Abstract
Introduction
The development of novel bifunctional chelates for attaching copper-64 to biomolecules has been an active area of research for several years. However, many of these 64Cu-chelates have poor in vivo stability or harsh radiolabeling conditions.
Methods
In this study, two triazacyclononane analogs; C-NE3TA (4-carboxymethyl-7-[2-(carboxymethyl-amino)-3-(4-nitro-phenyl)-propyl]-[1,4,7]triazo-nan-1-yl-acetic acid) and N-NE3TA (4-carboxymethyl-7-[2-[carboxymethyl-(4-nitro-benzyl)-amino]-ethyl]-[1,4,7]triazonan-1-yl-acetic acid) were evaluated for their labeling efficiency with 64Cu at room temperature and evaluated in vitro and in vivo. In vitro studies included complexation kinetics with Cu(II) using a spectrophotometric method and rat serum stability, while the in vivo study evaluated biodistribution in SCID mice.
Results
C-NE3TA and N-NE3TA were labeled at >95% efficiency up to ~3.4 Ci/μmol. Both C-NE3TA and N-NE3TA formed complexes with Cu(II) almost immediately, with the Cu(II) complexation by C-NE3TA being faster than the formation of Cu(II)-N-NE3TA. Both 64Cu-N-NE3TA and 64Cu-C-NE3TA were 96.1% and 90.5% intact after 48 h incubation in rat serum, respectively. This is compared to 64Cu complexes of the control chelators, p-NH2-Bn-DOTA and p-NH2-Bn-NOTA, with 93.9% and 97.9% retention of 64Cu in the complex, respectively. In vivo evaluation of 64Cu-N-NE3TA and 64Cu-C-NE3TA demonstrates good clearance from normal tissues except for the liver, where 59% and 51% of the radioactivity is retained at 24 h compared to 1 h for 64Cu-N-NE3TA and 64Cu-C-NE3TA, respectively. This compares to 78% and 3% retention for 64Cu-p-NH2-Bn-DOTA and 64Cu-p-NH2-Bn-NOTA.
Conclusions
These studies demonstrate that while N-NE3TA and C-NE3TA appear to be superior chelators for 64Cu than p-NH2-Bn-DOTA, they are not better than p-NH2- Bn-NOTA. Nevertheless, it may still be interesting to evaluate these chelators after conjugation to biomolecules.
Keywords: bifunctional chelators; copper-64; serum stability, biodistribution
1. Introduction
64Cu (t1/2=12.7h, Iβ+=17%, Iβ-=39%, IEC=43%, Emax=0.656 MeV) has become an attractive radionuclide in the development of a wide range of radiopharmaceuticals for positron emission tomography (PET) and radiotherapy due its half-life, beta emission, and the ability to produce it on large scale with high specific activity [1,2]. The intermediate half-life of 64Cu makes it a good candidate for PET evaluation of peptide and protein interactions with their cellular targets [3,4]. The increased use of 64Cu in PET and targeted radiotherapy has highlighted the need for bifunctional chelators (BFCs) that form copper complexes with high stability. Chemically reactive functional groups in BFCs can be coupled to various bioactive molecules such as peptides, nanoparticles, and proteins.
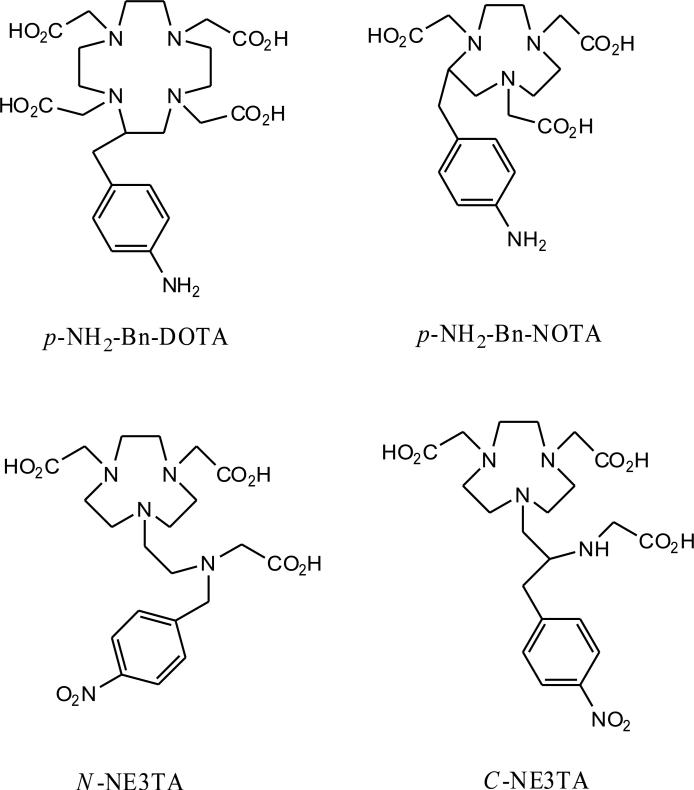
Earliest reported BFCs were derived from well established non-macrocyclic chelating agents e.g. ethylenediamine tetraacetic acid (EDTA). However, Cu complexes of nonmacrocyclic chelators such as EDTA and diethylenetriamine pentaacetic acid (DTPA) have been shown to be unstable in vivo although the complexes are thermodynamically stable [5,6]. In response to the kinetic instability of acyclic chelators, macrocyclic BFC derivatives of 1,4,7,10-tetra-azacylcododecane-N,N′,N″,N‴-tetraacetic acid (DOTA) and 1,4,8,11-tetraazacyclotetra decane-N,N′,N″,N‴-tetraacetic acid (TETA) derivatives have been developed for complexing with 64Cu. These macrocyclic Cu(II) complexes are kinetically more inert than Cu(II)-EDTA and Cu(II)-DTPA. However, they still have issues with regards to in vivo stability and slow complex formation rates [7] which can result in high uptake of activity by nontarget tissues such as the liver [8,9]. Cross-bridged cyclams offer improved kinetic stability to prevent in vivo transchelation reactions compared to DOTA and TETA [7,10,11]. However, ligands of this type require harsh conditions such as high temperatures to incorporate 64Cu. Thus, labeling antibodies and certain peptides is unfavorable with cross-bridged cyclams. Previous studies have shown the potential use of 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) as a BFC for labeling Cu-64 [12,13]. NOTA