Abstract
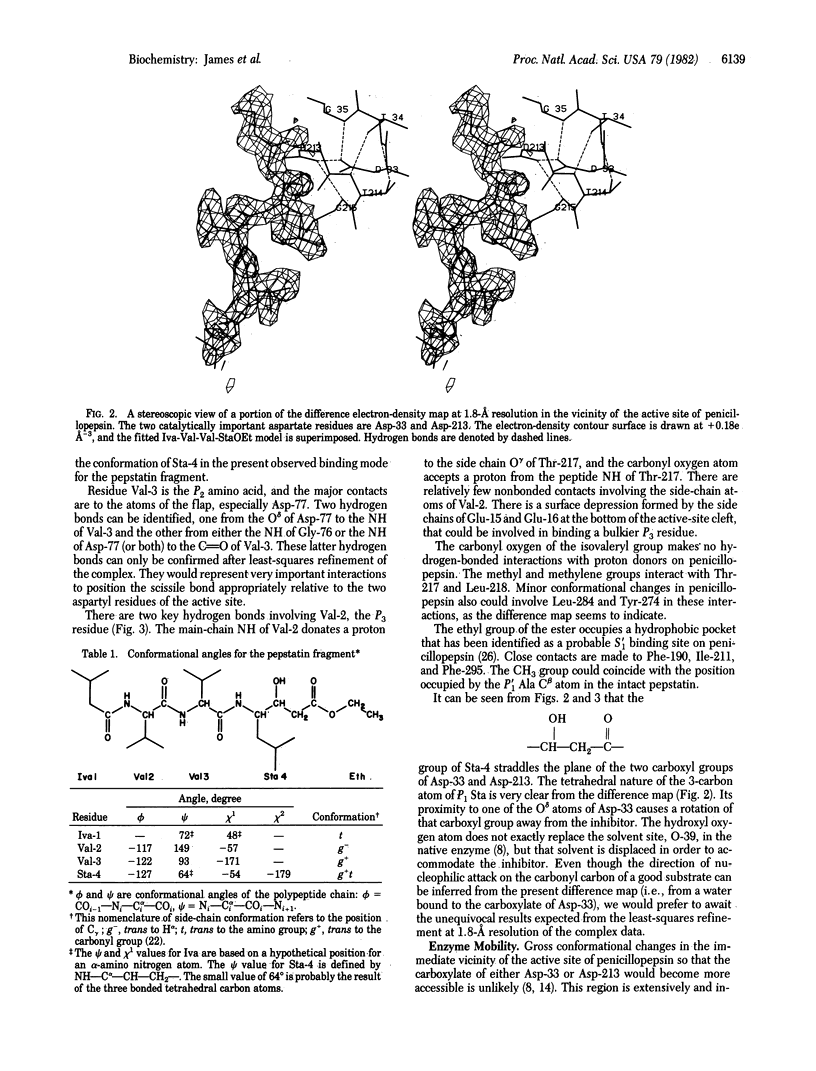
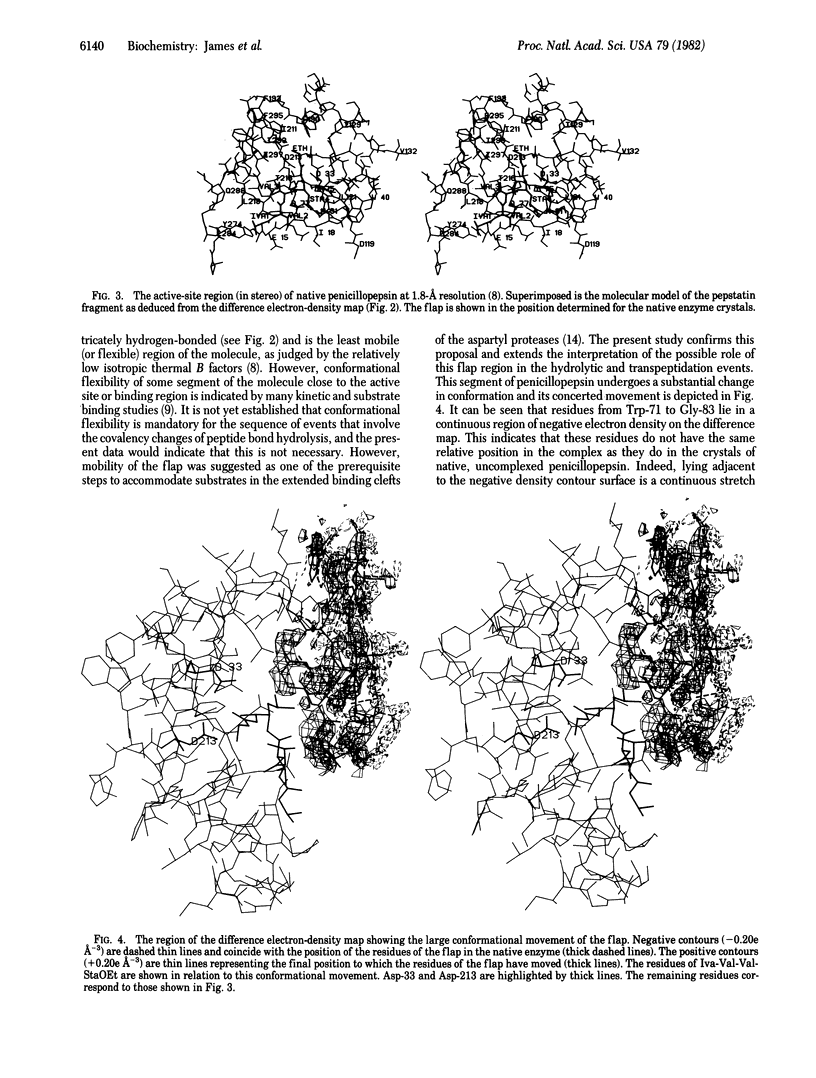
Crystals of the molecular complex between the esterified tripeptide fragment of pepstatin and the aspartyl proteinase penicillopepsin are isomorphous with crystals of native penicillopepsin. The difference electron-density map at 1.8-A resolution, computed by using the amplitude differences and refined phases of reflections from the crystal of native penicillopepsin, unambiguously showed the binding mode of isovaleryl-Val-Val-StaOEt, where StaOEt is the ethyl ester of statine [(4S,3S)-4-amino-3-hydroxyl-6-methylheptanoic acid]. In addition, a major conformational change in penicillopepsin involving the large beta loop of residues from Trp-71 to Gly-83 (the so-called "flap" region) occurs as a result of this inhibitor binding. This structural movement provides the first confirmation of the importance of enzyme flexibility in the aspartyl proteinase mechanism. The 3-hydroxyl group of the Statine residue and the carbonyl oxygen atom of the ethyl ester are situated on either side of the approximate plane containing the hydrogen-bonded carboxyl groups of Asp-33 and Asp-213. The observed binding mode of the pepstatin tripeptide fragment is similar to that predicted for the binding of good substrates with penicillopepsin [James, M. N. G. (1980) Can. J. Biochem. 58, 251-271].
