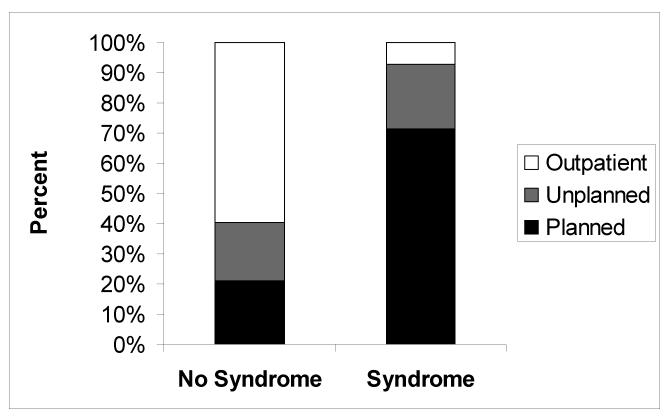
Among all severely obese children, those with severe systemic diseases/syndromes were more likely (10/14, 71.4%) to be scheduled as inpatients compared to those without such comorbidities (18/86, 20.9%) (P=0.001) (Figure 2). Among severely obese children scheduled as outpatients, the presence of a severe systemic disease/syndrome was univariately associated with an increased likelihood of unplanned admission (Table 3). Of children scheduled as outpatients, the percent undergoing procedures that were completed in the afternoon did not differ significantly between those who were admitted vs. not (35.0% vs. 32.7%, P=0.852).
Figure 2.
Distribution according to admission status among severely obese patients in regard to the presence or absence of severe systemic disease or syndrome.
Table 3.
Patient characteristics and comorbid diseases in severely obese children undergoing tonsillectomy according to the discharge status.
| Variable | Planned Admission (n=28) |
Scheduled as Outpatients (n=72) |
P | |
|---|---|---|---|---|
| Discharged (n=52) |
Unplanned Admission (n=20) |
|||
| Age at surgery (years) | 8.9 ±5.1 | 9.4 ±3.1 | 9.1 ±4.5 | 0.752 |
| Sex | 0.930 | |||
| Male | 11 (39.3) | 28 (53.8) | 11 (55.0) | |
| Female | 17 (60.7) | 24 (46.2) | 9 (45.0) | |
| Body mass index (kg/m2) | 28.1 ± 8.6 | 28.7 ±6.7 | 27.8 ±8.0 | 0.600 |
| Reactive airway disease | 7 (25.0) | 14 (26.9) | 5 (25.0) | 0.868 |
| Current upper respiratory infection | 0 (0) | 2 (3.8) | 1 (5.0) | 1.000 |
| Sleep disordered breathing | 26 (92.9) | 44 (84.6) | 17 (85.0) | 0.968 |
| Severe systemic disorder/syndrome | 10 (35.7) | 1 (19) | 3 (15.0) | <0.001 |
| Duration of anesthesia (hours) | 1.6 ±0.8 | 1.3 ±0.4 | 1.5 ±0.6 | 0.055 |
| Time surgery completed | 0.852 | |||
| Morning (08:00-11:59) | 14 (50.0) | 35 (67.3) | 13 (65.0) | |
| Afternoon (12:00-17:00) | 14 (50.0) | 17 (32.7) | 7 (35.0) | |
The data are presented as n (%) or mean ± standard deviation as appropriate. P values are from t-test for continuous variables and Chi square or Fisher’s exact test for nominal variables comparing outpatients discharged vs. unplanned admission.
DISCUSSION
The main finding of this study is that severe obesity was independently associated with an increased rate of severe perioperative adverse events, with most events related to airway or breathing complications and this finding is independent of the presence or absence of sleep disordered breathing.
Previous studies suggest that obesity is associated with increased perioperative risk for adverse respiratory events (7, 11, 12). It is possible that children who are severely obese may be at even higher risk, and this is not known as these children have not been the subject of analyses. Also, comorbid conditions which may be associated with obesity can complicate the consideration of obesity as an independent risk factor. In this analysis, we specifically examined severely obese children (defined here as a BMIA ≥98th percentile), and the impact of two conditions that may be associated with obesity: SDB and systemic disease/syndromes.
A history of reactive airway disease or SDB increases the risk of adverse perioperative events in pediatric patients undergoing non-cardiac surgery (5, 6). Recent studies find that SDB is associated with higher rates of respiratory complications after tonsillectomy (8, 11, 12, 14). Although tonsillectomy eventually improves SDB and quality of life in most patients (15), risk persists even after resection in the immediately postoperative period, with upper airway obstruction still occurring on the first postoperative night in children with SDB (16). Therefore, the surgical removal of anatomical “obstruction” does not immediately eliminate the risk for perioperative respiratory complications in these patients. SDB is common in obese children (17). The high rate of concurrence between these two conditions raises the question of whether any increase in risk associated with obesity could be explained by SDB. In our cohort, SDB was highly prevalent in both obese and normal weight patients, and SDB was the most prevalent surgical indication. When SDB was included as a factor in the analysis, severe obesity was still highly associated with adverse events.
