Abstract
Objectives
To determine the extent to which the co-occurrence of chronic obstructive pulmonary disease (COPD) and cognitive impairment affect adverse health outcomes in older adults.
Design
Multi-center longitudinal cohort study.
Setting
California, Pennsylvania, Maryland, and North Carolina.
Participants
Three thousand ninety-three community-dwelling adults aged ≥65 from the Cardiovascular Health Study. Four hundred thirty-one participants had chronic obstructive pulmonary disease (COPD) at study baseline.
Measurements
Follow-up began at the second CHS visit and continued for three years. Spirometric criteria for airflow limitation served to establish COPD, using the Lambda-Mu-Sigma method, which accounts for age-related changes in lung function. Cognitive impairment was evaluated by the modified Mini Mental State Exam and claims data. Outcomes were respiratory-related and all-cause hospitalizations and death.
Results
Participants with co-existing COPD and cognitive impairment had the highest rates of respiratory-related (adjusted hazard ratio [HR]=4.10, 95%CI=1.86–9.05) and all-cause hospitalizations (HR= 1.34, 95%CI=1.00–1.80) and death (HR=2.29, 95%CI=1.18–4.45). In particular, individuals with both conditions had a 48% higher rate of all-cause hospitalizations (adjusted synergy index [SI]=1.48, 95%CI=0.19–11.31) and nearly a three-fold higher rate of death (SI=2.74, 95%CI=0.43–17.32) than the sum of risks for each respective outcome associated with having COPD or cognitive impairment alone. However, tests for interaction were not statistically significant for the presence of synergism between both conditions relative to any of the outcomes. Therefore, we cannot conclude that the combined effect of COPD and cognitive impairment is greater than additive.
Conclusion
Co-existing COPD and cognitive impairment have an additive effect on respiratory-related and all-cause hospitalizations and death. Optimizing outcomes in older adults with COPD and cognitive impairment will require that we determine how to improve concurrent management of both conditions.
Keywords: Chronic obstructive pulmonary disease, cognitive impairment, health outcomes, hospitalizations, disability, death
INTRODUCTION
Knowledge of the clinical effects of co-existing chronic diseases is essential for understanding disease burden and guiding appropriate clinical care in older adults. Chronic obstructive pulmonary disease (COPD) and cognitive impairment are complex chronic conditions that increase in prevalence with aging and are each associated with substantial morbidity and mortality in older adults.1–4 Although COPD and cognitive impairment have been studied primarily as individual conditions, there is a growing body of evidence to indicate that these two conditions often co-exist and could even be inter-related. Published studies on U.S. Medicare beneficiaries have reported that between 17–25% of older adults with dementia concurrently have COPD.5,6 Recent work has further demonstrated an association between COPD severity and the risk of cognitive decline, specifically in chronically hypoxic or oxygen-dependent patients with COPD.7,8 Moreover, COPD and cognitive impairment have each been associated with high rates of hospitalizations9,10 and death9, 11–13 in older adults.
The presence of co-existing chronic conditions increases health care utilization and contributes to a higher risk of serious health outcomes.14 Prior research on the clinical effects of COPD and cognitive impairment has focused mainly on their impact as single conditions. What is not yet known is the extent to which co-existing COPD and cognitive impairment adversely impact health outcomes in older adults. While it can be expected that a greater comorbid disease burden elevates the risks of poor clinical outcomes, understanding whether COPD interacts synergistically with cognitive impairment to negatively affect outcomes will assist with prioritizing and designing enhanced treatment strategies to reduce morbidity and mortality, particularly in older adults who have a increased likelihood of experiencing adverse events and hospitalizations with multiple medication use and complex treatment regimens.15,16 Such knowledge also could guide clinical investigators as to where to target their research efforts and serve as the basis for evaluating new approaches to optimizing the management of co-existing COPD and cognitive impairment in older adults.
In a prospective cohort of community-dwelling adults aged ≥65, we determined the impact of co-existing COPD and cognitive impairment on respiratory-related and all-cause hospitalizations and death. We also assessed whether the co-occurrence of both conditions is associated with greater than expected rates of adverse health outcomes.
METHODS
Study population
The Cardiovascular Health Study (CHS) is a multi-center prospective cohort of randomly sampled Medicare-eligible adults from Sacramento County, California; Allegheny County (Pittsburgh), Pennsylvania; Washington County, Maryland; and Forsyth County, North Carolina. Eligible participants were aged ≥65, non-institutionalized, capable of providing informed consent, and planning to reside in their respective communities for at least three more years. Individuals who were wheelchair-bound at home or who were receiving hospice care, chemotherapy, or radiation treatment were excluded. Enrollment of the original cohort was initiated during 1989–1990 and included 5201 participants. An additional 687 African Americans were recruited from 1992–1993, resulting in a complete cohort of 5888 participants.
