Abstract
Over the past two decades, key advancements have been made in understanding the complex pathology that occurs following not only high levels of arsenic exposure (>1ppm) but also levels previously considered to be low (<100 ppb). Past studies have characterized the deleterious effects of arsenic on the various functions of cardiovascular, pulmonary, immunological, respiratory, endocrine and neurological systems. Other research has demonstrated an elevated risk of a multitude of cancers and increased rates of psychopathology, even at very low levels of arsenic exposure. The hypothalamic-pituitary-adrenal (HPA) axis represents a multisite integration center that regulates a wide scope of biological and physiological processes: breakdown within this system can generate an array of far-reaching effects, making it an intriguing candidate for arsenic-mediated damage. Using a mouse model, we examined the effects of perinatal exposure to 50 ppb sodium arsenate on the functioning of the HPA axis through the assessment of corticotrophin-releasing factor (CRF), proopiomelanocortin (Pomc) mRNA, adrenocorticotrophin hormone (ACTH), corticosterone (CORT), 11β-Hydroxysteroid Dehydrogenase Type 1 (11β-HSD 1), and glucocorticoid receptor (GR) protein and mRNA. Compared to controls, we observed that the perinatal arsenic-exposed offspring exhibit an increase in hypothalamic CRF, altered CORT secretion both at baseline and in response to a stressor, decreased hippocampal 11β-HSD 1 and altered subcellular GR distribution in the hypothalamus. These data indicate significant HPA axis impairment at post-natal day 35 resulting from perinatal exposure to 50 ppb sodium arsenate. Our findings suggest that the dysregulation of this critical regulatory axis could underlie important molecular and cognitive pathology observed following exposure to arsenic.
Keywords: Arsenic (As), Hypothalamic-Pituitary-Adrenal (HPA) axis, Glucocorticoid Receptor (GR), Neuroendocrine, corticotrophin-releasing factor (CRF), adrenocorticotrophin hormone (ACTH), corticosterone
1. Introduction
While the deleterious health effects induced by arsenic have widely been recognized, exposure remains nearly unavoidable due to the ubiquitous presence of arsenic in the environment. Several reports demonstrate that exposure to arsenic results in damage to a variety of biological systems including the cardiovascular, pulmonary, immunological, respiratory, neurological, and endocrine systems. Arsenic exposure is also implicated in an increased risk of bladder, kidney, lung, skin, liver, and prostate cancers (ATSDR 1999; U.S. EPA 2001). Although the effects associated with arsenic exposure are many, of particular interest are those acting on the central nervous system, as they can elicit an abundance of biochemical alterations that manifest in emotional, behavioral, and cognitive abnormalities. The body of literature investigating the neurological insults of arsenic has broadened considerably over the past few decades; however, many of the underlying neurotoxicological mechanisms still remain unclear.
Although arsenic has been shown to exert a myriad of detrimental effects, one area of focus that merits further investigation is the hypothalamic-pituitary-adrenal (HPA) axis. The HPA axis regulates neuroendocrine and behavioral responses and its proper functioning is critical for normal physiology and cognition (Young, 2004 for review). Perturbations of this axis are often observed in neuropsychiatric illness and can be accompanied by cognitive deficits (Bremner et al., 2004; Knapman et al., 2010). Recent advances in the literature indicate that arsenic interferes with the functioning of key components of the HPA axis. Corticotrophin-releasing factor (CRF) marks a starting point of the HPA axis. Release of CRF is in part regulated by the catecholamine norepinephrine (NE), (Conti and Foote, 1995), levels of which are modified by arsenic in the hypothalamus of adult mice (Mejía et al., 1997). Arsenic has also been linked to increases in plasma adrenocorticotrophin hormone (ACTH) in mice exposed to 20 ppm arsenite (Delgado et al., 2000), and at distinct micromolar concentrations, arsenic has both stimulatory and inhibitory effects on glucocorticoid receptor (GR) mediated gene activation in rat EDR3 hepatoma cells (Bodwell et al., 2004). Furthermore, Jana and colleagues (2006) found that treatment with 5 mg sodium arsenite/kg body weight per day increased plasma corticosterone (CORT) in adult male rats and produced changes in dopamine, NE, and 5-HT in both the hypothalamus and pituitary. These findings support the assertion that arsenic alters components of the stress axis, yet whether the damage induced by arsenic is primary or secondary in nature and where within the axis the damage occurs is unknown.
Much emphasis in the literature has been placed on studies that use moderate (ca.100 ppb) or even high (>1 ppm) levels of arsenic; the potential effects generated by lower doses on the developing HPA axis have received little attention. Previous work in our laboratory using perinatal exposure to 50 ppb sodium arsenate suggests abnormalities in the HPA axis as a result of this exposure (Martinez et al., 2008; Martinez-Finley et al., 2009). Notably, this exposure paradigm results in decreases in the hippocampal mineralocorticoid receptor (MR) and glucocorticoid receptors (GR) (Martinez-Finley et al., 2009) and an increase in baseline plasma corticosterone (Martinez et al., 2008) at post-natal day (PND) 35, thereby suggesting HPA axis dysregulation. Additionally, behavioral endpoints including cognitive deficits and depressive-like behaviors identified in our exposure model (Martinez et al., 2008) have also been linked to breakdown in HPA axis function (Young, 2004, for review). Arsenic readily crosses the placental barrier after maternal exposure (Hood et al., 1987; Hood et al., 1988) and therefore poses a neurotoxic threat to the developing fetus. Maternal factors play a critical role in the development of the fetal HPA axis. Our perinatal arsenic exposure model coincides with development of the HPA axis (embryonic day 10.5 to birth) when function is established and fine-tuned; thus, axis development in particular may be vulnerable to insult (Reichardt and Schütz, 1996). Because HPA function can be programmed during development (Levine, 1994), exposure to toxins during this critical period could have severe and enduring effects that may persist long after the initial exposure has been discontinued.
Expanding on our prior observations, the present work describes a systematic evaluation of components of the HPA axis to determine if perinatal exposure to 50 ppb sodium arsenate possesses the neurotoxic potential to disrupt the functioning of this critical regulatory system. Here, we present evidence of arsenic-induced HPA axis dysregulation through the assessment of CRF, ACTH, 11β-Hydroxysteroid Dehydrogenase Type 1 (11β-HSD 1), proopiomelanocortin (Pomc) mRNA, GR compartmentalization in the hypothalamus, and GR mRNA in the hippocampus and hypothalamus. Furthermore, we confirm that the changes we observe in components of the HPA axis translate into abnormalities in HPA axis response, as measured by secretion of CORT following exposure to a stressor. Our findings indicate that a perinatal exposure to 50 ppb arsenic alters the functioning of the HPA axis and we speculate that these changes are exacerbated by aberrant GR signaling within the hippocampus.
