Abstract
Polychlorinated biphenyls (PCBs) are synthetic chemicals primarily used as coolants and insulators in electrical equipment. Although banned for several decades, PCBs continue to exist in the environment because of their long half-life, continued presence in items produced before the ban, and poor disposal practices. Epidemiological and experimental studies have identified exposure to PCBs as a potential risk factor for Parkinson’s disease, perhaps more so in females. The objective of this work was to examine the association between PCB levels in post-mortem human brain tissue and the diagnosis of Parkinson’s disease, as well as the degree of nigral depigmentation. We also sought to determine if this association was more significant when patients were stratified by sex. Post-mortem brain samples from control patients and those diagnosed with Parkinson’s disease were obtained from the Emory University Brain Bank and from the Nun Study. Concentrations of eight prevalent PCB congeners were extracted from post-mortem brain tissue and analyzed using gas chromatography-mass spectrometry. PCB congeners 153 and 180 were significantly elevated in the brains of Parkinson’s disease patients. When stratified by sex, the female Parkinson’s disease group demonstrated significantly elevated concentrations of total PCBs and specifically congeners 138, 153, and 180 compared to controls, whereas PCB concentrations in males were not significantly different between control and Parkinson’s disease groups. In a separate population of women (Nun Study) who had no clinical signs or symptoms of PD, elevated concentrations total PCB and congeners 138, 153 and 180 were also observed in post-mortem brain tissue exhibiting moderate nigral depigmentation compared to subjects with mild or no depigmentation. These quantitative data demonstrate an association between brain PCB levels and Parkinson’s disease-related pathology. Furthermore, these data support epidemiological and laboratory studies reporting a link between PCB exposure and an increased risk for Parkinson’s disease, including greater susceptibility of females.
Keywords: Parkinson’s disease, polychlorinated biphenyl, PCB, sex, exposure, neurodegenerative disease, mass spectrometry
1. Introduction
Polychlorinated biphenyls (PCBs) are anthropogenic compounds primarily used as dielectric fluids in capacitors and transformers due to their chemical stability and physical properties. Although the manufacture, processing, and distribution of PCBs was discontinued in the United States (U.S.) in 1977 (ATSDR, 2000), total worldwide production through 1980 was estimated to be approximately 1.1 million metric tons (2.4 billion pounds), with the majority of production (60 %) occurring in the U.S. (Bletchly, 1983). Despite stringent regulatory measures, the use and servicing of “totally enclosed” PCBs, including electrical and railroad transformers, circuit breakers and liquid-filled cables, are still authorized in the U.S. (CFR, 2011). Due to the legacy of allowable uses, releases from hazardous waste sites, and improper disposal practices, combined with their persistence in the environment, PCBs continue to pose long-term threats to human health and the environment.