Follow-up for this study began at the second CHS visit during 1990–1991, when the modified Mini Mental State Exam (range 0–100) was first administered for the original sample. Follow-up continued for three years after the second CHS visit, which served as our study baseline. Eligible participants for this study included those who were aged 65–80, were white, and completed at least two American Thoracic Society (ATS)-acceptable spirometric measurements obtained during initial examination (1989–1990).17 To account for age-related changes in lung function over time, the Lambda-Mu-Sigma (LMS) method was chosen to evaluate the initial spirometric data in the present study.18 Because reference values for the LMS method are currently unavailable for non-whites and individuals >80 years old, analyses were limited to those who were white and aged 65–80. To focus on participants with COPD, which is characterized by airflow limitation that is not fully reversible, those with self-reported asthma were excluded (N=283). Although participants were not excluded based upon reproducibility criteria, as per current practice,19 participants who did not meet ATS standards for acceptable spirometry or were missing spirometry were excluded (N=469). Participants who demonstrated a spirometric restrictive pattern using the LMS method18 (N= 334) also were excluded from the analyses to enable specific comparisons between individuals with spirometrically-defined COPD vs. normal lung function. Of the 3158 participants who met eligibility criteria, who did not have self-reported asthma or restrictive lung function, who had ATS-acceptable spirometry, and who survived until the second CHS visit, 2.1% (N=65) did not have available data from cognitive assessment, medical history, and Medicare claims to ascertain the presence of cognitive impairment. After excluding those with missing data on cognitive function, the final analytic sample comprised 3093 participants. All participants provided informed consent. Approval for this study was obtained from the Human Investigation Committee at Yale University School of Medicine and the National Heart, Lung, and Blood Institute (NHLBI).
Data collection
Socio-demographic factors, smoking, prevalent health conditions, acute illnesses, and hospitalizations were surveyed either during semi-annual telephone calls or annual in-person interviews. Data on chronic diseases and conditions and hospitalizations were obtained by self-report, medical record abstraction, and health care utilization data. Measures of cognition, mood, and physical function were ascertained annually. Spirometry was performed using a water-sealed spirometer and assessed according to contemporary ATS protocols.17, 20 Extensive details about CHS data collection have been previously described.20 CHS research materials and limited-access data were obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center.
Chronic diseases and conditions
As described in prior CHS work, 21, 22 COPD was established according to an age-appropriate spirometric definition of airflow limitation, which is the physiologic hallmark of COPD. The measured FEV1/FVC was calculated from the largest combination of measurements for FEV1 and FVC during any of the spirometric maneuvers that met ATS acceptability criteria.19, 23 Airflow limitation, which characterizes COPD, was defined by a forced expiratory volume in one second to forced vital capacity (FEV1/FVC) less than the lower-limit of normal (LLN), using the Lambda-Mu-Sigma (LMS) method.18 The LMS method designates the LLN for FEV1/FVC as the 5th percentile of the distribution of Z scores (LMS-LLN5). The LMS-LLN5 threshold for defining COPD by spirometry has been clinically validated21 and more accurately reflects the relationship between age-related changes in pulmonary function and anthropometric features.18 Other spirometric definitions of airflow limitation that are more commonly used24,25 do not account for the effect of advancing age on lung physiology and differences in all elements of distribution, as reflected by the skewness (Lambda), median (Mu), and coefficient-of-variation (Sigma).18, 21, 26, 27 Participants were classified as having normal lung function if FEV1/FVC and FVC were both equal to or greater than LMS-LLN5. LMS-based COPD status, as determined by spirometry data from the initial examination, was carried forward to the second CHS visit (our study baseline) because spirometry was unavailable at this follow-up period.
Cognitive impairment was determined by Medicare claims data for dementia (ICD-9 code 331) or a modified Mini Mental State Exam (3MS) score of ≥1.5 standard deviations below the strata mean for education and race.28 This 3MS threshold has been previously demonstrated to have a high specificity for cognitive impairment.29 For cognitive impairment, claims data within two years prior to the second CHS visit were assessed. Of the 143 participants who were defined to have cognitive impairment, only 0.7% (N=1) were identified by claims data alone.
Based upon their COPD and cognitive status, participants were categorized into four groups: 1) neither COPD nor cognitive impairment, 2) COPD only, 3) cognitive impairment only, and 4) both COPD and cognitive impairment. Other co-existing chronic diseases and conditions were collectively measured using the Functional Comorbidity Index, excluding COPD and cognitive impairment, or dementia.30 This index was chosen because it is more sensitive than other comorbidity indices, such as the Charlson Index, in assessing the relationship between multiple chronic conditions and outcomes associated with physical function. Depressive symptoms were evaluated by the previously validated 10-item Center for Epidemiologic Studies Depression Scale (CES-D).31,32 A CES-D score ≥8 (range 0–30) indicates the presence of depressive symptoms with higher scores reflecting more severe symptoms.
Health outcomes
Health outcomes assessed in this study were respiratory-related and all-cause hospitalizations and death within three years following the second CHS visit. Hospitalizations for respiratory-related illnesses, which included acute COPD exacerbations, pneumonia, and influenza, were identified by ICD-9 codes from hospital claims data, which were also used to assess all-cause hospitalizations. Deaths were ascertained by medical records, death certificates, obituaries, and proxy interviews with next-of-kin.