2. Materials and Methods
Note: All arsenicals were handled with extreme caution in accordance with MSDS recommendations
2.1 Perinatal arsenic exposure paradigm
The University of New Mexico Health Sciences Center Institutional Animal Care and Use Committee has approved the arsenic-exposure paradigm used in these studies. C57BL/6 mice were maintained on a reverse 12-hour light/dark cycle (lights off from 0800 to 2000 h) in the Animal Resource Facility with food and water provided ad libitum. Fifty ppb arsenic water was prepared using sodium arsenate (Sigma, St. Louis, MO; #A-6756) and Milli-Q water from a Milli-Q Plus purification system (Millipore, Billerica, MA; # ZD5211584). Because arsenate is the predominant form in water sources, we chose this over the more toxic yet less common form, arsenite (U.S. EPA 2000; Welch et al., 1988). Water administered to control animals comprised of 50% Milli-Q water and 50% UNM tap water; all water was replaced weekly. Though tap water at UNM is estimated to contain approximately 5 ppb arsenic, a degree of fluctuation exists such that Milli-Q water was used to address this possibility. Mice were harem bred and then transferred to separate housing. Dams were administered arsenic treated water two weeks prior to breeding and were maintained on the arsenic treatment throughout gestation until pups reached weaning around PND 21. At weaning, pups were housed with litter-mates and the arsenic exposure was discontinued for the pups. Mice were sacrificed by decapitation at PND 31-40, the adolescent time point, since many of the intellectual consequences of arsenic exposure have been described in children (Calderón et al., 2001; Rosado et al., 2007; Wasserman et al., 2004; Wright et al., 2006). Only samples from males were analyzed in these studies. Multiple breeding rounds were generated so as to achieve a sufficient number of different litters from different dams for the treatment or control groups. In each study, no less than five different litters from different dams are represented. Thus, an n=5 represents the number of different litters used.
2.2 Tissue and plasma collection
Animals were sacrificed by decapitation and the hypothalamus, hippocampus, and pituitary were rapidly dissected on ice. Tissue samples were snap frozen by submersion in liquid nitrogen and stored at −80°C until use. Blood was collected using Safe-T-Fill EDTA capillary tubes (Ram Scientific Inc., Yonkers, NY; #077051). Plasma was separated by centrifugation at 1,000 × g for 10 minutes at 4°C and stored at −80°C until use.
2.3 ACTH concentration in plasma
An ELISA kit (MD Bioproducts, St Paul, MN; #M046006) was utilized to assay the concentration of ACTH in plasma. To achieve the required volume of plasma to assay in duplicate, 4-6 samples were combined. The ELISA was run as per manufacturer’s instructions and absorbance was read using an Infinite M200 (Tecan Group Ltd., Männedorf, Switzerland) with the wavelength set at 405 nm. A dose response curve of concentration versus absorbance was generated from the calibrators and used to calculate the concentration for experimental samples by four-parameter logistic curve (4PLC) using ReaderFit online software (Hitachi Solutions America Ltd, San Francisco, CA, 2012).
2.4 Sample preparation for western blotting
To analyze the cellular compartmentalization of the GR, cellular fractionation was conducted as described by Buckley and Caldwell (2004). CRF and 11β-HSD 1 were analyzed in the cell lysate, which was obtained by centrifugation at 1,000 × g for 4 minutes at 4°C after which the supernatant was collected. Total protein was determined by Bradford assay as described by Weeber and colleagues (2001) and used to load a pre-determined amount into each well for western blotting.
2.5 GR protein levels in the hypothalamus
Both nuclear and cytosolic fractions were probed to assess the translocation of the GR in the hypothalamus. All western blotting procedures were performed according to our established protocols (Martinez-Finley et al., 2011) with the following changes implemented: a 7 μg aliquot of total protein was separated and probed with either rabbit anti-GR (1:3,000 Santa Cruz Biotechnology, Santa Cruz, CA; #sc-1004) and a mouse anti-β-Actin (1:2,000 Sigma; #A5441-.2ML) antibody to serve as a loading control. Values are expressed as GR normalized to β-Actin.
2.6 CRF in the hypothalamus
Protein levels of CRF from whole cell lysate were assessed in a similar manner to our established western blotting protocol (Martinez-Finley et al., 2011) with the following changes implemented: 30 μg total protein separated, transferred to PVDF (BioRad, Hercules, CA; #162-0177), and probed with rabbit-anti-CRF (1:1,000 Santa Cruz Biotechnology; #sc-10718) and a mouse anti-β-Actin.
2.7 11β-Hydroxysteroid Dehydrogenase Type 1 protein levels in the hippocampus
Cell lysate from hippocampal tissue was probed for 11β-HSD 1 using western blotting methodology described by Martinez-Finley and colleagues (2011). Forty μg total protein were separated. A rabbit anti-11β-HSD 1 antibody (1:1,000 Santa Cruz Biotechnology; #sc-20175) was used to detect the protein and a mouse anti-β-Actin was again used as a loading control. In the mouse, 11β-HSD 1 can undergo N-linked glycosylation at amino acids 162 and 207 which could result in the appearance of three distinct bands in western blotting (UniProt ID: P50172). To differentiate between glycosylation at one amino acid, both amino acids, and nonglycosylated forms of 11β-HSD 1, an experiment was conducted to confirm the molecular weights of each of these. PNGase F, an amidase that cleaves N-linked glycosylation, was used to treat samples and the reduction of bands at the approximate molecular weight of the glycosylated forms was observed. A de-glycosylation kit (New England BioLabs, Ipswich, MA; #P0704L) was used to perform this. Briefly, an 86 μl aliquot of sample was incubated with 4 μl of 10X glycoprotein denaturing buffer and heated for 10 minutes at 100°C. Six μl of 0.5 M sodium phosphate buffer, 6 μl of 10% NP-40 and a 4 μl aliquot of PNGase F were then added to the samples and incubated for one hour at 37°C. Samples were probed for 11β-HSD 1 and the identity of each of the three bands was verified by the reduction of a band at the molecular weight expected for each glycosylated form. Negative controls that did not receive the PNGase F retained bands indicative of the glycosylated forms at the anticipated molecular weight.
2.8 Preparation of mRNA and cDNA synthesis
A 10-15 mg aliquot of frozen tissue was homogenized by mortar and pestle over liquid nitrogen. The lysate was transferred to a QIAshredder™ homogenizer (Qiagen, Valencia, CA; #79654) for thorough lysis and RNA was extracted using an RNeasy® Plus Mini Kit (Qiagen; #74134) as described by the manufacturer. The mRNA concentration was determined (OD 260 nm) using a NanoDrop® ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). Isolated mRNA was stored at −80°C. Reverse transcription was performed using a QuantiTect Reverse Transcription Kit (Qiagen; #205311) and a PTC-200 Peltier Thermal Cycler (MJ Research, Taunton, MA).
2.9 Real-Time PCR
RT-PCR was performed using a QuantiFast SYBR Green PCR Kit (Qiagen; #204054) on an ABI 7300 Real-Time PCR System (Applied Biosystems). No template controls and reverse transcription controls were used and a dissociation curve was evaluated. Additionally, primer efficiencies were validated. Primers for the Nr3c1 and Pomc genes encoding GR and POMC, respectively, were designed based on reported sequences from PrimerBank (PrimerBank ID: 6680103a and 6679415a1, respectively) as discussed in Spandidos et al., 2008 and 2010 and Wang and Seed, 2003. Hprt was run as an endogenous control and two sets of primers were used: Hprt (a) was run with Nr3c1 and Hprt (b) with Pomc. Hprt (a) primers were designed based on sequences from Caldwell et al., 2008, and Hprt (b) from Gilsbach et al., 2006, as shown in Table 1. Results were assessed using the Comparative CT (ΔΔCT) method and are described as the change in fold expression.
Table 1.