The spectrum of SDB encompasses various conditions, from habitual snoring to fully developed OSA, and includes intermediate cases with upper airway resistance syndrome and obstructive hypoventilation (18). The diagnosis of OSA cannot be based solely on clinical criteria and an accurate diagnosis requires polysomnography. This diagnosis may have implications for pathophysiology. In particular, children with true OSA have an increased systemic inflammatory response that may contribute to an increased risk for end-organ morbidity (18, 19). For example, children with OSA have altered endothelial function which may be reversed with improvement of obstruction after adenotonsillectomy (20). In our patients, most of the SDB diagnoses were established on clinical grounds, and only a small proportion of children underwent polysomnography testing before surgery. For this reason, we cannot comment on the frequency of true OSA within our study patients, or OSA as an independent risk factor for postoperative complications.
Severely obese children also had a higher frequency of associated systemic disorders and syndromes, which by itself can lead to obesity, or may be caused or exacerbated by obesity. It was not possible to determine with this analysis how these disorders may themselves contribute to risk, as their absolute prevalence was relatively low. However, when included as a factor in the analysis, severe obesity itself was still significantly associated with adverse events, suggesting that the higher frequency of comorbid conditions could not explain the association.
The proportion of surgical procedures scheduled as planned admissions was significantly greater in severely obese (28.0%) compared to normal weight children (13.0%), indicating that physicians are more likely to plan extended postoperative monitoring in these children. This makes it difficult to directly compare the rates of unplanned admission between severely obese and normal weight groups. Nonetheless, almost half of the severely obese group was admitted, with those children with a severe systemic disorder or syndrome having the highest admission rate (13 of 14) (Table 3, Figure 2). Others have demonstrated increased rates of admissions in obese children undergoing oral or airway procedures (5, 11, 12), and some proposed overnight observation for all obese children after tonsillectomy (12, 14, 21, 22). The current data do not bear directly on this recommendation; the lack of adverse events in those obese children discharged as outpatients should be interpreted with caution, as the numbers of these patients was relatively low (the upper bound of the 95% confidence interval for no events in a group of 52 children is 6.7%). In our study, completion of surgery in the afternoon in severely obese children was not associated with the increased rate of unplanned admissions. Intuitively, a longer monitoring period after tonsillectomy (by completing the surgery in the morning) may allow for more time to recovery which potentially may reduce the need for hospitalization. One study demonstrated (8) that children with severe SDB whose tonsillectomy is performed in the morning are less likely to exhibit episodes of oxyhemoglobin desaturation compared to surgery completed in the afternoon.
Limitations of the study
Our findings should be interpreted in light of several limitations. First, this is a retrospective study with all the inherent limitations of this design. While in the majority of our patients the indication for tonsillectomy was SDB, we do not know which children met the full criteria for obstructive sleep apnea as the indication for tonsillectomy was predominantly based on clinical diagnosis rather than by polysomnography. Therefore, differences in the severity of SDB between severely obese and normal weight children and the potential role of the severity of SDB as a contributor to postoperative complications could not be assessed. Although severely obese patients were more likely to be scheduled as inpatients, our study cannot elucidate whether these admissions were warranted or not.
Conclusions
Compared to normal weight children, severely obese pediatric patients undergoing adenotonsillectomy have higher rates of perioperative respiratory complications, and higher rates of planned and unplanned admissions. The risk of adverse respiratory events persists despite adjustment for SDB. Despite the increase in risk associated with obesity, it is important to note that 85% of our severely obese children did not experience adverse events, demonstrating that even for the severely obese children undergoing tonsillectomy, the majority do not experience severe perioperative complications.
Acknowledgments
This research was funded by National Institutes of Health, National Center for Research Resources, National Center for Advancing Translational Sciences, Center for Translational Science Activities (NIH/NCRR/NCATS CTSA) grant number UL1 RR024150 (RedCap®). This project was supported by the Department of Anesthesiology, College of Medicine, Mayo Clinic, Rochester, MN, 55905. We would also like to thank Mr. Andrew Hanson (statistical program analyst, Mayo Clinic Rochester, Minnesota) and Shonie Buenvenida, R.N. (study coordinator, Mayo Clinic Rochester, Minnesota).
Footnotes
Stephen J. Gleich and Michael D. Olson contributed equally to this work.