Statistical analyses
Descriptive statistics were calculated for participants based upon COPD and cognitive status at the second CHS visit, which reflects the baseline of this current study. Socio-demographic and clinical characteristics among disease groups were compared by chi-square analysis for categorical variables and by analysis of variance for continuous variables. We used proportional hazard models to assess the relationships between COPD, cognitive impairment, and their interaction at our study baseline and the time to respiratory-related and all-cause hospitalizations and death. Participants were censored at the time of death, when lost to follow-up, or at the end of the three-year follow-up interval. To account for individuals who died before becoming hospitalized or who were lost to follow-up prior to becoming hospitalized, we performed competing-risk Cox regression analyses which treated the occurrence of death and missingness as competing events.
To quantify the extent to which co-existing COPD and cognitive impairment contributed to each of the outcomes and to determine whether synergism between these conditions was present, we further assessed the synergy index (SI). The SI is a measure of synergism that evaluates for departure from additivity, which better reflects biologic significance than assessing for the presence of an interaction on a multiplicative scale, and may indicate an underlying causal relationship between risk factors.33, 34 The SI refers to the excess risk of an outcome due to co-existing COPD and cognitive impairment relative to the sum of risks contributed by each condition alone. A SI>1 indicates the presence of a synergistic interaction. When an interaction is absent, SI=1 or the 95% CI of SI will include 1. The SI was chosen as the primary measure of synergism because it is less likely than other measures to vary across strata defined by the covariates in multivariable regression models.35
Using the program developed by Li and colleagues,36 we calculated the SIs to examine whether COPD and cognitive impairment could have a synergistic effect on the risk of each outcome in older adults. Estimations of the hazard ratios (HRs) and calculations of the SIs were adjusted for age, gender, education, smoking, the Functional Comorbidity Index, and depressive symptoms. Statistical tests were two-tailed with a p-value level <.05 considered statistically significant. Analyses were conducted with SAS statistical software Version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Table 1 presents characteristics of the CHS participants at study baseline, categorized according to COPD and cognitive status. In this population (N=3093), 14.9% (N=460) of the participants had COPD, and 4.6% (N=143) had cognitive impairment. Moreover, 6.3% (N=29) of those with COPD had co-existing cognitive impairment, whereas 20.3% (N=29) of those with cognitive impairment had co-existing COPD. Of all study participants, 81.4% (N=2519) had neither COPD nor cognitive impairment; 13.9% (N=431) had COPD only; 3.7% (N=114) had cognitive impairment only; and 0.9% (N=29) had both conditions. Most participants from each disease group completed high school or received higher education. Of participants with co-existing COPD and cognitive impairment, the majority were male (65.5%; N=19). A higher frequency of participants with both conditions vs. one or neither condition were current smokers (P<.001). In addition, compared to those with only one or neither condition, participants with COPD and cognitive impairment demonstrated poorer cognitive function and had a higher prevalence of prior hospitalizations for respiratory-related illnesses (all Ps<.001).
Table 1.
Baseline Characteristics of Cardiovascular Health Study Participants By COPD and Cognitive Status*
| Neither COPD Nor Cognitive Impairment |
COPD Only |
Cognitive Impairment Only |
Both COPD & Cognitive Impairment |
|
|---|---|---|---|---|
| Total N=3093 | N=2519 | N=431 | N=114 | N=29 |
| Age (years), No. (%) | ||||
| 65–70 | 1209 (48.0) | 219 (43.7) | 76 (21.0) | 10 (21.3) |
| 71–76 | 943 (37.4) | 175 (34.9) | 91 (25.1) | 14 (29.8) |
| 77–80 | 367 (14.6) | 84 (16.8) | 117 (32.3) | 13 (27.7) |
| Gender, No. (%) | ||||
| Female | 1507 (59.8) | 210 (48.7) | 55 (48.3) | 10 (34.5) |
| Male | 1012 (40.2) | 221 (51.3) | 59 (51.8) | 19 (65.5) |
| Education, No. (%) | ||||
| <12th grade | 581 (23.1) | 134 (31.2) | 27 (23.7) | 5 (17.2) |
| High school graduate, or GED | 763 (30.4) | 121 (28.2) | 49 (43.0) | 15 (51.7) |
| >12th grade | 1169 (46.5) | 174 (40.6) | 38 (33.3) | 9 (31.3) |
| Smoking, No. (%) | ||||
| Never | 1268 (50.4) | 70 (16.2) | 51 (44.7) | 6 (20.7) |
| Former | 1055 (41.9) | 244 (56.6) | 46 (40.4) | 10 (34.5) |
| Current | 195 (7.7) | 117 (27.2) | 17 (14.9) | 13 (44.8) |
| Presence of depressive symptoms,† No. (%) | ||||
| No | 1970 (78.2) | 328 (76.3) | 84 (75.0) | 21 (72.4) |
| Yes | 548 (21.8) | 102 (23.7) | 28 (25.0) | 8 (26.6) |
| Functional Comorbidity Index,‡ mean (± SD) | 1.8 (1.6) | 1.9 (1.6) | 2.2 (2.0) | 2.1 (1.8) |
| Modified Mini Mental State Exam (0–100),§ mean (± SD) | 92.7 (5.3) | 92.0 (5.7) | 71.1 (13.8) | 75.5 (9.3) |
| Prior hospitalizations for respiratory-related illnesses,∥ No. (%) | 12 (0.5) | 20 (4.6) | 1 (0.9) | 2 (6.9) |
| Prior hospitalizations for all causes,¶ No. (%) | 323 (12.8) | 65 (15.1) | 25 (21.9) | 6 (20.7) |
Abbreviations: COPD, chronic obstructive pulmonary disease; GED, General Educational Development; SD, standard deviation.