Primers used to perform RT-PCR for GR mRNA (encoded by the Nr3c1 gene) and Pomc mRNA (encoded by the Pomc gene).
| Target | Forward Primer | Reverse Primer |
|---|---|---|
| Hprt (a) | AAGACTTGCTCGAGATGTCATGAA | ATCCAGCAGGTCAGCAAAGAA |
| Nr3c1 | AGCTCCCCCTGGTAGAGAC | GGTGAAGACGCAGAAACCTTG |
| Hprt (b) | TGACACTGGCAAAACAATGCA | GGTCCTTTTCACCAGCAAGCT |
| Pomc | ATGCCGAGATTCTGCTACAGT | TCCAGCGAGAGGTCGAGTTT |
2.10 Stress response
To examine the effects of arsenic exposure on the ability to mount a corticosteroid response to a perceived stressor, we conducted a predator scent exposure study and evaluated the concentration of CORT in plasma at 30 and 60 minutes post-stressor in control and arsenic PND 35 mice. Briefly, animals were transferred to individual housing in a holding room and allowed to habituate to the environment for one hour before beginning the exposure at 10:00 AM. Mice were maintained in the holding room until their time of use to prevent pre-exposure to the stressor. Animals were assigned to either a 30 minute or 60 minute group. A baseline plasma sample was collected for each animal from the submandibular vein using a 25 gauge needle (Golde et al., 2005) and Safe-T-Fill EDTA capillary tubes. Immediately following the baseline collection, animals were placed into an exposure cage containing a 1.5 × 3 cm section of filter paper saturated with 3% (v/v) 2,3,5-trimethyl-3-thiazoline (TMT) (Contech, Victoria, BC, Canada; #300000368), the chemical present in fox urine. After 10 minutes, animals were removed from the exposure cage and returned to individual housing where they remained until they were sacrificed by decapitation at 30 or 60 minutes post stressor-onset. Trunk blood was collected using the Safe-T-Fill EDTA capillary tubes described earlier and stored on ice until the plasma extraction was performed. The concentration of CORT in plasma was measured using the DetectX Corticosterone EIA Kit (Arbor Assays, Ann Arbor, MI; #K014-H1) per the manufacturer’s instructions. OD readings were taken on an Infinite M200 and data was analyzed by 4PLC using ReaderFit online software. This study was replicated on two different cohorts of mice several months apart.
2.11 Buried food pellet test
Because the stress response test (see above) is dependent upon an intact olfactory system, we conducted a buried food pellet (BFP) test to verify that arsenic-exposed offspring and control mice have similar olfactory abilities as measured by the ability to detect and locate a food reward. The BFP test was conducted as described by Nathan and colleagues (2004) with the modifications described by Allan and colleagues (2008). Briefly, mice were placed on a food-restricted diet for two days prior to the testing period and continued on the restricted diet for the duration of the four day testing period. A pellet of standard chow was placed in a randomized location inside a clean cage and covered with clean bedding. The amount of time between when the mouse was placed into the cage and when the food pellet was retrieved was measured. Mice were returned to their home cage after each testing trial. The mice used in this behavioral study were not reused in any of the other experiments.
2.12 Statistical analysis
Western blotting data were normalized to the average control value of each blot to correct for variations in OD intensity between different blots. For the quantification of 11β-HSD 1 and its glycosylated forms, the data were analyzed using a one-way ANOVA (SPSS, v.19; Chicago, IL). All other data were analyzed by t-test using GraphPad Software (GraphPad Software, v.4.03; San Diego, CA) with the following specifics: For the stressor response data, each CORT time course (baseline, 30, and 60 minutes) value was plotted and the area under the curve was calculated (GraphPad). The area under the curve values were then analyzed by t-test. For the analysis of Pomc mRNA, values were normalized to z-scores prior to analysis by t-test (GraphPad). Significance was set at p<.05.
3. Results
3.1 Arsenic exposure paradigm
Dam water consumption, dam body weight, litter size, pup body weight, and pup brain wet weights were not significantly altered by the arsenic exposure paradigm employed in these studies (Martinez et al., 2008; Martinez-Finley et al., 2009).
3.2 ACTH concentration in plasma
The concentration of ACTH in plasma was measured via ELISA. No difference in absorbance was reported between the arsenic-exposed animals and controls in an n=6 (t(10)=0.2473, p>.05).
3.3 GR protein in the hypothalamus
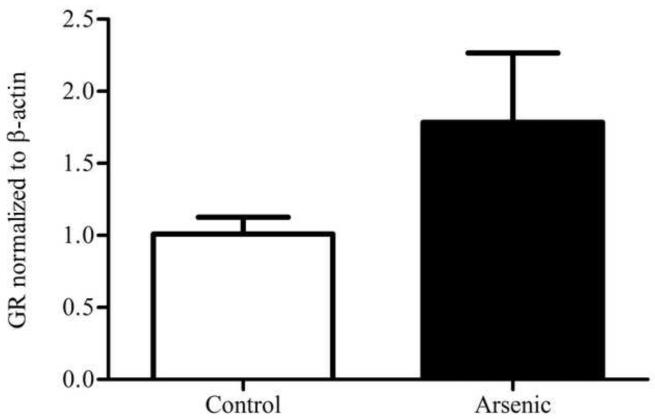
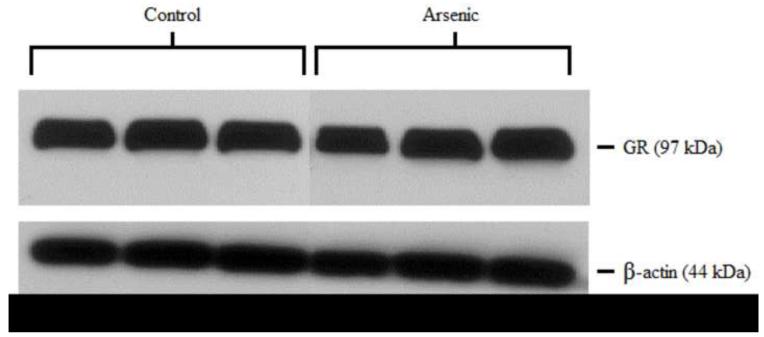
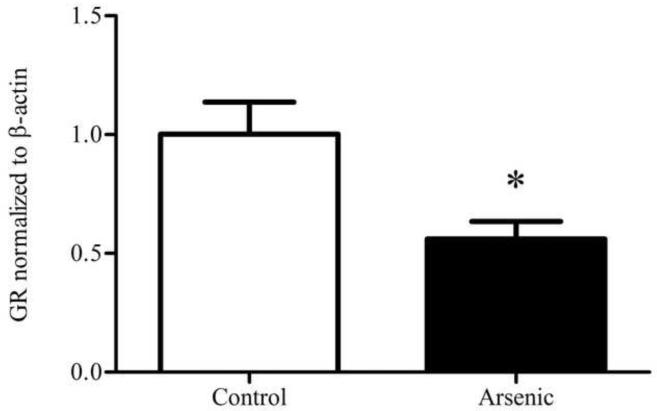
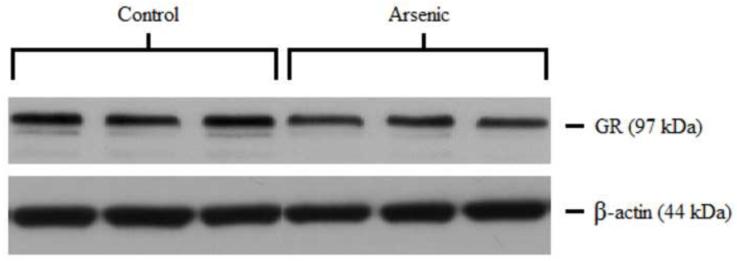
GR was semi-quantitatively measured by western blotting in both the cytosolic and nuclear compartments of the hypothalamus. Control and arsenic-exposed samples were analyzed in the same gel, and the OD of the immunoreactivities was quantified. Data were corrected to β-actin, a ubiquitously expressed housekeeping protein, and presented as percent of control. Analysis by t-test for n=8–9 revealed a statistically significant reduction in the GR immunoreactivities in the cytosolic fraction (t(16)=2.88, p=.011) in the arsenic-exposed animals. Interestingly, there was a modest increase in the GR immunoreactivities in the nuclear fraction, (t(15)=1.657, p=.118) though this was not of statistical significance (Figure 1).