REFERENCES
- 1.Baugh RF, Archer SM, Mitchell RB, et al. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144:S1–30. doi: 10.1177/0194599810389949. [DOI] [PubMed] [Google Scholar]
- 2.Blair RL, McKerrow WS, Carter NW, et al. The Scottish tonsillectomy audit. The Audit Sub-Committee of the Scottish Otolaryngological Society. J Laryngol Otol Suppl. 1996;20:1–25. [PubMed] [Google Scholar]
- 3.Erickson BK, Larson DR, St Sauver JL, et al. Changes in incidence and indications of tonsillectomy and adenotonsillectomy, 1970-2005. Otolaryngol Head Neck Surg. 2009;140:894–901. doi: 10.1016/j.otohns.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 4.Nafiu OO, Reynolds PI, Bamgbade OA, et al. Childhood body mass index and perioperative complications. Paediatr Anaesth. 2007;17:426–430. doi: 10.1111/j.1460-9592.2006.02140.x. [DOI] [PubMed] [Google Scholar]
- 5.Setzer N, Saade E. Childhood obesity and anesthetic morbidity. Paediatr Anaesth. 2007;17:321–326. doi: 10.1111/j.1460-9592.2006.02128.x. [DOI] [PubMed] [Google Scholar]
- 6.Tait AR, Voepel-Lewis T, Burke C, et al. Incidence and risk factors for perioperative adverse respiratory events in children who are obese. Anesthesiology. 2008;108:375–380. doi: 10.1097/ALN.0b013e318164ca9b. [DOI] [PubMed] [Google Scholar]
- 7.Cote CJ, Sheldon SH. Obstructive sleep apnea and tonsillectomy: do we have a new indication for extended postoperative observation? Can J Anaesth. 2004;51:6–12. doi: 10.1007/BF03018539. [DOI] [PubMed] [Google Scholar]
- 8.Koomson A, Morin I, Brouillette R, et al. Children with severe OSAS who have adenotonsillectomy in the morning are less likely to have postoperative desaturation than those operated in the afternoon. Can J Anaesth. 2004;51:62–67. doi: 10.1007/BF03018549. [DOI] [PubMed] [Google Scholar]
- 9.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 10.Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. Natl Health Stat Report. 2010:1–5. [PubMed] [Google Scholar]
- 11.Nafiu OO, Green GE, Walton S, et al. Obesity and risk of peri-operative complications in children presenting for adenotonsillectomy. Int J Pediatr Otorhinolaryngol. 2009;73:89–95. doi: 10.1016/j.ijporl.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Fung E, Cave D, Witmans M, et al. Postoperative respiratory complications and recovery in obese children following adenotonsillectomy for sleep-disordered breathing: a case-control study. Otolaryngol Head Neck Surg. 2010;142:898–905. doi: 10.1016/j.otohns.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong J, Reilly JJ. Breastfeeding and lowering the risk of childhood obesity. Lancet. 2002;359:2003–2004. doi: 10.1016/S0140-6736(02)08837-2. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad R, Abdullah K, Amin Z, et al. Predicting safe tonsillectomy for ambulatory surgery. Auris Nasus Larynx. 2010;37:185–189. doi: 10.1016/j.anl.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Schwengel DA, Sterni LM, Tunkel DE, et al. Perioperative management of children with obstructive sleep apnea. Anesth Analg. 2009;109:60–75. doi: 10.1213/ane.0b013e3181a19e21. [DOI] [PubMed] [Google Scholar]
- 16.Nixon GM, Kermack AS, McGregor CD, et al. Sleep and breathing on the first night after adenotonsillectomy for obstructive sleep apnea. Pediatr Pulmonol. 2005;39:332–338. doi: 10.1002/ppul.20195. [DOI] [PubMed] [Google Scholar]
- 17.Shelton KE, Woodson H, Gay S, et al. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis. 1993;148:462–466. doi: 10.1164/ajrccm/148.2.462. [DOI] [PubMed] [Google Scholar]
- 18.Dayyat E, Kheirandish-Gozal L, Gozal D. Childhood Obstructive Sleep Apnea: One or Two Distinct Disease Entities? Sleep Med Clin. 2007;2:433–444. doi: 10.1016/j.jsmc.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gozal D, Serpero LD, Sans Capdevila O, et al. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med. 2008;9:254–259. doi: 10.1016/j.sleep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gozal D, Kheirandish-Gozal L, Serpero LD, et al. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation. 2007;116:2307–2314. doi: 10.1161/CIRCULATIONAHA.107.696823. [DOI] [PubMed] [Google Scholar]
- 21.McColley SA, April MM, Carroll JL, et al. Respiratory compromise after adenotonsillectomy in children with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 1992;118:940–943. doi: 10.1001/archotol.1992.01880090056017. [DOI] [PubMed] [Google Scholar]
- 22.Rosen GM, Muckle RP, Mahowald MW, et al. Postoperative respiratory compromise in children with obstructive sleep apnea syndrome: can it be anticipated? Pediatrics. 1994;93:784–788. [PubMed] [Google Scholar]