forms a 6-coordinate distorted prismatic complex with Cu(II) by coordinating the lone pairs of the three nitrogen atoms and the three carboxylate groups of the chelator [14]. The previous work by Kukis et al. with 67Cu showed less than 1% loss of the radioactivity per day in human serum from a functionalized NOTA wherein one carboxylate group was functionalized for conjugation to a biomolecule [12]. Further, Prasanphanich et al. showed that a peptide conjugated to NOTA [15] displayed considerably lower accumulation of radioactivity in the liver and kidney compared to the 64Cu-DOTA-labeled peptide [8,16,17]. Therefore, the evaluation of novel triazacyclononane-based BFCs that can form stable 64Cu-complexes is warranted. Chong et al. have synthesized several triazacyclononane-derived BFCs and evaluated them in vitro and in vivo as the chelators of 153Gd, 90Y, or 177Lu [18-27]. These studies showed high in vitro serum stability and good in vivo biodistribution of the radiolabeled complexes [26-28]. 2,2’-(7-((carboxymethyl)amino)ethyl)-1,4,7-triazonanane-1,4-diyl)diacetic acid (NE3TA) was previously radiolabeled with 64Cu and the complex showed high stability in rat serum.[24] The bifunctional versions of NE3TA, (4-carboxymethyl-7-[2-(carboxymethyl-amino)-3-(4-nitro-phenyl)-propyl]-[1,4,7]triazonan-1-yl-acetic acid) (C-NE3TA) and (4-carboxymethyl-7-[2-[carboxymethyl-(4-nitro-benzyl)-amino]-ethyl]-[1,4,7]triazonan-1-yl-acetic acid) (N-NE3TA), were synthesized (Figure 1) [23,27]. C-NE3TA and N-NE3TA differ in that the 4-nitro-phenyl group that will be used for conjugation to proteins is attached either to the nitrogen (N-NE3TA) or a carbon (C-NE3TA) of the amino ethyl side chain. This small change was made to determine how it would affect the complexation with copper. In this work, we demonstrate the efficient and high specific activity radiolabeling of C-NE3TA and N-NE3TA with 64Cu at room temperature. The complex kinetics of C-NE3TA and N-NE3TA with Cu(II) were determined and the 64Cu radiolabeled C-NE3TA and N-NE3TA were evaluated for in vitro stability and compared to the known Cu(II) chelators, 2-(4-aminobenzyl)-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (p-NH2-Bn-DOTA) and S-2-(4-aminobenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid (p-NH2-Bn-NOTA). Biodistribution studies were also performed in normal SCID mice to evaluate the tissue uptake and clearance of the 64Curadiolabeled NE3TA analogues and compare results to that of 64Cu-DOTA and 64Cu-NOTA BFCs.
Figure 1.
Structure of bifunctional chelators (BFCs)
2. Material and methods
2.1 Reagents and Analyses
Reaction vials were acid washed and all solvents and reagents were used as purchased without further purification. Milli-Q water was chelexed before use. P-NH2-Bn-NOTA and p-NH2-Bn-DOTA were purchased from Macrocyclics, Inc. (Dallas, TX). Waters C18 silica gel TLC plates (KC18F, 60 Å) were purchased from Fisher Scientific (Pittsburgh, PA). Radio-TLCs were developed with 10% NH4Oac:MeOH (3:7) and analyzed using a Bioscan 200 imaging scanner (Bioscan, Inc., Washington, DC). Copper-64 was prepared on CS-15 cyclotron at Washington University Medical School, St. Louis, MO according to the previous reported method [2]. Radioactivity was counted with a Beckman Gamma 8000 counter containing a NaI crystal (Beckman Instruments, Inc., Irvine, CA). 64CuCl2 was diluted with a 10-fold excess of 0.1 M ammonium acetate (NH4Oac), pH ~5.5 for radiolabeling. High-performance liquid chromatography (HPLC) purification (radiosynthetic and nonradiosynthetic) was performed by Phenomenex C-18 Luna 5 μm, 10 × 250 mm2 analytical column with a mobile phase of water (0.1% TFA) (solvent A) and acetonitrile (0.1% TFA) (solvent B). A linear gradient of 100% solvent A to 100% solvent B over 40 min at 2 mL/min was used. HPLC purification was performed in Agilent Technologies 1200 series HPLC equipped with a diode array detector. Radioactivity signals were detected by Beckman 8000 automated well- type gamma counter (Fullerton, CA) and UV absorbencies were monitored at 220 nm and 254 nm. All absorbance measurements for complexation formation kinetics were obtained on an Agilent 8453 diode array spectrophotometer equipped with a 8-cell transport system (designed for 1 cm cells).