The second visit of the Cardiovascular Health Study (CHS) represents the baseline of this current study. Differences among all four disease groups were statistically significant for age, gender, completed education, smoking status, mean Modified Mini Mental State Exam scores, and prior respiratory-related and all-cause hospitalizations (all with P<.001 except for prior all-cause hospitalizations with P=.02). The presence of depressive symptoms (P=.60) and the mean Functional Comobidity Index scores (P=.09) were not statistically significant across the four disease groups.
A 10-item Center for Epidemiologic Studies Depression Scale score (0–30) ≥8 indicates the presence of depressive symptoms. Higher scores reflect more severe symptoms.
The Functional Comorbidity Index is a composite score based upon the count of up to 18 possible co-existing chronic medical conditions, which are associated with physical function. COPD and cognitive impairment, or dementia, were excluded from the calculation of this index.
A Modified Mini Mental State Exam score ≥1.5 standard deviations below the strata mean for race and educational level is highly specific for cognitive impairment. Lower Modified Mini Mental State Exam (3MS) scores reflect poorer cognitive function.
Hospitalizations for acute COPD exacerbations, pneumonia, and influenza infections were ascertained two years prior to study baseline and confirmed by Medicare claims data.
Hospitalizations for all causes were ascertained two years prior to study baseline and confirmed by Medicare claims data.
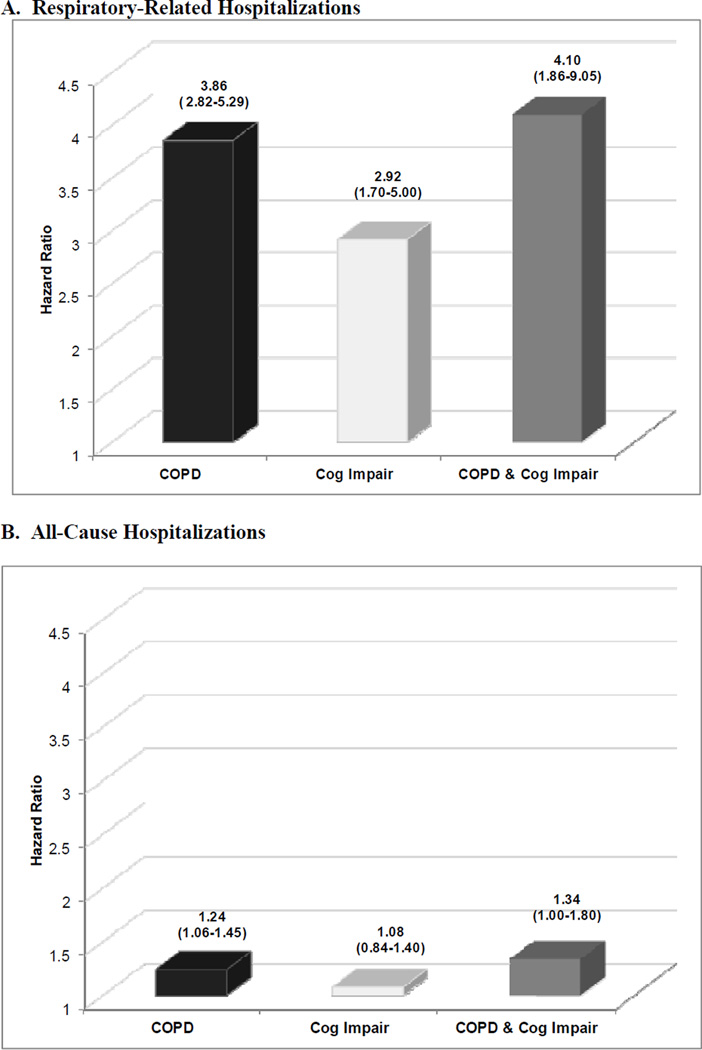
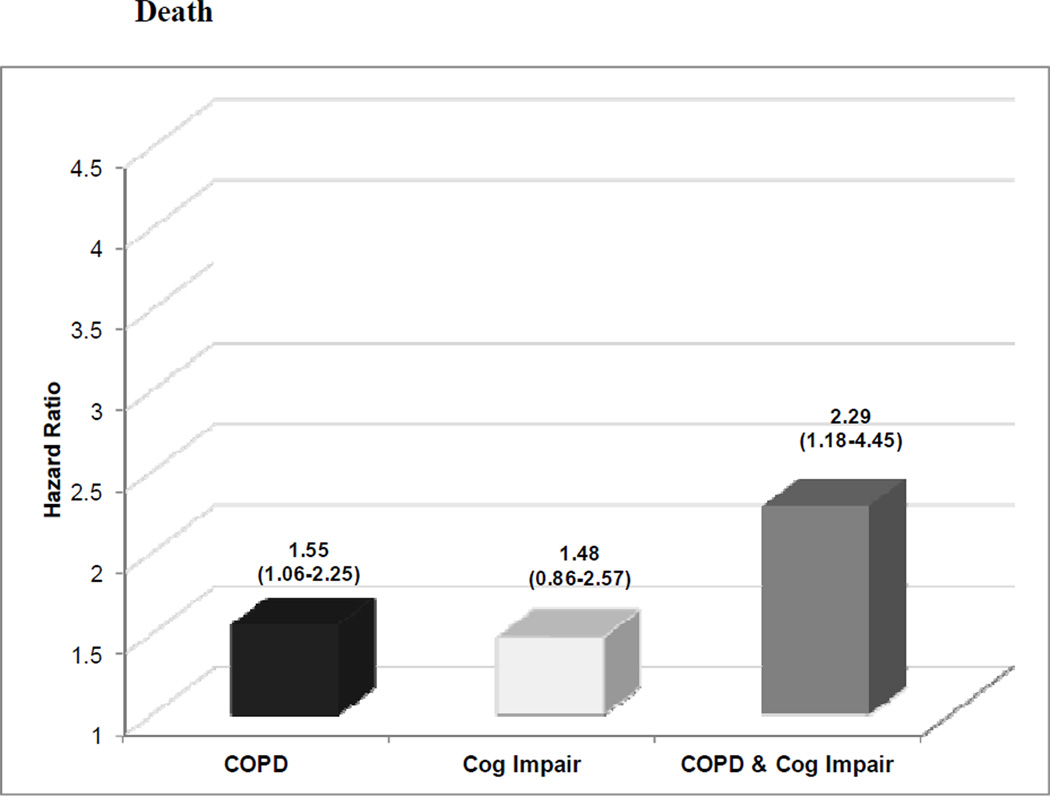
The extent to which COPD and cognitive impairment affected hospitalization rates is graphically displayed in Figure 1. After adjusting for socio-demographic characteristics, smoking status, and other co-existing chronic conditions, co-existing COPD and cognitive impairment, as compared to each alone, was associated with the highest rates of hospitalizations for respiratory-related illnesses (adjusted hazard ratio [HR]=4.10, 95%CI=1.86–9.05) and all-causes (HR= 1.34, 95%CI=1.00–1.80). In particular, participants with both conditions demonstrated a 48% higher rate of all-cause hospitalizations (adjusted synergy index [SI]=1.48, 95%CI=0.19–11.31) than the sum of risks associated with having COPD or cognitive impairment alone. Individuals with both conditions, as compared to each alone, also had the greatest mortality risk (HR=2.29, 95%CI=1.18–4.45). Co-existence of COPD and cognitive impairment was associated with a nearly three-fold higher rate of death (SI=2.74, 95%CI=0.43–17.32) than the sum of risks associated with having each condition alone (Figure 2). However, tests for interaction were not statistically significant for the presence of synergism between both conditions relative to respiratory-related and all-cause hospitalizations or death. Therefore, we cannot conclude that the combined effect of COPD and cognitive impairment is greater than additive for any of the outcomes.
Figure 1.