Of the 209 possible PCB congeners, 84 were present in commercial mixtures (e.g., Aroclors) at concentrations of greater than 0.5 weight percent (Frame et al., 1996). Of these congeners, six are widely considered to be “indicators” of PCB exposure (PCB 28, 52, 101, 138, 153, 180) based on their persistence in the environment and accumulation in animal and human tissue (Bachour et al., 2000). Of these congeners, PCBs 138, 153, and 180 were also elevated in mouse brain following exposure to Aroclor 1254:1260 mixture (Caudle et al., 2006). Due to their lipophilic (hydrophobic) properties and low rates of metabolism, the more highly chlorinated PCB congeners preferentially accumulate in fatty tissues and readily pass the blood-brain barrier. Dewailly and colleagues (1999) measured the concentrations of 14 PCB congeners in subcutaneous fat, omental fat, brain, and liver autopsy tissue from an Inuit population living in Greenland and found that congeners 138, 153 and 180 accounted for 63-68 % of the PCB burden. In brain tissue samples, concentrations of PCB congeners 138, 153 and 180 ranged from approximately 30 to 400 ppb (ug/kg lipid) (Dewailly et al., 1999)
Exposure to PCBs has been associated with a range of neurological effects, including neurobehavioral abnormalities in newborns and young children (e.g., Branchi et al., 2005; Jacobson et al., 1990; Schantz et al., 2003) and neurochemical alterations in laboratory animals (Bemis and Seegal, 2004; Malkiewicz et al., 2006; Mariussen and Fonnum, 2001; Seegal et al., 2002). A consistent finding of both in vitro and in vivo studies is the significant reduction in dopamine concentrations in the striatal tissue following acute exposures to Aroclor 1016, 1254, or 1260 (Chishti et al., 1996; Richardson and Miller, 2004; Seegal et al., 1991). Previous work in our laboratory demonstrates that exposure of mice to moderate levels of PCB mixtures (7.5 or 15 mg/kg/day of 1:1 Aroclor 1254:1260) results in dose-dependent reductions in striatal dopamine transporter (DAT) expression and function, although no observable changes in dopamine levels or tyrosine hydroxylase (TH) expression were detected (Caudle et al., 2006). Several studies have identified the importance of the DAT and the vesicular monoamine transporter 2 (VMAT2) in the regulation of dopamine levels in the striatum (Fon et al., 1997; Gainetdinov et al., 1998; Miller et al., 1999a,b). The primary function of DAT is to remove dopamine from the synapse following exocytosis, while VMAT2 sequesters cytosolic dopamine into vesicles for release. Altered transporter (i.e., DAT and VMAT2) function can impede dopamine sequestration and storage, resulting in elevated levels of dopamine in the cytoplasm, where it is available to react to form oxidized byproducts (e.g., 3,4-dihydroxyphenylacetic acid) and reactive oxygen species (ROS). Such PCB-induced oxidative stress can lead to dopaminergic cell damage, and ultimately death, as demonstrated by Lee and colleagues (2006).
Epidemiological studies have shown an increased incidence of Parkinson’s disease (PD) in humans, especially females (Standardized Mortality Ratio [SMR] = 2.95) who were occupationally-exposed to PCBs (Steenland et al., 2006). In a study of 8 Parkinson’s disease patients (3 female, 5 male) and 7 controls, elevated concentrations of PCB congeners 153 (p < 0.05) and 180 (p < 0.01) were detected in the caudate nucleus of Parkinson’s disease patients (Corrigan et al., 1998). Since these data were published, few, if any, studies have attempted to relate concentrations of PCB measured in human tissue to the incidence of Parkinson’s disease, despite the apparent association. Over this same time period, advancements in analytical instrumentation, and in particular mass spectrometry, have resulted in greatly improved mass resolution and lower detection limits. This is particularly relevant to PCBs, for which gas-chromatography (GC) combined with electron capture detection (ECD) has been the accepted analytical technique for nearly 30 years (e.g. EPA, 2000; Erickson, 1997). However, PCB quantification by GC-ECD suffers from two important limitations, (a) poor resolution that can result in co-elution of congeners that cannot be distinguished without extended runs times (e.g., > 2 hr) and/or sophisticated separation techniques (e.g., two-dimensional analysis) and (b) nonspecific response of ECDs, especially to oxygenated species, which is particularly problematic for tissue and plasma samples (Hardin et al., 1990).
The objective of this research was to quantify selected PCB congeners in post-mortem brain tissue from patients with Parkinson’s disease or Alzheimer’s disease (AD)-related neuropathology and age-matched controls using advanced gas-chromatography-mass spectrometry (GC-MS) techniques. Correlations between individual PCB congeners and summed concentrations, sex, and degree of neuropathological features are evaluated in two independent cohorts obtained from the Emory Brain Bank and the Nun Study. Confirmation of elevated PCB concentrations in the brain tissue of patients from an additional cohort exhibiting Parkinson’s disease neuropathology provides further insight into PD etiopathogenesis and supports the potential link between PCB exposures and neurodegeneration.