2.2 64Cu radiolabeling to determine the maximum specific activity
The maximum specific activities were determined experimentally via titrating 64CuCl2 in 0.1 M NH4Oac (pH ~ 5.5) with the new BFCs, N-NE3TA and C-NE3TA. Briefly, for each chelator, six reaction vials were prepared in 0.1 M NH4Oac (pH ~ 5.5) via dilution to give final BFC masses in the range 0.002 to 0.1 μg. 3.7 MBq (100 μCi) of 64Cu in 0.1 M NH4Oac (pH ~ 5.5) was added to each vial and adjusted the final volume to 100 μL (final pH ~ 5.5) and vortexed for 10-15 seconds. The reactions were incubated on a rotator at 37 °C for 1 h. After incubation, 1 μL aliquots were withdrawn from reaction vials and analyzed by TLC (C-18) with a mixture of 10% NH4Oac:MeOH (3:7) as a mobile phase for labeling percentage. All reactions were done in triplicate. The data were plotted as % labeling vs. amount of BFC reacted and the amount of mass required to achieve 50% labeling was then determined. This mass was then multiplied by 2 to obtain the minimal mass for 100% labeling and the maximum specific activity. For HPLC analysis, the BFCs were reacted with excess cold CuCl2 under the same reaction conditions as mentioned above.
2.3 Complexation kinetics
The complexation kinetics of N-NE3TA and C-NE3TA with Cu(II) was investigated using a spectrophotometric method that employs a competing reaction with Arsenazo III (AAIII). AAIII is known to form weak complexes with Cu(II) and the complex absorbs at 652 nm. However, uncomplexed AAIII absorbs little at this wavelength. The kinetic studies were carried out as described previously [26,29]. Briefly, a stock solution of the Cu(II)-AAIII reagent was prepared in 0.15 M NH4Oac, pH 5.0 (metal free) by adding an aliquot of copper atomic absorption solution (45.2 μL, 1.55 x 10-2 M) into a 14 μM solution of AAIII (100 mL) to afford a 7 μM solution of Cu(II). The resulting Cu(II)-AAIII mixture was protected from the light to avoid degradation over time and stirred for 2 h for complete equilibration. A multicell transport of the UV/visible spectrometer was zeroed against a cell filled with 2 mL of AAIII solution. The solution in each sample cell was removed and filled with 2 mL of Cu(II)-AAIII solution. 15 μL of Cu(II)-AAIII solution was then removed from each sample cell, and each ligand (1 mM, 15 μL) was added into their respective sample cells. Kinetic spectra were collected every 30 sec at 610 nm for 1 h.
2.4 Determination of pH for 64Cu labeling
Carrier free 64Cu (3.7MBq) was added to N-NE3TA or C-NE3TA (0.05 μg) in different buffer solutions. Buffers consisted of 10 mM Glycine (pH 2, 3, 8, or 9) or 10 mM NaOAc (pH 4, 5, 6, or 7). The final reaction volumes were adjusted to 100 μL and incubated on a rotator for 1 h at 37°C. All reactions were performed in triplicate and labeling efficiency was determined by TLC with 10% NH4Oac:MeOH (3:7) as a mobile phase.
2.5 Serum Stability
The stability of the radiolabeled BFCs was analyzed in vitro in rat serum (Sigma Aldrich) according to the following procedure. 10 μL of 64Cu-labeled chelator (~ 3.7 MBq) was added to 90 μL of rat serum and incubated at 37 °C. Aliquots were removed at different time points (1, 2, 4, 24 and 48 h) and analyzed by TLC as described above and the percentage of bound 64Cu was determined.
2.6 Biodistribution Studies
All animal studies were performed in compliance with the Guidelines for Care and Use of Research Animals established by Washington University's Animal Studies Committee. Biodistribution studies were carried out in female SCID mice. The tissue uptake of the new ligands was compared to that of p-NH2-Bn-DOTA and p-NH2-Bn-NOTA. Mice (n = 4/group) were injected via tail vain with 64Cu-labeled BFCs (0.74 MBq in 100 μL of saline per animal). At 1, 4 and 24 h post-injection, mice were anesthetized with 1-2% isoflurane and sacrificed by cervical dislocation. Subsequently, the tissues of interest were harvested, weighed, and measured in a gamma counter. Samples were corrected for radioactive decay to calculate percent injected-dose per gram (%ID/g) of tissue.