Effect of chronic obstructive pulmonary disease and cognitive impairment on the risk of (A) respiratory-related and (B) all-cause hospitalizations. COPD indicates chronic obstructive pulmonary disease; Cog Impair, cognitive impairment. COPD and cognitive impairment additively increased the risk of respiratory-related and all-cause hospitalizations. The numbers above the bar graphs are the hazard ratios (HRs). Below these point estimates within parentheses are the 95% confidence intervals (CIs). Participants with neither COPD nor cognitive impairment were the reference group. HRs were adjusted for age, gender, education, smoking, and other comorbidities.
Figure 2.
Effect of chronic obstructive pulmonary disease (COPD) and cognitive impairment on the risk of death. COPD indicates chronic obstructive pulmonary disease; Cog Impair, cognitive impairment. COPD and cognitive impairment additively increased the risk of death. The numbers above the bar graphs are the hazard ratios (HRs). Below these point estimates within parentheses are the 95% confidence intervals (CIs). Participants with neither COPD nor cognitive impairment were the reference group. HRs were adjusted for age, gender, education, smoking, and other comorbidities.
DISCUSSION
Understanding the extent to which co-existing COPD and cognitive impairment contribute to major clinical outcomes in older adults will assist with establishing disease risk, focusing treatment priorities, and informing the design of enhanced approaches to disease management. In this prospective population-based study of older adults, we found that the co-existence of COPD and cognitive impairment had an additive, but not synergistic, effect on respiratory-related and all-cause hospitalizations and death over a three-year period, accounting for other co-morbid chronic conditions and factors associated with these outcomes. These results provide new information about the extent to which the co-existence of COPD and cognitive impairment could adversely contribute to health outcomes.
Several possibilities exist that could account for why COPD and cognitive impairment were associated with additively increased rates of respiratory-related and all-cause hospitalizations as well as death. Cognitive impairment may represent an important COPD-related comorbidity that manifests as a consequence of hypoxemia8 due to increasing COPD severity or a heightened pro-inflammatory state associated with COPD and the common extrapulmonary comorbidities, such as cardiovascular disease, diabetes mellitus, and depression, which characterize its clinical spectrum.37 Optimal management of any complex chronic condition such as COPD requires the ability to learn new skills as well as to initiate, organize, and execute essential self-care functions and daily treatment regimens. Cognitively impaired older adults who have COPD or other complex chronic conditions, including heart failure and diabetes mellitus, may be more vulnerable to hospitalization than their cognitively intact counterparts because they no longer command the executive skills that are necessary for performing important disease self-management tasks which promote tertiary prevention. Regardless of its underlying etiology, cognitive impairment has considerable clinical implications for health outcomes not only in COPD, but also in other complex chronic conditions.