2. Materials and methods
2.1. Chemicals
Ten analytical grade PCB congeners (28, 65, 101, 118, 138, 149, 153, 166, 170, and 180) were obtained from Accustandard (New Haven, CT). Acetone (Certified ACS, 99.6% purity), hexane (Certified ACS, 99.7% purity), and anhydrous sodium sulfate (Certified ACS, 99.4% purity, 10–60 mesh) were purchased from Fisher Chemicals (Fair Lawn, NJ). All other reagents were obtained from Sigma–Aldrich (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA).
2.2. Human samples
Human brain samples were obtained from the Emory Alzheimer’s Disease Research Center Brain Bank, Center for Neurodegenerative Disease, Department of Neurology at Emory University, tertiary care facility (Miller et al., 1997; Miller et al., 1999a). All subjects with Parkinson’s disease pathology were confirmed histopathologically by the depletion of midbrain dopaminergic neurons and identification of Lewy bodies, and all subjects with (AD) pathology were confirmed using Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) criteria for definite AD (Mirra et al., 1991). AD subjects had no evidence of PD pathology. PD subjects may or may not exhibit AD pathology. Patients were seen by experienced board-certified neurologists at the Emory University Movement Disorders Clinic. Inclusion criteria for PD were specific; three of the four findings of resting tremor, bradykinesia, rigidity and asymmetric onset, with disease duration of three years or more, and absence of atypical features or other causes of Parkinsonism. Control brains obtained by Emory University autopsy service were obtained from patients who died from non-neurologic causes such as myocardial infarct, pulmonary embolus, or cancer, and whose brains were free of evidence of neurodegenerative disease. Frozen post-mortem brain samples were placed immediately in -80°C until use. Fixed post-mortem samples were obtained from the opposite hemisphere as frozen and were placed in 10 % formalin until use.
Fixed post-mortem brain tissue samples from the Nun Study (Snowdon et al., 1996) were provided by Dr. Snowdon and colleagues at the University of Kentucky. The design of the Nun Study has been described elsewhere (e.g., Snowdon et al., 2000; Mortimer et al., 2004). Briefly, between 1991 and 1993, all members from the School Sisters of Notre Dame born before 1917 and living in Midwestern, eastern, and southern United States communities were invited to join the Nun Study. Of 1,031 eligible Catholic sisters aged 75 years and older, 678 (66 %) agreed to participate in the study, which included consent to the collection of archival and medical records, annual cognitive and physical assessments, and brain donation after death. Participants did not significantly differ from nonparticipants in mean age, mortality rate, race, or country of birth. Of these subjects, 40 were analyzed for nigral depigmentation using a semi-quantitative scoring system (i.e., none, mild, moderate, or severe depigmentation). A neuropathologist, blinded to the subjects cognitive test score, performed gross and microscopic evaluations of the substantia nigra (Mortimer et al., 2004). The average age of the subjects at death was 95 years, and did not exhibit clinical criteria for PD. Degree of nigral depigmentation in the Nun study subjects was categorized as follows: none (no depigmentation, n = 30), mild (< 10 % loss, n = 6), moderate (10-50 %, n = 4), and severe (> 50% loss; n = 0).