Figure 1.
Subcellular distribution of GR in the hypothalamus of control and arsenic exposed animals. (a) Levels of GR protein are elevated in the nuclear fraction of the hypothalamus (p=.118), though this was not of statistical significance. (b) Representative western blot of nuclear GR in control and perinatal arsenic-exposed offspring. (c) GR protein is decreased in the cytosolic fraction of the hypothalamus (p=.011). Data are presented as corrected to β-actin and normalized to controls. (d) Representative western blot of cytosolic GR in control and perinatal arsenic-exposed offspring. Data are presented as mean±SEM for n=8-9 litters.
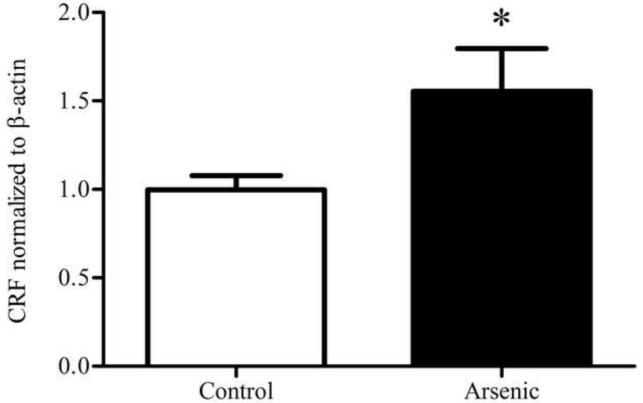
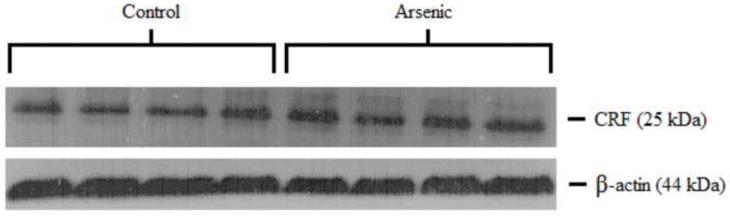
3.4 CRF in the hypothalamus
CRF was semi-quantitatively assessed in the hypothalamus via western blotting. Control and arsenic-exposed samples were analyzed in the same gel and the OD of immunoreactivities was quantified. To serve as a loading control, data were corrected to β-actin and presented as percent of control. Analysis by t-test in an n=8 showed a statistically significant increase in the OD of CRF immunoreactivities in the hypothalamus in the arsenic-exposed animals as pictured in Figure 2 (t(14)=2.20, p=.045).
Figure 2.
CRF in the hypothalamus (a) CRF in the hypothalamus is significantly elevated in the arsenic-exposed offspring. (b) Representative western blot for hypothalamic CRF. Data are presented as normalized to β-actin and corrected to controls (p=.045). Results are presented as mean±SEM for n=8 litters.
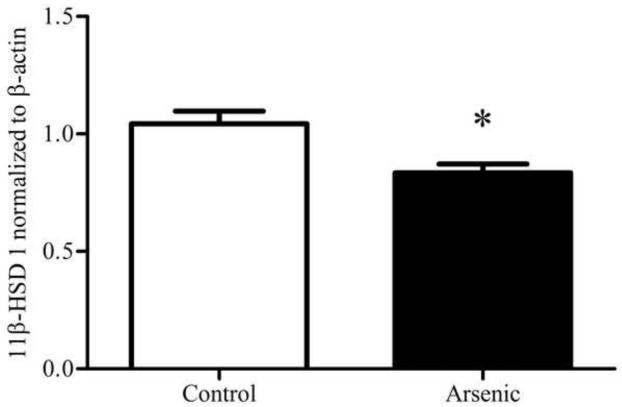
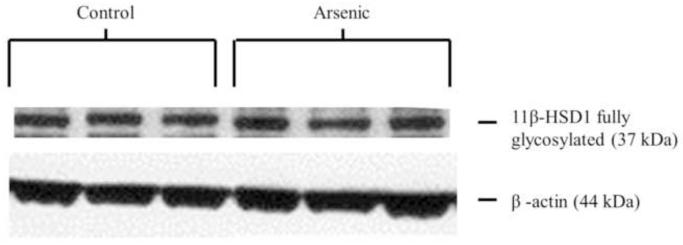
3.5 11β-HSD 1 protein in the hippocampus
11β-HSD 1 is a ketoreductase that converts 11-dehydrocorticosterone to corticosterone and is a powerful regulator of intracellular CORT concentration. Thus, we examined 11β-HSD 1 in the hippocampus, a brain region in which we have previously demonstrated decreased GR and MR translocation to the nucleus (Martinez-Finley et al., 2009) to determine if lower levels of the 11β-HSD 1 enzyme could underlie this observation. 11β-HSD 1 and its glycosylated forms were assessed via western blotting in hippocampal tissue. Analysis by one-way ANOVA for an n=8 in control and n=10 in arsenic revealed a statistically significant decrease in the OD of immunoreactivities of the fully glycosylated form of 11β-HSD 1 in the arsenic-exposed samples (F(1, 16)=10.55, p=.005), shown in Figure 3. No difference was detected in the immunoreactivities of the total forms or partially glycosylated form of 11β-HSD 1.
Figure 3.
11β-HSD 1 in the hippocampus control and perinatal arsenic-exposed offspring. (a) The fully glycosylated form of 11β-HSD 1 is significantly decreased in the arsenic-exposed group (p=.005). (b) Representative western blot for the glycosylated and un-glycosylated forms of 11β-HSD 1 in the arsenic-exposed group and controls. Data were normalized to β-actin and corrected to controls. Data are presented as mean±SEM for n=8 litters in control and n=10 litters in the arsenic group.
3.6 GR mRNA in the hypothalamus and hippocampus
To determine the expression of the Nr3c1 gene that encodes the GR, mRNA for GR was quantified by RT-PCR in the hypothalamus and the hippocampus. In the hippocampus, GR mRNA was unchanged in the arsenic-exposed animals (t(18)=1.33, p>.05) in an n=10. In the hypothalamus of the arsenic-exposed animals there was a trend towards increased expression for an n=6 but significance was not reached (t(10)=2.04, p=.069).
3.7 Pomc mRNA in the pituitary
POMC is a precursor molecule that is cleaved to form ACTH. Pomc mRNA was assessed by RT-PCR from pituitary tissue samples. We found that Pomc mRNA levels were unchanged by the arsenic treatment for an n=7 (t(14)=0.189, p>.05).