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fruton J. S. Fluorescence studies on the active sites of proteinases. Mol Cell Biochem. 1980 Sep 15;32(2):105–114. doi: 10.1007/BF00227803. [DOI] [PubMed] [Google Scholar]
- Fruton J. S. The mechanism of the catalytic action of pepsin and related acid proteinases. Adv Enzymol Relat Areas Mol Biol. 1976;44:1–36. doi: 10.1002/9780470122891.ch1. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A. Radiation damage in protein crystallography. J Mol Biol. 1976 Sep 25;106(3):889–893. doi: 10.1016/0022-2836(76)90271-0. [DOI] [PubMed] [Google Scholar]
- Hsu I. N., Delbaere L. T., James M. N., Hofmann T. Penicillopepsin from Penicillium janthinellum crystal structure at 2.8 A and sequence homology with porcine pepsin. Nature. 1977 Mar 10;266(5598):140–145. doi: 10.1038/266140a0. [DOI] [PubMed] [Google Scholar]
- Hsu I. N., Delbaere L. T., James M. N., Hofmann T. Penicillopepsin: 2.8 A structure, active site conformation and mechanistic implications. Adv Exp Med Biol. 1977;95:61–81. doi: 10.1007/978-1-4757-0719-9_5. [DOI] [PubMed] [Google Scholar]
- James M. N., Hsu I. N., Delbaere L. T. Mechanism of acid protease catalysis based on the crystal structure of penicillopepsin. Nature. 1977 Jun 30;267(5614):808–813. doi: 10.1038/267808a0. [DOI] [PubMed] [Google Scholar]
- Janin J., Wodak S. Conformation of amino acid side-chains in proteins. J Mol Biol. 1978 Nov 5;125(3):357–386. doi: 10.1016/0022-2836(78)90408-4. [DOI] [PubMed] [Google Scholar]
- Kelly J. A., Sielecki A. R., Sykes B. D., James M. N., Phillips D. C. X-ray crystallography of the binding of the bacterial cell wall trisaccharide NAM-NAG-NAM to lysozyme. Nature. 1979 Dec 20;282(5741):875–878. doi: 10.1038/282875a0. [DOI] [PubMed] [Google Scholar]
- Marciniszyn J., Jr, Hartsuck J. A., Tang J. Mode of inhibition of acid proteases by pepstatin. J Biol Chem. 1976 Nov 25;251(22):7088–7094. [PubMed] [Google Scholar]
- Marshall G. R. Structure--activity relations of antagonists of the renin--angiotensin system. Fed Proc. 1976 Nov;35(13):2494–2501. [PubMed] [Google Scholar]
- Morishima H., Takita T., Umezawa H. The chemical synthesis of pepstatin A. J Antibiot (Tokyo) 1972 Sep;25(9):551–552. doi: 10.7164/antibiotics.25.551. [DOI] [PubMed] [Google Scholar]
- Rich D. H., Sun E. T., Ulm E. Synthesis of analogues of the carboxyl protease inhibitor pepstatin. Effects of structure on inhibition of pepsin and renin. J Med Chem. 1980 Jan;23(1):27–33. doi: 10.1021/jm00175a006. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Hofmann T. Acyl intermediates in penicillopepsin-catalysed reactions and a discussion of the mechanism of action of pepsins. Biochem J. 1975 Jun;147(3):549–563. doi: 10.1042/bj1470549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa H., Aoyagi T., Morishima H., Matsuzaki M., Hamada M. Pepstatin, a new pepsin inhibitor produced by Actinomycetes. J Antibiot (Tokyo) 1970 May;23(5):259–262. doi: 10.7164/antibiotics.23.259. [DOI] [PubMed] [Google Scholar]
- Wang T. T., Hofmann T. Acyl and amino intermediates in penicillopepsin-catalysed reactions and activation by nonsubstrate peptides. Can J Biochem. 1977 Apr;55(4):286–294. doi: 10.1139/o77-040. [DOI] [PubMed] [Google Scholar]
- Wang T. T., Hofmann T. Acyl and amino intermediates in reactions catalysed by pig pepsin. Analysis of transpeptidation products. Biochem J. 1976 Mar 1;153(3):691–699. doi: 10.1042/bj1530691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman R. J., Burkitt D. W. Pepsin inhibition by a high specific activity radioiodinated derivative of pepstatin. Arch Biochem Biophys. 1979 Apr 15;194(1):157–164. doi: 10.1016/0003-9861(79)90605-2. [DOI] [PubMed] [Google Scholar]