2.3. PCB analytical methods
2.3.1. Extraction procedure
The tissue extraction procedure was based on methods by Corrigan and colleagues (2000) and Grandjean and colleagues (2001), and was performed following the general previously described procedures (Caudle et al., 2006; Hatcher et al., 2007). Frozen human occipital cortex tissue (ca. 500mg) was added to 10 ml of a 1:1 mixture of hexane and acetone in an amber glass vial. PCB concentrations from this brain region were considered representative because organochlorines are evenly distributed throughout the brain (Hatcher et al., 2007). A 10 uL aliquot of hexane containing PCB congeners 65 and 166 was added to each sample to serve as internal standards at final concentrations of 10 μg/L (ppb). The tissue was homogenized and the vials were placed in a sonication bath for 15 minutes, vortexed for an additional 2 minutes and then allowed to incubate at room temperature overnight. The supernatant was separated by centrifugation for 10 minutes at 1,250 × g and transferred to a 35 ml glass centrifuge tube. This extraction sequence was repeated an additional four times, to yield a total extract volume for each tissue sample of approximately 25 ml. The extract solution was then reduced by evaporating the contents of each tube at 45°C in an exhaust fume hood with an analytical nitrogen evaporator (Organomation; Berlin, MA). The dry residue was weighed and dissolved in 2 ml of 1:1 hexane:acetone. The 2 ml volume, plus two 1 ml rinses, were transferred to a 25 ml solid-phase extraction column (Alltech Assoc., Model no. 227950, Deerfield, IL) containing 5 g of Florisil™ and 1 g of anhydrous sodium sulfate, that was preconditioned with 8 ml of hexane. The Florisil™ column was then eluted with 5 ml of methyl tertiary-butyl ether (MTBE), and the process was repeated four times to yield an effluent volume of approximately 25 ml. The hexane/MTBE extract was then evaporated at 45°C with nitrogen gas as described above. The residue was resuspended in 1.0 ml hexane volume, plus two 0.5 ml hexane rinses, and transferred to glass autosampler vials, which were immediately sealed with PTFE-lined screw caps and stored at 4°C in the dark prior to analysis.
2.3.2. Quantification
Brain tissue extracts were analyzed for selected PCB congeners using an Agilent 6890N GC equipped with an Agilent 5975 inert mass selective detector (MSD). Separation was achieved with an HP-5MS capillary column (30 m × 0.25 mm diameter × 0.25 μm film thickness, Agilent) operated at an initial temperature of 100 °C, ramped to 180°C (25°C/min), increased to 250°C (2°C/min), and finally ramped to 280°C (20°C/min) and held for 5 minutes to yield a total run time of 48 minutes. Electron impact ionization (EI) was employed due the stability of PCBs, and the MSD was operated in selective ion monitoring (SIM) mode, with a minimum of three ions collected for each PCB congener over a specified elution time window (Table 1). Quantification was achieved using a 5-point calibration curve constructed from individual PCB congeners prepared in hexane by serial dilution. Mass spectral matching was based individual PCB standards and confirmed using the NIST/EPA/NIH Mass Spectral Library (NIST02). The method detection limit (MDL) for individual PCB congeners ranged from 0.09 to 0.7 ppb, while spiked recovery efficiencies ranged from 97 to 105% for PCB congeners 101, 118, 138, 149, 153, 166, 170, and 180 (Table 1).
Table 1.
Summary of analytical method parameters for PCB congeners.
| PCB Congener | Chlorine Position | Ret. Time (min) | Quant. Ion (SIM) | ID Ions (SIM) | Recovery (%) | MDL (ppb) |
|---|---|---|---|---|---|---|
| PCB 28 | 2,4,4’ | 13.03 | 256 | 186, 258, 260 | 87.9 ± 3.7a | 0.23 |
| PCB 65b | 2,3,5,6 | 15.13 | 292 | 220, 222, 290, 294 | 95.9 ± 2.5 | 0.09 |
| PCB 101 | 2,2’,4,5,5’ | 19.83 | 326 | 254, 291, 324, 328 | 97.4 ± 1.5 | 0.55 |
| PCB 149 | 2,2’3,4’,5’,6 | 23.75 | 360 | 254, 256, 324, 326 | 102.3 ± 0.8 | 0.68 |
| PCB 118 | 2,3’,4,4’,5 | 24.18 | 326 | 254, 256, 324, 328 | 101.8 ± 0.9 | 0.31 |
| PCB 153 | 2,2’,4,4’,5,5’ | 25.71 | 360 | 288, 290, 358, 362 | 103.7 ± 0.8 | 0.29 |
| PCB 138 | 2,2’,3,4,4’,5’ | 27.56 | 360 | 288, 290, 358, 362 | 103.8 ± 0.8 | 0.34 |
| PCB 166b | 2,3,4,4’,5,6 | 28.60 | 360 | 288, 290, 292, 362 | 105.1 ± 0.5 | 0.71 |
| PCB 180 | 2,2’,3,4,4’,5,5’ | 32.89 | 396 | 322, 324, 392, 396 | 105.7 ± 0.9 | 0.23 |
| PCB 170 | 2,2’,3,3’4,4’,5 | 35.06 | 396 | 324, 326, 392, 396 | 104.5 ± 0.8 | 0.19 |
= mean ± standard error of mean,
=internal standard, SIM = selective ion monitoring, MDL = method detection limit.