3.8 Stress response
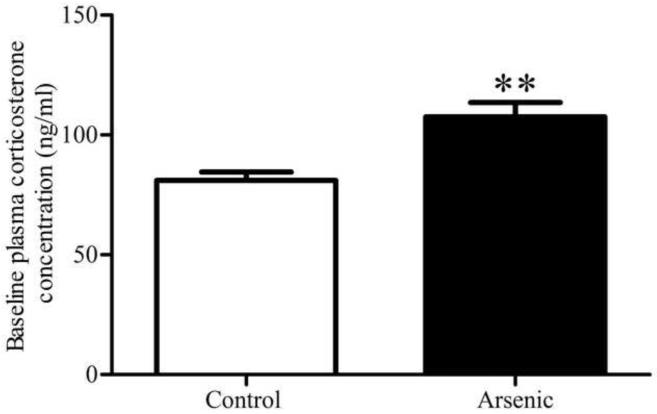
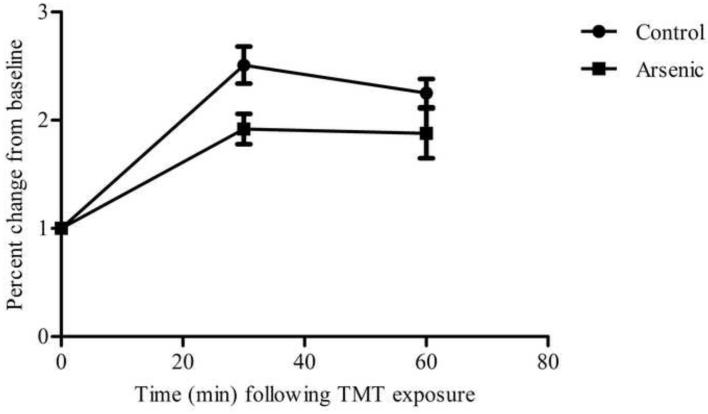
Arsenic-exposed offspring and controls were exposed to TMT for 10 minutes and their stress response was measured by changes in CORT concentration at 30 and 60 minutes post-stressor onset. The data reveal that, though the arsenic animals had increased baseline concentration of plasma CORT (t(16)=3.498, p=.003) in an n=8 and 10 for control and arsenic, respectively, the arsenic offspring exhibited a blunted CORT response and have a smaller change in plasma CORT concentration following a predator-scent stressor compared to controls. CORT concentration in plasma at baseline, 30 minute and 60 minute time points was graphed and the area under the curve was analyzed in an n=7, revealing a statistically significant difference between the arsenic and control mice (t(12)=2.648, p=.02), as shown in Figure 4.
Figure 4.
Baseline and stress-induced plasma corticosterone concentration in arsenic and control groups. (a) Baseline plasma CORT concentration was significantly increased in the arsenic-exposed offspring (p=.003), a replication of our previous finding included in Martinez-Finley et al., 2008. Data are presented as mean±SEM for n=8 litters in control and n=10 litters in the arsenic group. (b) Stress-induced plasma corticosterone at 30 and 60 minutes post stressor-onset: area under curve was significantly decreased in the arsenic-exposed offspring (p=.02) in n=7 litters.
3.9 Buried food pellet test
Perinatal arsenic and control mice (n=8) were evaluated for their ability to detect and locate a buried food pellet. There was no difference in the latency to retrieve a hidden food pellet between the arsenic-exposed offspring and control mice, suggesting equivalent olfactory abilities (Table 2).
Table 2.
The perinatal arsenic-exposed offspring do not differ from controls in their olfactory abilities, as measured by latency to detect a hidden food pellet (p>.05).
Latency (in seconds) to retrieve food pellet: average of 2 trials per day for 5 days
| Control (mean ± SEM) | Arsenic |
| 7.2 (±0.8) | 6.8 (±1.0) |
4. Discussion
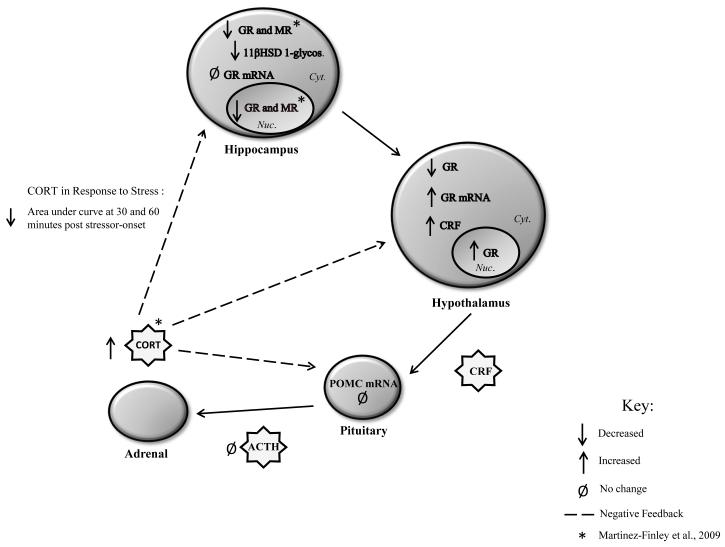
Collectively, these findings show that perinatal exposure to 50 ppb of arsenic induced multiple alterations in the HPA axis that were observable at ~PND 35 in mice (Figure 5). The dysregulation of the HPA axis occurred at multiple levels; some of the alterations may be direct targets of the arsenic exposure while others may represent compensatory changes that manifest only in response to arsenic-mediated damage that has occurred elsewhere. Altered glucocorticoid signaling in the hippocampus and deficits in hippocampal-dependent learning and memory suggest that the proper functioning of this brain region is compromised in our perinatal arsenic exposure model (Martinez et al., 2008; Martinez-Finley et al., 2009). We speculate that the arsenic-mediated hippocampal damage, either directly or indirectly, likely contributes to a breakdown in HPA axis feedback and regulation.
Figure 5.
Summary of alterations induced by perinatal exposure to 50 ppb sodium arsenate in male, ~PN35 mice.
The hippocampus is sensitive to the damaging effects of elevated CORT (see McEwen 1999, for review) and a variety of cellular mechanisms are engaged to protect against further damage in the face of increased and sustained levels of circulating CORT. The occurrence of compensatory regulation in our model is indicated by a decrease in the glucocorticoid and mineralocorticoid receptors in the hippocampus (Martinez-Finley et al., 2009) which would slow the damaging effects of sustained corticosterone activation. In the present study, we observed a decrease in the fully glycosylated form of 11β-HSD 1 enzyme in the hippocampus. 11βHSD 1 acts as a reductase in the hippocampus and is a powerful regulator of intracellular CORT concentration acting to convert the inert 11-keto form (11-dehydrocorticosterone) to its active form (corticosterone) (Holmes and Seckl, 2006; see Wyrwoll et al., 2011 for review). Decreasing 11βHSD 1 is one mechanism of reducing intracellular CORT levels. In a study by Yau and colleagues (2007), 11βHSD 1 knockout mice displayed drastically reduced intracellular glucocorticoid levels, despite elevated plasma glucocorticoids. Likewise, Harris and colleagues (2001) report that mice deficient in 11βHSD 1 show attenuated glucocorticoid negative feedback in the presence of elevated levels of basal plasma CORT. Findings such as these have illuminated the intriguing possibility that regulation by 11βHSD 1 may be a mechanism by which intracellular CORT signaling is controlled without changing circulating plasma CORT levels (Harris et al., 2001; Yau et al., 2007). In our study, we found a decrease in the levels of the glycosylated form of the enzyme, which some maintain is the active form of the enzyme, although other studies have shown that the 11βHSD1 does not need to be fully glycosylated to be enzymatically active (Blum et al., 2000; Walker et al., 2001). To assess 11βHSD 1, we evaluated hippocampal homogenates for protein levels. One caveat of these findings is that protein levels do not necessarily reflect enzymatic activity, though the two usually do exhibit a strong correlation. However, 11βHSD 1 will exhibit bidirectional activity in cellular homogenates from the hippocampus which would render the evaluation of enzyme activity unsuitable for our purposes (Rajan et al., 1996). Down-regulation of the GR and the 11βHSD 1 enzyme serve as protective mechanisms against the damaging effects of uncontrolled elevated CORT, yet will ultimately deteriorate control over the HPA axis as glucocorticoid signaling in hippocampal neurons plays a role in decreasing HPA axis drive (Sapolsky et al., 1984).