3. Results and discussion
2.1 64Cu radiolabeling
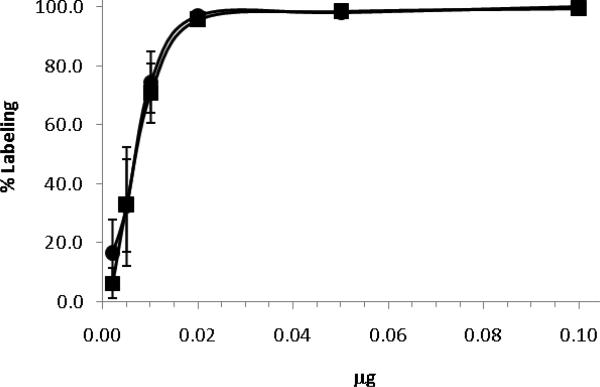
The BFCs were labeled with 64Cu in 0.1 M NH4Oac buffer (pH 5.5). The high labeling efficiency (> 95%) was achieved in 1 h for both N-NE3TA and C-NE3TA. These results show that relatively fast and efficient radiolabeling can be achieved for the new BFCs under mild conditions. The specific activity was determined by titrating BFCs with 64Cu (Figure 2). The maximum specific activity was ~3.4 Ci/μmol for both N-NE3TA and C-NE3TA. Thus, the position and presence of the p-NO2-Bn group in the BFCs did not disturb 64Cu coordination of the parent NE3TA chelating backbone.
Figure 2.
Percent labeling of C-NE3TA (squares) and N-NE3TA (circles) using a fixed amount of 64Cu and various amounts of BFCs.
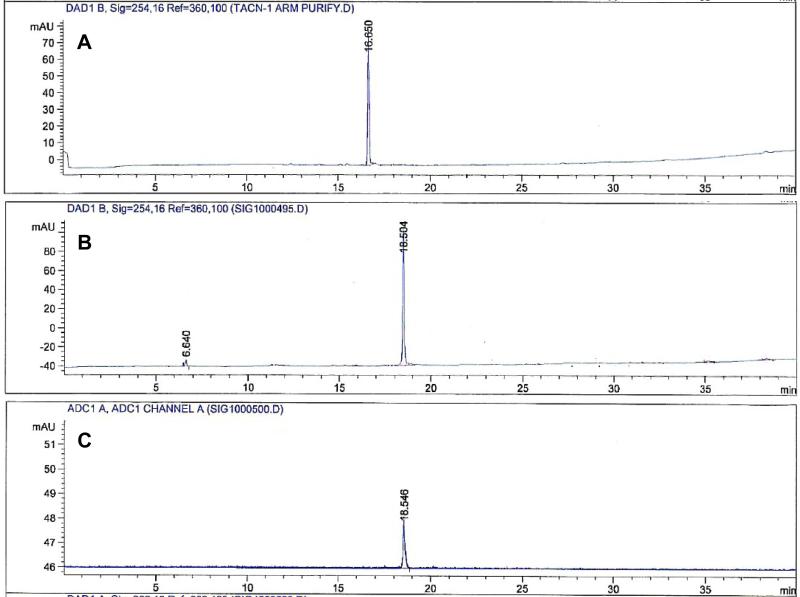
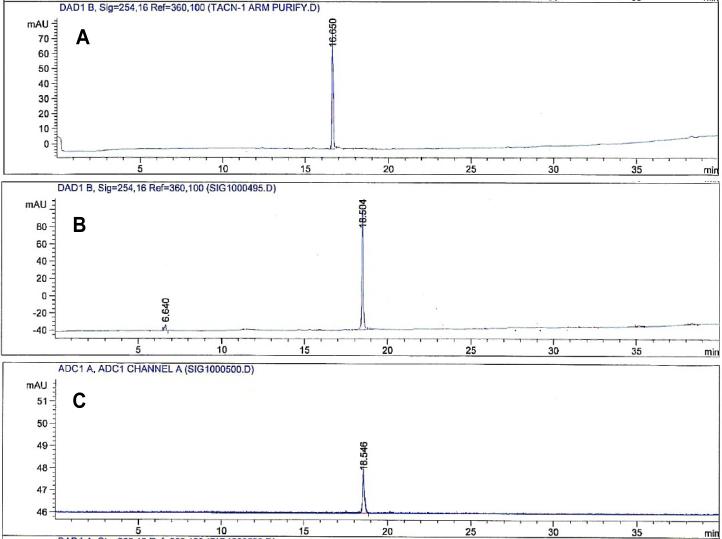
Non-radioactive Cu(II) complexes of the BFCs were synthesized as standards to identify 64Cu labeled BFCs and HPLC retention times of non-radioactive Cu complexes of N-NE3TA and C-NE3TA were compared with corresponding radioactive BFCs (Figures 3 and 4). The non-radioactive Cu(II) complexes of N-NE3TA and C-NE3TA eluted slightly earlier than their radiolabeled counterparts since the non-radioactive complexes are detected first by the UV lamp followed by the radioactive detector. The extra carboxylic acid group has increased the polarity of C-NE3TA and detected slightly earlier than N-NE3TA.
Figure 3.