2.4. Statistical Analysis
Statistics for the two human data sets were based on comparisons in disease groups (i.e., control, PD, and AD) for the Emory brain bank cohort, and degree of depigmentation (i.e., none, mild, and moderate) for the Nun Study. Data were analyzed using non-parametric Wilcoxon rank sum tests using SAS® statistical software package (SAS Institute, Cary, NC). Exact tests (two-sided) were all Wilcoxon tests, with the exception of the comparsion of disease groups with both sexes combined, where a normal approximation (two-sided) was used. In addition, Spearman rank-order and Pearson correlation tests were performed for the Emory cohort to assess associations between PCB levels and subject age and disease duration, respectively.
3. Results
3.1. Patient demographics (Emory Cohort)
Patients in each disease group (control, PD, AD) were compared according to age at death, disease duration (if applicable), and post-mortem interval (PMI) (Table 2). The male:female ratio in the Parkinson’s disease and Alzheimer’s disease subjects was approximately 3:1, and 1:1 in controls. No significant correlation (Spearman r value) was observed between age and either individual representative PCB congeners (138, p = 0.21 to 0.79; 153, p = 0.83 to 0.91; 180 p = 0.50 to 0.84) or total PCBs (sum of 8 congeners; p = 0.41 to 0.91) for male, female and the combined (male+female) patient groups. Additionally, Pearson correlations p values ranged from 0.12 (138) to 0.54 (sum), indicating that there was no significant correlation between disease duration and measured PCB levels.
Table 2.
Patient demographics.
| Control | PD | AD | |
|---|---|---|---|
| No. of Subjects | 13 | 45 | 14 |
| Age (yrs) | 66.31 ± 1.69 | 71.91 ± 1.27 | 70.93 ± 1.83 |
| Male/Femalea | 6/7 | 33/10 | 11/3 |
| Duration (Yrs)b | ---- | 12.20 ± 1.00 | 7.10 ± 1.11 |
| PMI (hrs)c | 7.68 ± 1.11 | 10.03 ± 1.02 | 7.36 ± 1.82 |
= Number of males and females in each diagnosis. Sex was not available for all subjects.
= Disease duration was not available for all subjects, expressed as mean ± S.E.M. calculated from 41 PD subjects and 11 AD subjects.
= Post-mortem interval (PMI) was not available for all subjects, expressed as mean ± standard error of mean calculated from 12 controls, 39 PD subjects, and 11 AD subjects.