The hippocampus is an important factor in maintaining an inhibitory tone over the HPA axis. Our laboratory has previously described an elevation in baseline CORT in the arsenic-exposed mice (Martinez-Finley et al., 2008), which could potentially compromise the integrity of the hippocampus. Damage sustained to this brain area will interfere with the ability to appropriately regulate the HPA axis (Sapolsky et al., 1984; Rubin et al., 1966; Dunn and Orr, 1984). Corticosteroid receptors are of particular importance as decreased numbers of these receptors in the hippocampus results in HPA axis hypersecretion (Sapolsky et al., 1984). Moreover, lesions of the fimbria-fornix transections and lateral fimbria-fornix fiber tracts, which serve as a primary inhibitory communication pathway from the hippocampus to the hypothalamus, elevate CRF mRNA in the medial parvocellular PVN of the hypothalamus (Herman et al., 1992). Hypothalamic CRF was significantly increased in the arsenic exposed offspring which could suggest that the hypothalamic PVN is receiving an excitatory signal. Several studies have reported that stimulation of discrete regions within the hippocampus decreases plasma corticosteroid secretion, demonstrating that the hippocampus is able to alter corticosteroid levels (Rubin et al., 1966; Dunn and Orr, 1984). Thus, an increased HPA axis tone could result from a failure of the hippocampus to communicate appropriate inhibitory information.
Though there is increased CRF in the hypothalamus, the arsenic-exposed animals do not display measurable differences in Pomc mRNA, the precursor for ACTH. These results indicate that elevated levels of CRF are not increasing Pomc mRNA transcription to a detectable level. In agreement with this is the finding of no measurable differences in plasma ACTH concentration. These results could be attributed to desensitization of CRF receptors in the pituitary which would result in an ACTH concentration in plasma that is not increased, even with an increase in CRF (Teli et al., 2005). However, the arsenic-exposed offspring still display an elevation in plasma CORT concentration despite normal plasma ACTH concentrations. One possible explanation for this finding is that the arsenic-exposed offspring exhibit increased adrenal sensitivity, resulting in an exaggerated CORT response from normal levels of ACTH. Sustained increases in HPA activity can produce hypersensitivity to ACTH in the adrenal gland (Droste et al., 2003; Groenink et al., 2002; Revsin et al., 2008) making this a plausible interpretation of our observations, though this would require direct testing.
Negative feedback is a distributed process occurring at multiple levels, including the hippocampus, pituitary, and hypothalamus. In the presence of elevated CORT, negative feedback should ultimately terminate the hormonal stress response via GR activation (Sapolsky et al., 1984). To assess GR-mediated negative feedback in the hypothalamus, we measured GR distribution in the cytosolic and nuclear compartments. Hypothalamic GR was significantly decreased in the cytosolic fraction in the perinatal arsenic offspring while nuclear GR was maintained at slightly elevated, but non-significant levels. The reduction in the cytosolic GR could result from either overall lower levels of GR or depletion of GR due to increased trafficking to the nucleus. However, the latter possibility is unlikely since under supraphysiological concentrations of CORT, cytosolic GR depletion has been demonstrated (Pariante et al., 2001; Spencer et al., 2000), but lower, biologically relevant doses of CORT in vivo do not produce significant depletion in cytosolic GR (Conway-Campbell et al., 2007; Kitchener et al., 2004). Thus, it is more plausible that the reduction in cytosolic GR is a result of down-regulation of the receptor and not depletion due to increased nuclear trafficking. In the present study, the modest increase in nuclear GR levels indicates that despite lower cytosolic GR levels, there may be enough CORT activation to maintain nuclear GR at levels that are normal or even slightly above those seen in controls. This would suggest that GR-mediated negative feedback is occurring in the hypothalamus, but ultimately, the stress response is not terminated, perhaps due to inappropriate GR signaling in the hippocampus.
Because decreases in hippocampal GR can dampen the effectiveness of negative feedback, a signaling process critical for regulation of HPA axis tone, we evaluated whether or not the decrease in the GR protein was due to arsenic-induced disruption at the transcriptional level. One possibility is that changes in the GR are rooted in epigenetic alterations that interfere with transcription of the gene, resulting in lower levels of the receptor. In pursuit of this possibility, we measured GR mRNA in both the hippocampus and hypothalamus to determine if abnormalities may reflect epigenetic changes that interfere with transcription. Our findings show no changes in hippocampal GR mRNA, and a slight increase in hypothalamic GR mRNA. Because GR transcripts are maintained at normal or slightly elevated levels in the arsenic-exposed offspring, it is unlikely that alterations are occurring at the transcriptional level that would satisfactorily explain the reductions in the protein levels of the GR. Our lab is currently investigating other post-transcriptional alterations that could result in the decreased levels of the glucocorticoid receptor.
Finally, we examined the ability of the arsenic-exposed offspring to respond appropriately after exposure to a predator scent stressor as compared to control mice. Because this experiment is dependent upon sense of smell, we conducted a buried food pellet test and verified that arsenic-exposed offspring and control mice have similar olfactory abilities, as measured by latency to detect a hidden pellet of food. We hypothesized that alterations in multiple components of the HPA axis will ultimately lead to changes in stress reactivity, measured by plasma CORT concentration, when exposed to a severe stressor. The data reveal that the arsenic exposed offspring have a significantly blunted CORT response measured by the area under the curve of CORT secretion at baseline, 30 minutes, and 60 minutes post-stressor exposure. Although the arsenic mice display significantly increased baseline CORT concentration in plasma, they show a smaller change from baseline after exposure to stress as compared to controls. One interpretation of this finding is that the arsenic-exposed animals exhibit a deficit in their ability to activate the HPA axis response in the face of a severe stressor that results from the alterations in baseline levels of critical HPA axis components. It appears that the arsenic-exposed animals have a decreased slope (b= 0.014) between the 30 and 60 minute time points compared to controls (b=0.022), suggesting the arsenic-exposed mice return to baseline more slowly. However, additional time-points over an extended period of time would be needed for this conclusion to be statistically validated. If there were to be a reduced rate of return in the perinatal arsenic mice this could be mediated by decreased numbers of hippocampal corticosteroid receptors which would slow the return to a baseline CORT concentration.
The present work expanded upon preliminary data from our earlier publications suggesting the possibility of HPA axis modification due to perinatal exposure to arsenic (Martinez et al., 2008; Martinez-Finley et al., 2009). Here, we carefully examined components of the HPA axis, identified precise changes that occur, and validated our hypothesis that perinatal exposure to 50 ppb arsenic induces persisting HPA axis dysregulation in a rodent model. Endocrine disruption is likely to be key factor in arsenic pathology. Understanding the mechanisms by which arsenic is able to program this pathology is crucial to untangling the broad spectrum of deleterious effects that manifest after exposure, and our findings suggest that the programming of the HPA axis during the perinatal period could be an integral component. Future research should aim to identify therapeutic agents that might mitigate arsenic-induced damage to the HPA axis. Finally, we should note that studies have demonstrated that the form of arsenical speciation is important to its effect on hippocampal function (Krüger et al., 2007; 2009) and identifying possible species specific actions may be important to understanding the mechanism involved in arsenics actions on the GR.