HPLC chromatograms of N-NE3TA (A), Cu(II)-N-NE3TA (B), and 64Cu-N-NE3TA (C). Both A and B are UV traces, while C is the radioactive trace.
Figure 4.
HPLC chromatograms of C-NE3TA (A), Cu(II)-C-NE3TA (B), and 64Cu-C-NE3TA (C). Both A and B are UV traces, while C is the radioactive trace.
A wide range of pHs were tested to evaluate the robustness of labeling our BFCs. Both chelators gave high radiochemical yields (> 97%) over the pH range tested. A slight decrease in the percent labeling (87-89%) was observed at pH 2. The observed results might be due to protonation of electron donor nitrogen and oxygen atoms at very low pH.
2.2 Complexation Kinetics
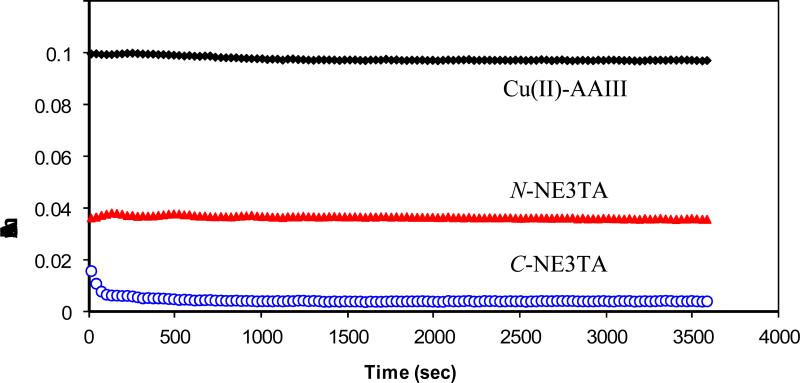
The results of the complexation kinetics at pH 5.0 are shown in Figure 5. The complexation kinetics of the ligands with Cu(II) was determined using a spectroscopic competing reaction with Arsenazo III (AAIII). The absorbance (A610) for the Cu(II)–AAIII complex was measured in the absence and in the presence of the ligands over 1 h at room temperature. The data in Figure 5 indicate that both C-NE3TA and N-NE3TA rapidly formed complexes with Cu(II), while the complexation with C-NE3TA was faster than that observed with N-NE3TA.
Figure 5.
Complexation kinetics of N-NE3TA (1 mM) and C-NE3TA (1 mM) with Cu(II) (7 μM) using the AAIII(14 μM) based assay at room temperature in 0.15 M NH4Oac, pH 5.0. The data is plotted as the absorbance of Cu(II)AAIII alone or after mixing with N-NE3TA or CNE3TA over time.
2.3 Serum Stability
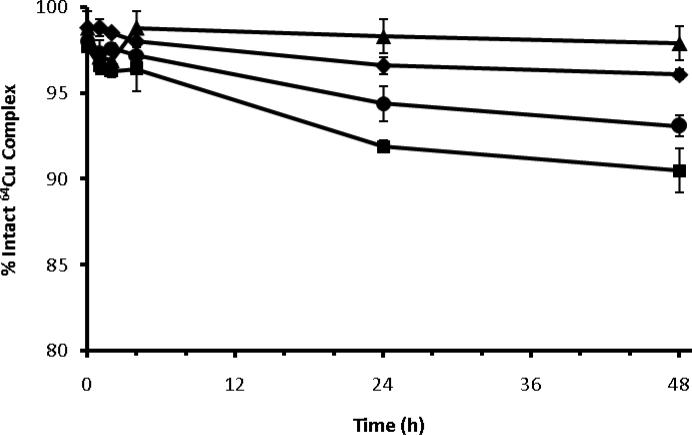
In vitro serum stability of 64Cu-N-NE3TA and 64Cu-C-NE3TA was analyzed and compared with commercially available BFCs p-NH2-Bn-NOTA and p-NH2-Bn-DOTA at different time points (Figure 6). All four BFCs showed high stability in rat serum at 37 °C at 4 h. At 24 h, 91.9 ± 0.4% of 64Cu-C-NE3TA remained intact and only dropped to 90.5 ± 1.3% at 48 h. Comparatively, 96.6 ± 0.5% of 64Cu-N-NE3TA remained intact at 24 h, which was greater than 64Cu-p-NH2-Bn-DOTA (94.4 ± 1.0%), but not as high as 64Cu-p-NH2-Bn-NOTA (98.3 ± 0.3%). A similar trend comparing the BFCs was observed at 48 h. These data would predict stability of the complexes as 64Cu-p-NH2-Bn-NOTA > 64Cu-N-NE3TA > 64Cu-p-NH2-Bn-DOTA > 64Cu-C-NE3TA.
Figure 6.
In vitro serum stability of 64Cu-N-NE3TA (diamonds), 64Cu-C-NE3TA (squares), 64Cu-p-NH2-Bn-DOTA (circles), and 64Cu-p-NH2-Bn-NOTA (triangles). The data is plotted as the percentage of intact copper complex as determined by TLC over time.