3.2. PCB concentrations in post-mortem brain tissue of control, AD, and PD patients
In order to determine if a relationship existed between PCB concentrations in human post-mortem brain tissue and the diagnosis of neurodegenerative disease, we used GC-MS methods to identify and quantify concentrations of eight PCB congeners at ppb levels in brain tissue samples obtained from the Emory Alzheimer’s Disease Research Center Brain Bank. Examination of PCB congeners revealed significant elevations of congeners 153 and 180 in the Parkinson’s disease group versus controls. The concentration of PCB 153 was 2.08-fold higher in Parkinson’s disease brains than in controls (p = 0.006), while PCB 180 was 2.49-fold higher in Parkinson’s disease brains (p = 0.003) (Table 3). Total PCB congener levels were 1.39-fold higher in the Parkinson’s disease group when compared to control (p = 0.07). None of the other PCB congeners were significantly elevated in Parkinson’s disease brains, and none were significantly elevated in the Alzheimer’s disease subjects (Table 3). When stratified by sex (Fig. 1), females with Parkinson’s disease exhibited significantly elevated concentrations of total PCBs (1.98-fold; p =0.03) as well as the specific congeners 138, 153, and 180. The concentration of PCB 138 was 2.60-fold higher in Parkinson’s disease brains than in controls (p = 0.013), PCB 153 was 4.66-fold higher (p = 0.007) and PCB 180 was 3.64-fold higher in Parkinson’s disease brains versus controls (p = 0.003). There were no significant differences in PCB concentrations between female AD and control patients. We did not observe any significant differences in the concentrations of individual PCB congeners in the male disease groups (Fig. 1).
Table 3.
Concentrations of several PCB congeners in post-mortem brain tissue from Parkinson’s disease (PD), Alzheimer’s disease (AD), and control patients.
| PD (n=45) | AD (n=14) | Control (n=13) | |
|---|---|---|---|
| Total PCBs | 9.49 ± 5.67 | 7.66 ± 4.31 | 6.82 ± 4.43 |
| 28 | ND | ND | ND |
| 101 | 0.73 ± 0.62 | 0.75 ± 0.80 | 0.96 ± 0.69 |
| 118 | 1.11 ± 0.98 | 1.29 ± 0.70 | 1.30 ± 1.25 |
| 138 | 1.41 ± 0.94 | 1.16 ± 0.59 | 1.26 ± 1.48 |
| 149 | 0.55 ± 0.48 | 0.52 ± 0.39 | 0.64 ± 0.34 |
| 153 | 2.56 ± 1.81** | 1.88 ± 1.60 | 1.23 ± 1.40 |
| 170 | 0.95 ± 1.17 | 0.63 ± 0.70 | 0.57 ± 0.61 |
| 180 | 2.17 ± 1.87** | 1.41 ± 1.37 | 0.87 ± 0.57 |
ND = Not detectable; Values expressed as mean ± standard error of mean (ng/g wet tissue);
p-value < 0.01 vs. controls
Fig 1. Total and individual PCB concentrations in post-mortem human brain tissue stratified by gender (Emory Cohort).
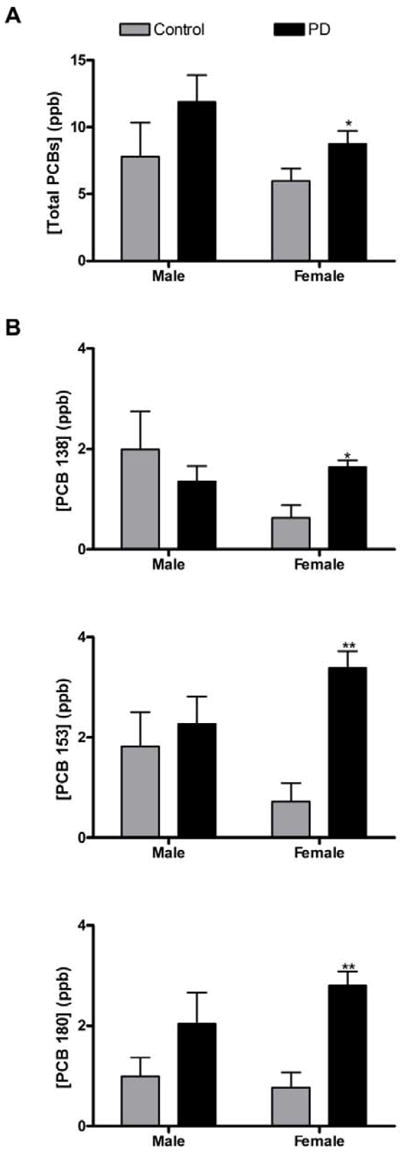
A. Post-mortem brains from females with PD exhibited significantly elevated concentrations of total PCBs. B. PCB congeners 138, 153, and 180 are also significantly elevated in post-mortem brains from females with PD. Significant differences (p < 0.05) were not observed in total or individual PCB concentrations in brains from males with or without PD (* p < 0.05 vs. control; ** p < 0.01 vs. control). Bars represent the standard error of mean (S.E.M.).