Although COPD and cognitive impairment were associated additively with respiratory-related and all-cause hospitalizations and death, the co-occurrence of these two conditions was not linked to synergistically higher rates of these outcomes. One plausible explanation for these study findings is that equally important determinants of hospitalizations and death over time, such as baseline functional limitation, advancing age, and other influential complex chronic conditions, could diminish the impact of co-existing COPD and cognitive impairment on these outcomes.38, 39 Another consideration is that our study population only included whites and individuals between 65–80 years old because spirometric reference values using the LMS method have not yet been established for ethnic minorities and the oldest old. Although the LMS method has been clinically validated both cross-sectionally and longitudinally in several large cohort studies besides CHS, 21, 22, 40–42 it led to a restricted sample size, which may have reduced our ability to detect any synergistic effect between co-existing COPD and cognitive impairment on each of the outcomes.
Results from this study suggest that enhanced strategies may be needed to evaluate and manage COPD and cognitive impairment concurrently in order to optimize health outcomes. Along these lines, perhaps taking pre-emptive measures to screen for cognitive impairment in patients with COPD early on or to ascertain whether cognitively impaired patients have adequate assistance with disease management tasks may uncover opportunities for earlier intervention, such as connecting patients with home health agencies or instructing caregivers on how to supervise medication use more directly. Incorporating similar approaches in the evaluation and management of index chronic conditions other than COPD, which are also strongly associated with cognitive impairment, could further reduce the risks of poor clinical outcomes in complex older adults and, in turn, potentially improve their overall health.
Given the nationwide movement to reduce 30-day hospital readmissions,43 current efforts which are directed toward developing interventions to reduce re-hospitalization rates should focus on identifying older adults with co-existing COPD and cognitive impairment prior to discharge and important risks factors, such as co-existing COPD and cognitive impairment, which are strongly associated with hospital readmissions. Moreover, identifying the precipitating and perpetuating factors which increase the mortality rate of individuals with co-existing COPD and cognitive impairment would provide further insight into enhanced strategies to modify key risk factors. Future research also is needed to identify potential predisposing factors of poor health outcomes that are associated with declining cognitive function in COPD and other inflammatory-related chronic conditions, such as heart failure and diabetes mellitus, which can present either independently or as comorbidities of COPD.44–46
In this study, several caveats should be taken into consideration regarding our approach. In our ascertainment of COPD, we were unable to adjust for disease severity according to the extent of airflow limitation because spirometry was also used to establish COPD based upon the LMS method. However, we created a separate categorical variable for LMS-based COPD comprising four groups: those with mild, moderate, or severe COPD and those with normal lung function. In separate regression models, we tested for the presence of a synergistic interaction between level of COPD severity, according to the LMS method, and cognitive impairment,but did not find a significant interaction effect.40,47 Further analyses of our study sample also demonstrated that there were no statistically significant differences among the prevalence rates of cognitive impairment in participants with greater severity of COPD (P=0.40). Of those with mild COPD, 3.9% (N=4) had co-existing cognitive impairment. Of those with moderate COPD, 5.7% (N=7) had co-existing cognitive impairment. Of those with severe COPD, 7.7% (N=18) had co-existing cognitive impairment.
Another limitation is that we did not update COPD and cognitive status longitudinally in our analyses because the data were not uniformly available. Nevertheless, the number of cases that were overlooked because disease status was not updated should only be minimal due to the short three-year follow-up interval. A third caveat is that participants in our study sample were all white and aged 65–80. Therefore, our results may not be generalized to non-whites and those who are >80 years old. Once LMS-derived reference equations become available for ethnic minorities and the oldest old, our findings can be validated in non-whites and those aged >80 years.18, 48 Our study sample also excluded participants who did not have available spirometry or were unable to provide acceptable spirometric maneuvers (N=732). These individuals were more likely to have cognitive impairment (N=44; 6.1%) than those who successfully completed spirometry (N=143; 4.6%). Because cognitive impairment was likely to be prevalent in the groups who were excluded from our study, by including individuals with unavailable or unacceptable spirometry, we would have underestimated the frequency of older adults with co-existing COPD and cognitive impairment in this cohort.
Moreover, our findings need to be interpreted bearing in mind that our baseline CHS cohort may represent a healthier and younger population of older adults than the general population of Medicare beneficiaries in prior epidemiologic studies who were sampled from administrative claims data, which include patients in skilled nursing facilities. Nursing home residents are more likely to have cognitive impairment and a greater comorbid disease burden than compared to community-dwelling older adults, particularly CHS participants who were required to have sufficient cognitive capacity to provide informed consent prior to study entry. Finally, because LMS-based COPD status was ascertained during the first CHS visit and then carried forward into the second CHS visit (our study baseline), when spirometry was unavailable, we may have underestimated the prevalence of COPD in CHS participants. This would render our point estimates more conservative than expected.