2.4 Biodistribution Studies
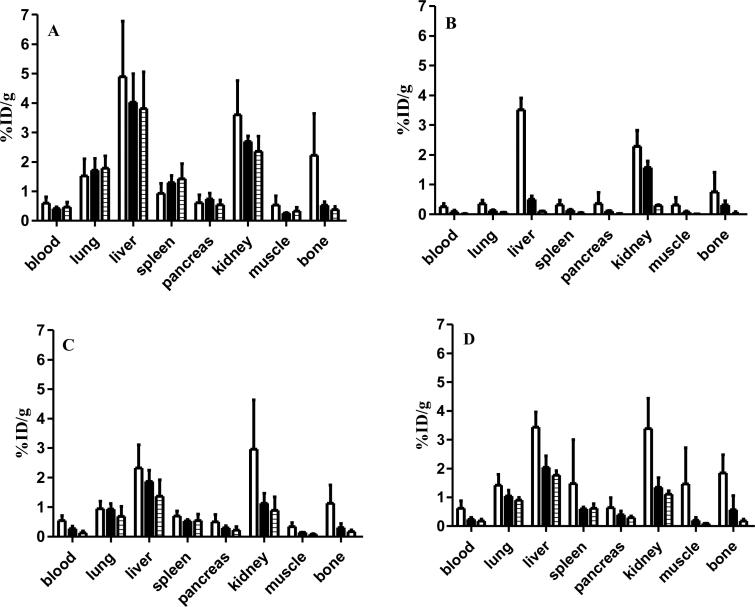
To investigate the organ uptake and clearance properties of the radiotracers, biodistribution studies were carried out with 64Cu-N-NE3TA and 64Cu-C-NE3TA and the results were compared with that of 64Cu-p-NH2-Bn-DOTA and 64Cu-p-NH2-Bn-NOTA. The results are summarized in Figure 7. Rapid clearance of activity was observed through the lung, spleen, pancreas, kidney, muscles and blood for both 64Cu-N-NE3TA and 64Cu-C-NE3TA. Radioactivity in kidney began to clear after 1 h and by 24 h the %ID/g decreased by 60% for both N-NE3TA and C-NE3TA. Compared to other tissues, the liver showed the highest activity at 24 h post-injection. At 24 h, the %ID/g in liver was 1.8 ± 0.2 and 1.4 ± 0.6 for 64Cu-C-NE3TA and 64Cu-N-NE3TA, respectively. This represents retention of radioactivity in the liver of 51% and 59% compared to 1 h for 64Cu-C-NE3TA and 64Cu-N-NE3TA, respectively. Compared to 64Cu-p-NH2-Bn-DOTA, 64Cu-N-NE3TA and 64Cu-C-NE3TA showed lower uptake in kidney and liver at all three time points. Here, the biodistribution data indicate more transchelation of 64Cu to liver proteins occurs with 64Cu-p-NH2-Bn-DOTA than 64Cu complexes of the new BFCs. The liver retention was 78% at 24 h when compared to 1 h for 64Cu-p-NH2-Bn-DOTA. As shown in Figure 7 (A, C and D), the amount of 64Cu in the blood at 1 h post-inection were approximately the same for all three chelators (C-NE3TA, N-NE3TA and p-NH2-Bn-DOTA). However, at 24 h post-injection, the radioactivity in blood for p-NH2-Bn-DOTA (%ID/g = 0.47 ± 0.17) was roughly threefold greater than that of C-NE3TA (%ID/g = 0.18 ± 0.06) and N-NE3TA (%ID/g = 0.13 ± 0.06). The same trend was observed for the radioactive uptake in the muscle for these chelators. Hence, the faster blood and muscle clearances of radioactivity for our new BFCs will increase target:blood and target:muscle ratios with time and thereby will increase the PET imaging quality compared to that of p-NH2-Bn-DOTA. After 24 h post-injection, our new BFCs chelators overall had lower activities in all tissues compared to that of 64Cu-p-NH2-Bn-DOTA. This is important for both PET imaging as well as therapy with 64Cu-BFC bimolecular conjugates. It is not clear why this differs from the serum stability studies in which 64Cu-CNE3TA appears to be less stable than 64Cu-p-NH2-Bn-DOTA. However, 64Cu-p-NH2-Bn-NOTA appears to be the most stable of all BFCs evaluated and showed the lowest %ID/g for all tissues at all time points (Figure 7). The liver retention of 64Cu-p-NH2-Bn-NOTA was only 3% at 24 h when compared to 1 h. This is in agreement with the serum stability data.
Figure 7.
Biodistribution of 64Cu-p-NH2-Bn-DOTA (A), 64Cu-p-NH2-Bn-NOTA (B), 64Cu-NNE3TA (C), and 64Cu-C-NE3TA (D) in female SCID mice. The animals (n = 4 per group) were injected with 64Cu-labeled chelators and were sacrificed 1 (white bars), 4 (black bars), and 24 (hatched bars) hours later. The blood, lung, liver, spleen, pancreas, kidney, muscle, and bone were harvested, weighed, and counted in the gamma counter. The percent injected dose per gram of tissue (%ID/g) was determined by decay correction of each sample normalized to weight. The data for each group represent the mean ± SD.