Acknowledgements
The authors would like to thank Dr. Kevin Caldwell for content review pertaining to the HPA axis as well as Christina Tyler for editorial assistance. Additionally, the authors would like to thank Dr. Daniel Savage for generously providing the TMT used in the stressor response study. The project described was supported by 1RO1ES019583-01 (AMA) from the National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- Allan AM, Liang X, Luo Y, Pak C, Li X, Szulwach KE, Chen D, Jin P, Zhao X. The loss of methyl-CpG binding protein 1 leads to autism-like behavioral deficits. Human Molecular Genetics. 2008;17(13):2047–57. doi: 10.1093/hmg/ddn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR . Toxicological Profile for Arsenic (Update) Agency for Toxic Substances and Disease Registry, SUDHHS, PHS; Washington, DC: 1999. [PubMed] [Google Scholar]
- Blum A, Martin HJ, Maser E. Human 11beta-hydroxysteroid dehydrogenase type 1 is enzymatically active in its nonglycosylated form. Biochem Biophys Res Commun. 2000;276(2):428–34. doi: 10.1006/bbrc.2000.3491. [DOI] [PubMed] [Google Scholar]
- Buckley CT, Caldwell KK. Fear conditioning is associated with altered integration of PLC and ERK signaling in the hippocampus. Pharmacol Biochem Behav. 2004;79:633–40. doi: 10.1016/j.pbb.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Bodwell JE, Kingsley LA, Hamilton JW. Arsenic at very low concentrations alters glucocorticoid receptor (GR) –mediated gene activation but not GR-mediated gene repression: complex dose-response effects are closely correlated with levels of activated GR and require a functional GR DNA binding domain. Chem Res Toxicol. 2004;17:1064–76. doi: 10.1021/tx0499113. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Anderson G, Newcomer JW, Charney DS. Effects of glucocorticoids on declarative memory function in major depression. Biological Psychiatry. 2004;55:811–5. doi: 10.1016/j.biopsych.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Calderón J, Navarro ME, Jimenez-Capdeville ME, Santos-Diaz MA, Golden A, Rodriguez-Leyva I, Borja-Aburto V, Díaz-Barriga F. Exposure to arsenic and lead and neuropsychological development in Mexican children. Environ Res. 2001;85(2):69–76. doi: 10.1006/enrs.2000.4106. [DOI] [PubMed] [Google Scholar]
- Caldwell K, Sheema S, Paz R, Samudio-Ruiz S, Laughlin M, Spence N, Roehlk M, Alcon S, Allan A. Fetal alcohol spectrum disorder-associated depression: Evidence for reductions in the levels of brain-derived neurotrophic factor in a mouse model. Pharmacology, Biochemistry and Behavior. 2008;90:614–24. doi: 10.1016/j.pbb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway-Campbell BL, McKenna MA, Wiles CC, Atkinson HC, De Kloet ER, Lightman SL. Proteasome-dependent down-regulation of activated nuclear hippocampal glucocorticoid receptors determines dynamic responses to corticosterone. Endocrinology. 2007;148:5470–7. doi: 10.1210/en.2007-0585. [DOI] [PubMed] [Google Scholar]
- Conti LH, Foote SL. Effects of pretreatment with corticotropin-releasing factor on the electrophysiological responsivity of the locus coeruleus to subsequent corticotropin-releasing factor challenge. Neuroscience. 1995;69:209–219. doi: 10.1016/0306-4522(95)00222-5. [DOI] [PubMed] [Google Scholar]
- Delgado JM, Dufour L, Grimaldo JI, Carrizales L, Rodríguez VM, Jiméz-Capdeville ME. Effects of arsenite on central monoamines and plasmatic levels of adrenocorticotropic hormone (ACTH) in mice. Toxicology Letters. 2000;117:61–7. doi: 10.1016/s0378-4274(00)00240-x. [DOI] [PubMed] [Google Scholar]
- Droste SK, Gesing A, Ulbricht S, Müller MB, Linthorst ACE, Reul JMHM. Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology. 2003;144(7):3012–23. doi: 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- Dunn JD, Orr SE. Differential plasma corticosterone responses to hippocampal stimulation. Exp Brain Res. 1984;54(1):1–6. doi: 10.1007/BF00235813. [DOI] [PubMed] [Google Scholar]
- Gilsbach R, Kouta M, Bonisch H, Bruss M. Comparison of in vitro and in vivo reference genes for internal standardization of real-time PCR data. Biotechniques. 2006;40:173–7. doi: 10.2144/000112052. [DOI] [PubMed] [Google Scholar]
- Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Animal. 2005;34(9):39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- Groenink L, Dirks A, Verdouw PM, Schipholt ML, Veening JG, van der Gugten J, Olivier B. HPA axis dysregulation in mice overexpressing corticotropin releasing hormone. Biological Psychiatry. 2002;51(11):875–81. doi: 10.1016/s0006-3223(02)01334-3. [DOI] [PubMed] [Google Scholar]
- Harris HJ, Kotelevtsev Y, Mullins JJ, Seckl JR, Holmes MC. Intracellular regeneration of glucocorticoids by 11beta-hydroxysteroid dehydrogenase (11beta-HSD-1) plays a key role in regulation of the hypothalamic-pituitary-adrenal axis: analysis of 11beta-HSD-1-deficient mice. Endocrinology. 2001;142:114–20. doi: 10.1210/endo.142.1.7887. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Young EA, Akil H, Watson SJ. Selective forebrain fiber tract lesions implicate ventral hippocampal structures in tonic regulation of paraventricular nucleus CRH and AVP mRNA expression. Brain Res. 1992;592:228–38. doi: 10.1016/0006-8993(92)91680-d. [DOI] [PubMed] [Google Scholar]
- Holmes MC, Seckl JR. The role of 11 beta-hydroxysteroid dehydrogenases in the brain. Mol Cell Endocrinol. 2006;248:9–14. doi: 10.1016/j.mce.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Hood RD, Vedel-Macrander GC, Zaworotko MJ, Tatum FM, Meeks RG. Distribution, metabolism, and fetal uptake of pentavalent arsenic in pregnant mice following oral or intraperitoneal administration. Teratology. 1987;35:19–25. doi: 10.1002/tera.1420350104. [DOI] [PubMed] [Google Scholar]
- Hood RD, Vedel GC, Zaworotko MJ, Tatum FM, Meeks RG. Uptake, distribution, and metabolism of trivalent arsenic in the pregnant mouse. J. Toxicol. Environ. Health. 1988;25:423–34. doi: 10.1080/15287398809531221. [DOI] [PubMed] [Google Scholar]
- Jana K, Jana S, Samanta PK. Effects of chronic exposure to sodium arsenite on hypothalamo-pituitary-testicular activities in adult rats: possible estrogenic mode of action. Reprod. Biol. Endocrinol. 2006;4:9. doi: 10.1186/1477-7827-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchener P, Di BJ, Borrelli E, Piazza PV. Differences between brain structures in nuclear translocation and DNA binding of the glucocorticoid receptor during stress and the circadian cycle. Eur. J. Neurosci. 2004;19:1837–46. doi: 10.1111/j.1460-9568.2004.03267.x. [DOI] [PubMed] [Google Scholar]