Despite these limitations, our study has several major strengths. To our knowledge, this is the first study to investigate the extent to which co-existing COPD and cognitive impairment impact multiple adverse health outcomes in a large cohort of community-dwelling older adults. The prospective study design further supports the possibility that the co-occurrence of these conditions is linked to respiratory-related and all-cause hospitalizations and death. By following participants longitudinally, we were able to establish a temporal relationship between co-existing COPD and cognitive impairment and each outcome over a three-year interval.
Optimizing the clinical care of older adults with COPD will require that we determine how to improve the concurrent management of not only the respiratory abnormalities and symptoms associated with COPD, but also co-occurring extrapulmonary conditions, such as cognitive impairment, which contribute to disease progression and poor clinical outcomes. Elucidation of the disease-specific or multi-systemic mechanisms that heighten the risk of hospitalizations and death will enhance our ability to develop the most appropriate strategies to improve the health of older adults with co-existing COPD and cognitive impairment.
ACKNOWLEDGMENTS
This research was funded in part by the John A. Hartford Foundation for Excellence in Geriatric Medicine at Yale University (Grant 2007-0009), the Yale Claude D. Pepper Older Americans Independence Center (National Institute on Aging P30 AG021342), and Clinical Translational Science Award (Grant UL1 RR024139/ KL2 RR024138) from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the opinions or official views of the John A Hartford Foundation, National Institute on Aging, NCATS/NIH, National Heart, Lung, and Blood Institute, or Cardiovascular Health Study.
Sponsor’s Role: None
Footnotes
This paper was presented in abstract and poster form at the 2011 American Geriatrics Society Annual Scientific Meeting in National Harbor, MD.
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Dr. Chang had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Chang and Tinetti. Acquisition of data: Chang, McAvay, and Tinetti. Analysis and interpretation of data: Chang, Chen, McAvay, and Tinetti. Drafting of manuscript: Chang. Critical revision of the manuscript for important intellectual content: Chang, Chen, McAvay, and Tinetti. Statistical analysis: Chang and Chen. We would like to acknowledge Heather G. Allore, PhD for her input on the statistical approach for this study.
REFERENCES
- 1.Mannino DM, Buist AS. Global burden of COPD: Risk factors, prevalence, and future trends. Lancet. 2007;370:765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 2.American Lung Association Epidemiology and Statistics Unit, Research and Program Services. [Accessed April 30, 2010];Trends in chronic bronchitis and emphysema: Morbidity and mortality. Available at: http://www.lungusa.org/finding-cures/for-professionals/trend-reports/copd-trend-report.pdf.
- 3.Langa KM, Larson EB, Karlawish JH, et al. Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity? Alzheimers Dement. 2008;4:134–144. doi: 10.1016/j.jalz.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.2011 Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7:208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Hill JW, Futterman R, Duttagupta S, et al. Alzheimer’s Disease and related dementias increase costs of comorbidities in managed medicare. Neurology. 2002;58:62–70. doi: 10.1212/wnl.58.1.62. [DOI] [PubMed] [Google Scholar]
- 6.Bynum JP, Rabins PV, Weller W, et al. The relationship between a dementia diagnosis, chronic illness, medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52:187–194. doi: 10.1111/j.1532-5415.2004.52054.x. [DOI] [PubMed] [Google Scholar]
- 7.Hung WW, Wisnivesky JP, Siu AL, et al. Cognitive decline among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:134–137. doi: 10.1164/rccm.200902-0276OC. [DOI] [PubMed] [Google Scholar]
- 8.Thakur N, Blanc PD, Julian LJ, et al. COPD and cognitive impairment: The role of hypoxemia and oxygen therapy. Int J Chron Obstruct Pulmon Dis. 2010;5:263–269. doi: 10.2147/copd.s10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mannino DM, Davis KJ. Lung function decline and outcomes in an elderly population. Thorax. 2006;61:472–477. doi: 10.1136/thx.2005.052449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chodosh J, Seeman TE, Keeler E, et al. Cognitive decline in high-functioning older adults is associated with an increased risk of hospitalization. J Am Geriatr Soc. 2004;52:1456–1462. doi: 10.1111/j.1532-5415.2004.52407.x. [DOI] [PubMed] [Google Scholar]
- 11.Mannino DM, Reichert MM, Davis KJ. Lung function decline and outcomes in an adult population. Am J Respir Crit Care Med. 2006;173:985–990. doi: 10.1164/rccm.200508-1344OC. [DOI] [PubMed] [Google Scholar]
- 12.Stump TE, Callahan CM, Hendrie HC. Cognitive impairment and mortality in older primary care patients. J Am Geriatr Soc. 2001;49:934–940. doi: 10.1046/j.1532-5415.2001.49184.x. [DOI] [PubMed] [Google Scholar]
- 13.Agüero-Torres H, Fratiglioni L, Guo Z, et al. Mortality from dementia in advanced age: A 5-year follow-up study of incident dementia cases. J Clin Epidemiol. 1999;52:737–743. doi: 10.1016/s0895-4356(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 14.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162:2269–2276. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- 15.Nolan L, O’Malley K. Part I: Sensitivity of the elderly to adverse drug reactions. J Am Geriatr Soc. 1988;36:142–149. doi: 10.1111/j.1532-5415.1988.tb01785.x. [DOI] [PubMed] [Google Scholar]
- 16.Colley CA, Lucas LM. Polypharmacy: The cure becomes the disease. J Gen Intern Med. 1993;8:278–283. doi: 10.1007/BF02600099. [DOI] [PubMed] [Google Scholar]
- 17.American Thoracic Society. Lung function testing: Selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 18.Stanojevic S, Wade A, Stocks J, et al. Reference ranges for spirometry across all ages: A new approach. Am J Respir Crit Care Med. 2008;177:253–260. doi: 10.1164/rccm.200708-1248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Resp J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 20.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 21.Vaz Fragoso CA, Concato J, McAvay G, et al. The ratio of the forced expiratory volume in 1-second to forced vital capacity in establishing chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:446–451. doi: 10.1164/rccm.200909-1366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaz Fragoso CA, Concato J, McAvay G, Van Ness PH, Gill TM. Respiratory impairment and COPD hospitalization in older adults—a competing risk analysis. Eur Resp J. 2012 [Epub] doi: http://erj.ersjournals.com/content/early/2012/01/19/09031936.00128711.abstract. [Google Scholar]
- 23.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 24.Global Initiative for Chronic Obstructive Lung Disease (GOLD) [Accessed March 29, 2012];Global Strategy for the Diagnosis, Management and Prevention of COPD. 2011 Available from: http://www.goldcopd.org/.