3.3. Contribution of individual PCB congeners to total PCB burden
The percent contribution of each of the eight PCB congeners to the total PCB load was examined in each of the disease groups. The summed concentration of PCB congeners 28, 101, 118, 138, 149, 153, 170, 180 was greater than 99.7% of the total PCBs measured in all groups (data not shown). A related goal of this project was to determine whether we could expand our analyses into formalin-fixed brain tissue thus permitting access to a wider array and larger number of tissue samples. We compared tissue from eight post-mortem subjects for which we had access to both frozen and formalin-fixed tissue from the same patient. Analysis revealed that while the summed levels of PCBs were slightly lower in the formalin-fixed samples of matched PD samples (n = 4), they were not significantly different from the concentrations in the frozen tissue (p > 0.05, data not shown).
3.4. Correlation of PCB concentrations with nigral depigmentation in post-mortem human brain tissue
To determine if the PCB associations seen in the Emory population of post-mortem samples could be extrapolated to other populations, we compared PCB concentrations in human brain to the degree of depigmentation of nigral neurons in patients without clinical symptoms of Parkinson’s disease using post-mortem brain tissue from the Nun Study. A total of 40 tissue samples were obtained, which were graded according to degree of depigmentation of nigral neurons: none, mild, or moderate (none of the subjects had Parkinson’s disease, and thus none had severe depigmentation). As shown in Figure 2, analysis of total PCB concentrations versus degree of pigmentation revealed higher concentrations of PCBs in the moderately depigmented group when compared to those with no depigmentation (1.75-fold, p = 0.07), and versus those with mild depigmentation (1.92-fold higher, p = 0.17). There was no significant correlation between age and total PCB levels based on a Spearmen correlation test (-0.03, p = 0.71), and thus age did not confound the observed findings. When concentrations of individual PCB congeners were examined, PCB congeners 138, 153 and 180 were elevated in the moderately depigmented patient group. The concentration of PCB 138 was 2.01-fold higher in moderately depigmented brains compared to those with no depigmentation (p = 0.09) and 3.21-fold higher than those with mild depigmentation (p = 0.02). The concentration of PCB 153 was 1.96-fold higher in moderately depigmented brains than those with no depigmentation (p = 0.13) and 3.01-fold higher than those with mild depigmentation (p = 0.26). The concentration of PCB 180 was 2.22-fold higher in moderately depigmented brains compared to those with no depigmentation (p 0.09), and 2.32-fold higher than those with mild depigmentation (p = 0.25). Although the p values obtained for these two-sided comparison of means did not fall below the p = 0.05 level except in the case of PCB 138, which was anticipated given the small sample size, the fact that measured PCB levels were 2 to 3 times higher in the moderate depigmentation group versus the mild and no depigmentation group provides supporting evidence for the association between PCB exposures and neuropathology.
Fig 2. Increased concentrations of total and individual PCB concentrations correlate with degree of depigmentation of nigral depigmentation in post-mortem brain tissue (Nun Study).

A. Total PCB concentrations are elevated in post-mortem brain tissue with moderate nigral depigmentation when compared to subjects with no or mild nigral depigmentation. B. PCB congeners 138 and 180 are elevated in post-mortem brain tissue with moderate nigral depigmentation when compared to subjects with no or mild nigral depigmentation (## p < 0.05, moderate vs. mild; * p < 0.10, moderate vs. none). Bars represent standard error of mean (S.E.M.).