- Knapman A, Heinzmann JM, Hellweg R, Holsboer F, Landgraf R, Touma C. Increased stress reactivity is associated with cognitive deficits and decreased hippocampal brain-derived neurotrophic factor in a mouse model of affective disorders. Journal of Psychiatric Research. 2010;44:566–75. doi: 10.1016/j.jpsychires.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Krüger K, Repges H, Hippler J, Hartmann LM, Hirner AV, Straub H, Binding N, Musshoff U. Effects of dimethylarsinic and dimethylarsinous acid on evoked synaptic potentials in hippocampal slices of young and adult rats. Toxicol Applied Pharmacol. 2007;225:40–46. doi: 10.1016/j.taap.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Krüger K, Straub H, Hirner AV, Hippler J, Binding N, Musshoff U. Effects of monomethylarsonic and monomethylasonous acid on evoked synaptic potentials in hippocampal slices of adult and young rats. Toxicology and Applied Pharmacology. 2009;236:115–123. doi: 10.1016/j.taap.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Levine S. The ontogeny of the hypothalamic-pituitary-adrenal axis. The influence of maternal factors. Ann. NY Acad. Sci. 1994;746:275–88. doi: 10.1111/j.1749-6632.1994.tb39245.x. [DOI] [PubMed] [Google Scholar]
- Martinez EJ, Kolb BL, Bell A, Andrea AM. Moderate perinatal arsenic exposure alters neuroendocrine markers associated with depression and increases depressive-like behaviors in adult mouse offspring. Neurotoxicology. 2008;29(4):647–55. doi: 10.1016/j.neuro.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Finley EJ, Ali AMS, Allan AM. Learning deficits in C57BL/6J mice following perinatal arsenic exposure: Consequence of lower corticosterone receptors? Pharmacol Biochem Behav. 2009;94:271–7. doi: 10.1016/j.pbb.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Finley EJ, Goggin SL, Labrecque MT, Allan AM. Reduced expression of MAPK/ERK genes in perinatal arsenic-exposed offspring induced by glucocorticoid receptor deficits. Neurotoxicol Teratol. 2011;33(5):530–7. doi: 10.1016/j.ntt.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejía JJ, Díaz-Barriga F, Calderón J, Ríos C, Jiméz-Capdeville ME. Effects of lead-arsenic combined exposure on central monoaminergic systems. Neurotoxicol. Teratol. 1997;19:489–97. doi: 10.1016/s0892-0362(97)00066-4. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–22. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Yost J, Litherland MT, Struble RG, Switzer PV. Olfactory function in apoE knockout mice. Behav Brain Res. 2004;150:1–7. doi: 10.1016/S0166-4328(03)00219-5. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Pearce BD, Pisell TL, Su C, Miller AH. The steroid receptor antagonists RU40555 and RU486 activate glucocorticoid receptor translocation and are not excreted by the steroid hormones transporter in L929 cells. J. Endocrinol. 2001;169:309–20. doi: 10.1677/joe.0.1690309. [DOI] [PubMed] [Google Scholar]
- Rajan V, Edwards CR, Seckl JR. 11 beta-Hydroxysteroid dehydrogenase in cultured hippocampal cells reactivates inert 11-dehydrocorticosterone, potentiating neurotoxicity. J. Neurosci. 1996;16:65–70. doi: 10.1523/JNEUROSCI.16-01-00065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt HM, Schütz G. Feedback control of glucocorticoid production is established during fetal development. Mol Med. 1996;2(6):735–44. [PMC free article] [PubMed] [Google Scholar]
- Revsin Y, van Wijk D, Saravia FE, Oitzl MS, De Nicola AF, de Kloet ER. Adrenal hypersensitivity precedes chronic hypercorticism in streptozotocin-induced diabetes mice. Endocrinology. 2008;149(7):3531–9. doi: 10.1210/en.2007-1340. [DOI] [PubMed] [Google Scholar]
- Rosado JL, Ronquillo D, Kordas K, Rojas O, Alatorre J, Lopez P, Garcia-Vargas G, Del Carmen Caamaño M, Cebrián ME, Stoltzfus RJ. Arsenic exposure and cognitive performance in Mexican schoolchildren. Environ Health Perspect. 2007;115(9):1371–5. doi: 10.1289/ehp.9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin RT, Mandell AJ, Crandall PH. Corticosteroid responses to limibic stimulation in man: Localization of stimulus sites. Science. 1966;153(3737):767–8. doi: 10.1126/science.153.3737.767. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc. Natl. Acad. Sci. USA. 1984;81:6174–7. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandidos A, Wang X, Dragnev S, Thurber T, Seed B. A comprehensive collection of experimentally validated primers for Polymerase Chain Reaction quantitation of murine transcript abundance. BMC Genomics. 2008;9:633. doi: 10.1186/1471-2164-9-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandidos A, Wang X, Wang H, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucl. Acids Res. 2010;38:D792–9. doi: 10.1093/nar/gkp1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RL, Kalman BA, Cotter CS, Deak T. Discrimination between changes in glucocorticoid receptor expression and activation in rat brain using Western blot analysis. Brain Res. 2000;868:275–86. doi: 10.1016/s0006-8993(00)02341-6. [DOI] [PubMed] [Google Scholar]
- Teli T, Markovic D, Levine MA, Hillhouse EW, Grammatopoulos DK. Regulation of corticotropin-releasing hormone receptor type 1alpha signaling: structural determinants for G protein-coupled receptor kinase-mediated phosphorylation and agonist-mediated desensitization. Mol Endocrinol. 2005;19(2):474–90. doi: 10.1210/me.2004-0275. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency (U.S. EPA) Arsenic occurrence in public drinking water supplies. 2000 EPA-815-R-00-023. [Google Scholar]
- United States Environmental Protection Agency (U.S. EPA) National primary drinking water regulations; arsenic and clarifications to compliance and new source contaminants monitoring. Fed Regist. 2001;66(14):6975–7066. [Google Scholar]
- Walker EA, Clark AM, Hewison M, Ride JP, Stewart PM. Functional expression, characterization, and purification of the catalytic domain of human 11-beta-hydroxysteroid dehydrogenase type 1. J Biol Chem. 2001;276(24):21343–50. doi: 10.1074/jbc.M011142200. [DOI] [PubMed] [Google Scholar]
- Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Research. 2003;31(24):e154, 1–8. doi: 10.1093/nar/gng154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, van Geen A, Slavkovich V, Lolacono NJ, Cheng Z, Hussain I, Momotaj H, Graziano JH. Water arsenic exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2004;112(13):1329–33. doi: 10.1289/ehp.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeber EJ, Savage DD, Sutherland RJ, Caldwell KK. Fear conditioning-induced alterations of phospholipase C-beta1a protein level and enzyme activity in rat hippocampal formation and medial frontal cortex. Neurobiol Learn Mem. 2001;76(2):151–82. doi: 10.1006/nlme.2000.3994. [DOI] [PubMed] [Google Scholar]
- Welch AH, Lico M, Hughes J. Arsenic in ground water of the western United States. Ground water. 1988;26(3):333–47. [Google Scholar]
- Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicology. 2006;27(2):210–6. doi: 10.1016/j.neuro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Wyrwoll CS, Holmes MC, Seckl JR. 11B-Hydroxysteroid dehydrogenases and the brain: From zero to hero, a decade of progress. Frontiers in Neuroendocrinology. 2011;32:65–286. doi: 10.1016/j.yfrne.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JL, McNair KM, Noble J, Brownstein D, Hibberd C, Morton N, Mullins JJ, Morris RG, Cobb S, Seckl JR. Enhanced hippocampal long-term potentiation and spatial learning in aged 11beta-hydroxysteroid dehydrogenase type 1 knock-out mice. J. Neurosci. 2007;27:10487–96. doi: 10.1523/JNEUROSCI.2190-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AH. Cortisol in mood disorders. Stress. 2004;7(4):205–8. doi: 10.1080/10253890500069189. [DOI] [PubMed] [Google Scholar]