- 25.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 26.Hansen JE, Sun XG, Wasserman K. Spirometric criteria for airway obstruction: Use percentage of FEV1/FVC ratio below the fifth percentile, not < 70% Chest. 2007;131:349–355. doi: 10.1378/chest.06-1349. [DOI] [PubMed] [Google Scholar]
- 27.Vaz Fragoso CA, Gill TM. Defining chronic obstructive pulmonary disease in an aging population. J Am Geriatri Soc. 2010;58:2224–2226. doi: 10.1111/j.1532-5415.2010.03128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teng EL, Chui HC. The modified mini-mental state (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 29.Yaffe K, Fiocco AJ, Lindquist K, et al. Predictors of maintaining cognitive function in older adults: The Health ABC Study. Neurology. 2009;72:2029–2035. doi: 10.1212/WNL.0b013e3181a92c36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58:595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Andresen EM, Malmgren JA, Carter WB, et al. Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 32.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1:385–401. [Google Scholar]
- 33.Greenland S, Lash TL, Rothman KJ. Concepts of interaction. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. 3rd Ed. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 71–83. [Google Scholar]
- 34.Ahlbom A, Alfredsson L. Interaction: A word with two meanings creates confusion. Eur J Epidemiol. 2005;20:563–564. doi: 10.1007/s10654-005-4410-4. [DOI] [PubMed] [Google Scholar]
- 35.Skrondal A. Interaction as departure from additivity in case-control studies: A cautionary note. Ann J Epidemiol. 2007;36:1111–1118. doi: 10.1093/aje/kwg113. [DOI] [PubMed] [Google Scholar]
- 36.Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol. 2007;17:227–236. doi: 10.1016/j.annepidem.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 38.Marengoni A, Von Strauss E, Rizzuto D, et al. The impact of chronic multimorbidity and disability on functional decline and survival in elderly persons. A community-based, longitudinal study. J Intern Med. 2009;265:288–295. doi: 10.1111/j.1365-2796.2008.02017.x. [DOI] [PubMed] [Google Scholar]
- 39.Newman AB, Sachs MC, Arnold AM, et al. Total and cause-specific mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2009;64:1251–1261. doi: 10.1093/gerona/glp127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaz Fragoso CA, Gill TM, McAvay G, et al. Use of lambda-mu-sigma-derived Z score for evaluating respiratory impairment in middle-aged persons. Respir Care. 2011;56:1771–1777. doi: 10.4187/respcare.01192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaz Fragoso CA, Gill TM, McAvay G, et al. Respiratory impairment and mortality in older persons: a novel spirometric approach. J Investig Med. 2011;59:1089–1095. doi: 10.231/JIM.0b013e31822bb213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaz Fragoso CA, Enright PL, McAvay G, et al. Frailty and respiratory impairment in older persons. Am J Med. 2012;125:79–86. doi: 10.1016/j.amjmed.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. NEJM. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 44.Vogels RL, Scheltens P, Schroeder-Tanka JM, et al. Cognitive impairment in heart failure: A systemic review of the literature. Eur J Resp Heart Fail. 2007;9:440–449. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Yaffe K, Blackwell T, Kanaya AM, et al. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 46.Xu W, Caracciolo B, Wang HX, et al. Accelerated cognitive decline from mild cognitive impairment to dementia in people with diabetes. Diabetes. 2010;59:2928–2935. doi: 10.2337/db10-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaz Fragoso CA, Concato J, McAvay G, et al. Staging the severity of chronic obstructive pulmonary disease in older persons based on spirometric Z-scores. J Am Geriatr Soc. 2011;59:1847–1854. doi: 10.1111/j.1532-5415.2011.03596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. [Accessed March 20, 2012];Spirometry (LMS) Available at: http://www.lungfunction.org/growinglungs/all-age.html.