4. Conclusion
We have determined the optimal 64Cu radiolabeling conditions for both C-NE3TA and NNE3TA and demonstrated that they form complexes with Cu(II) in a rapid manner. Both 64Cu-N-NE3TA and 64Cu-C-NE3TA were > 90% intact in serum for 48 h. Biodistribution studies showed more favorable in vivo clearance of the 64Cu-labeled N-NE3TA and C-NE3TA relative to 64Cu-p-NH2-Bn-DOTA. However, 64Cu-p-NH2-Bn-NOTA cleared the best from tissues and is superior to the other chelators investigated. Nevertheless, investigation of the new bifunctional chelators, C-NE3TA and N-NE3TA after conjugation to biomolecules and radiolabeling with 64Cu may be warranted.
Acknowledgements
This work was supported by the Department of Radiation Oncology, Washington University School of Medicine and NIH grant R01 CA136695. Drs. De Silva and Jain were supported by a DOE Integrated Research Training Program of Excellence in Radiochemistry (DE-SC0002032). Dr. Nilantha Bandara is thanked for his assistance in assembling of the manuscript.
Financial Support: Supported by the Department of Radiation Oncology, Washington University School of Medicine, NIH grant RO1 CA136695 and a DOE Integrated Radiochemistry Research Project of Excellence Grant (DE-SC00002032).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson C, Welch MJ. Radiometal-labeled agents (non-technetium) for diagnostic imaging. Chem Rev. 1999;99:2219–2234. doi: 10.1021/cr980451q. [DOI] [PubMed] [Google Scholar]
- 2.McCarthy DW, Shefer RE, Klinkowstein RE, Bass LA, Margeneau WH, Cutler CS, et al. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl Med Biol. 1997;24:35–43. doi: 10.1016/s0969-8051(96)00157-6. [DOI] [PubMed] [Google Scholar]
- 3.Bryan JN, Jia F, Mohsin H, Sivaguru G, Miller WH, Anderson CJ, et al. Comparative uptakes and biodistributions of internalizing vs. noninternalizing copper-64 radioimmunoconjugates in cell and animal models of colon cancer. Nucl Med Biol. 2005;32:851–858. doi: 10.1016/j.nucmedbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Li M, Meares CF. Synthesis, metal chelate stability studies, and enzyme digestion of a peptide-linked DOTA derivative and its corresponding radiolabeled immunoconjugates. Bioconjug Chem. 1993;4:275–283. doi: 10.1021/bc00022a005. [DOI] [PubMed] [Google Scholar]
- 5.Cole WC, DeNardo SJ, Meares CF, McCall MJ, DeNardo GL, Epstein AL, et al. Serum stability of 67Cu chelates: comparison with 111In and 57Co. Int J Rad Appl Instrum B. 1986;13:363–368. doi: 10.1016/0883-2897(86)90011-5. [DOI] [PubMed] [Google Scholar]
- 6.Moi MK, Meares CF, McCall MJ, Cole WC, DeNardo SJ. Copper chelates as probes of biological systems: stable copper complexes with a macrocyclic bifunctional chelating agent. Anal Biochem. 1985;148:249–253. doi: 10.1016/0003-2697(85)90653-0. [DOI] [PubMed] [Google Scholar]
- 7.Boswell CA, Sun X, Niu W, Weisman GR, Wong EH, Rheingold AL, et al. Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J Med Chem. 2004;47:1465–1474. doi: 10.1021/jm030383m. [DOI] [PubMed] [Google Scholar]
- 8.Rogers BE, Bigott HM, McCarthy DW, Della Manna D, Kim J, Sharp TL, et al. MicroPET imaging of a gastrin-releasing peptide receptor-positive tumor in a mouse model of human prostate cancer using a 64Cu-labeled bombesin analog. Bioconjug Chem. 2003;14:756–763. doi: 10.1021/bc034018l. [DOI] [PubMed] [Google Scholar]
- 9.Rogers BE, Della Manna D, Safavy A. In vitro and in vivo evaluation of a 64Cu-labeled polyethylene glycol bombesin conjugate. Cancer Biother Radiopharm. 2004;19:25–34. doi: 10.1089/108497804773391649. [DOI] [PubMed] [Google Scholar]
- 10.Sprague JE, Peng Y, Sun X, Weisman GR, Wong EH, Achilefu S, et al. Preparation and biological evaluation of copper-64-labeled Tyr3-octreotate using a cross-bridged macrocyclic chelator. Clin Cancer Res. 2004;10:8674–8682. doi: 10.1158/1078-0432.CCR-04-1084. [DOI] [PubMed] [Google Scholar]
- 11.Sun X, Wuest M, Weisman GR, Wong EH, Reed DP, Boswell CA, et al. Radiolabeling and in vivo behavior of copper-64-labeled cross-bridged cyclam ligands. J Med Chem. 2002;45:469–477. doi: 10.1021/jm0103817. [DOI] [PubMed] [Google Scholar]
- 12.Kukis DL, Diril H, Greiner DP, DeNardo SJ, DeNardo GL, Salako QA, et al. A comparative study of copper-67 radiolabeling and kinetic stabilities of antibody-macrocycle chelate conjugates. Cancer (Suppl) 1994;73:779–786. doi: 10.1002/1097-0142(19940201)73:3+<779::aid-cncr2820731306>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Kukis DL, Li M, Meares CF. Selectivity of antibody-chelate conjugates for binding copper in the presence of competing metals. Inorg Chem. 1993;32:3981–3982. [Google Scholar]
- 14.Vab der Merwe MJ, Boeyens JCA, Hancock RD. Optimum ligand hole sizes for stabilizing nickel(III). Structure of the nickel(III) complex of 1,4,7-triazacyclononane-N,N′,N″-triacetate. Inorg Chem. 1983;22:3489–3490. [Google Scholar]
- 15.Prasanphanich AF, Nanda PK, Rold TL, Ma L, Lewis MR, Garrison JC, et al. [64Cu-NOTA-8-Aoc-BBN(7-14)NH2] targeting vector for positron-emission tomography imaging of gastrin-releasing peptide receptor-expressing tissues. Proc Natl Acad Sci USA. 2007;104:12462–12467. doi: 10.1073/pnas.0705347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Park R, Hou Y, Tohme M, Shahinian AH, Bading JR, et al. MicroPET and autoradiographic imaging of GRP receptor expression with 64Cu-DOTA-[Lys3]bombesin in human prostate adenocarcinoma xenografts. J Nucl Med. 2004;45:1390–1397. [PubMed] [Google Scholar]
- 17.Yang YS, Zhang X, Xiong Z, Chen X. Comparative in vitro and in vivo evaluation of two 64Cu-labeled bombesin analogs in a mouse model of human prostate adenocarcinoma. Nucl Med Biol. 2006;33:371–380. doi: 10.1016/j.nucmedbio.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Chong HS, Brechbiel MW. A short and efficient synthesis of mono-substituted 1,4,7-triazacyclononanes. Synth Comm. 2003;33:1147–1154. [Google Scholar]
- 19.Chong HS, Garmestani K, Bryant LH, Jr., Milenic DE, Overstreet T, Birch N, et al. Synthesis and evaluation of novel macrocyclic and acyclic ligands as contrast enhancement agents for magnetic resonance imaging. J Med Chem. 2006;49:2055–2062. doi: 10.1021/jm051009k. [DOI] [PubMed] [Google Scholar]
- 20.Chong HS, Garmestani K, Ma D, Milenic DE, Overstreet T, Brechbiel MW. Synthesis and biological evaluation of novel macrocyclic ligands with pendent donor groups as potential yttrium chelators for radioimmunotherapy with improved complex formation kinetics. J Med Chem. 2002;45:3458–3464. doi: 10.1021/jm0200759. [DOI] [PubMed] [Google Scholar]
- 21.Chong HS, Lim S, Baidoo KE, Milenic DE, Ma X, Jia F, et al. Synthesis and biological evaluation of a novel decadentate ligand DEPA. Bioorg Med Chem Lett. 2008;18:5792–5795. doi: 10.1016/j.bmcl.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chong HS, Ma X, Le T, Kwamena B, Milenic DE, Brady ED, et al. Rational design and generation of a bimodal bifunctional ligand for antibody-targeted radiation cancer therapy. J Med Chem. 2008;51:118–125. doi: 10.1021/jm070401q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chong HS, Ma X, Lee HS, Bui P, Song HA, Birch N. Synthesis and evaluation of novel polyaminocarboxylate-based antitumor agents. J Med Chem. 2008;51:2208–2215. doi: 10.1021/jm701307j. [DOI] [PubMed] [Google Scholar]
- 24.Chong HS, Mhaske S, Lin M, Bhuniya S, Song HA, Brechbiel MW, et al. Novel synthetic ligands for targeted PET imaging and radiotherapy of copper. Bioorg Med Chem Lett. 2007;17:6107–6110. doi: 10.1016/j.bmcl.2007.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong HS, Milenic DE, Garmestani K, Brady ED, Arora H, Pfiester C, et al. In vitro and in vivo evaluation of novel ligands for radioimmunotherapy. Nucl Med Biol. 2006;33:459–467. doi: 10.1016/j.nucmedbio.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Chong HS, Song HA, Birch N, Le T, Lim S, Ma X. Efficient synthesis and evaluation of bimodal ligand NETA. Bioorg Med Chem Lett. 2008;18:3436–3439. doi: 10.1016/j.bmcl.2008.03.084. [DOI] [PubMed] [Google Scholar]
- 27.Chong HS, Song HA, Ma X, Milenic DE, Brady ED, Lim S, et al. Novel bimodal bifunctional ligands for radioimmunotherapy and targeted MRI. Bioconjug Chem. 2008;19:1439–1447. doi: 10.1021/bc800050x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chong HS, Song HA, Lim S, Macrenaris K, Ma X, Lee H, et al. A novel cholic acid-based contrast enhancement agent for targeted MRI. Bioorg Med Chem Lett. 2008;18:2505–2508. doi: 10.1016/j.bmcl.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 29.Brady ED, Chong HS, Milenic DE, Brechbiel MW. Development of a spectroscopic assay for bifunctional ligand-protein conjugates based on copper. Nucl Med Biol. 2004;31:795–802. doi: 10.1016/j.nucmedbio.2004.04.004. [DOI] [PubMed] [Google Scholar]