4. Discussion
There is compelling epidemiological evidence to indicate that environmental factors, including PCBs, are associated with an increased risk of Parkinson’s disease (Corrigan et al., 1998; Hatcher et al., 2008; Steenland et al., 2006). However, the specific compounds and related mechanisms responsible for this association are still unclear. PCBs are a class of synthetic compounds that are persistent in the environment. From a toxicological standpoint, these compounds exhibit many features that make them ideal candidates for being involved in the risk of developing Parkinson’s disease. These compounds exhibit low volatility, chemical stability, and lipophilic properties, making them extremely persistent in the environment. In addition, the strong tendency of PCBs to bioaccumulate and biomagnify increases the risk of human exposure and accumulation at toxic levels.
In this study, we report that concentrations of selected PCB congeners are elevated in post-mortem brain tissue from patients with Parkinson’s disease. These congeners represent the most abundant PCB congeners found in the mouse brain after exposure to a mixtures of Aroclor 1254:1260, the most common form in which PCBs where used (Caudle et al., 2006). These findings corroborate other studies reporting associations between PCB exposure and an increased risk of Parkinson’s disease. These results are also consistent with previous animal studies, in which exposure of mice to moderate doses of a 1:1 mixture of Aroclor 1254 and 1260 resulted in decreased expression of dopaminergic markers, including DAT and TH (Caudle et al., 2006). When stratified by sex, we found that the association between brain PCB concentrations was primarily driven by females. This is especially interesting given that women typically have a lower incidence of Parkinson’s disease in the general population. Steenland and colleagues reported a similar increased risk in Parkinson’s disease and other neurodegenerative diseases in a cohort of PCB-exposed female workers (Steenland et al., 2006).
This study is not a mere replication of previous studies showing elevated PCBs in post-mortem human brain (Corrigan et al., 1998). The fact that these samples were collected over a decade later than previous studies demonstrate a continued association between PCBs and Parkinson’s disease. Advances in mass spectrometry over the past decade now allow for precise identification and quantification of PCBs in human samples at ppb levels. Given the long prodromal period in the disease, exposures that occurred in the last decades of the past century are very relevant to patients with recent diagnosis. Exposures from birth to age 50’s could have a significant impact on the development and progression of the disease. Therefore, even with the continued decline in environmental levels of PCBs, they remain relevant for individuals at the average age of diagnosis of Parkinson’s disease.
The Nun Study represents a unique collection of postmortem brain samples. In contrast to the participants from the Emory Cohort of post-mortem tissues who spent at least some time in their life in the southeast United States, participants in the Nun Study represent a Midwestern population with different chemical exposure profiles. While none of the 40 subjects had a diagnosis of Parkinson’s disease, which with an average age of 95 is striking, many did have neuropathological evidence of nigrostriatal degeneration. The fact that the same three PCB congeners (i.e., 138, 153, and 180) were elevated in samples with moderate nigrostriatal degeneration is remarkable as these patients represent a completely different geographical cohort from the Emory population.
The methods presented in this study provide useful means to identify chemical exposures without the obstacles of recall bias and inability to identify specific exposures. Patients with neurodegenerative diseases could have been exposed to these compounds as early as their teens, and continuing up through the point of diagnosis. Persistence of these compounds in the brain provides the cumulative and toxicokinetic features indicative of chronically-acting neurotoxicants. In light of the long prodromal period in Parkinson’s disease, such persistent neurotoxicants are in prime position to be lead candidates for the link between environmental exposure and Parkinson’s disease. It is important to note that the elevated levels of the various PCB congeners do not necessarily mean that they cause disease. Rather, the unbiased identification of these compounds, when combined with previous epidemiological and toxicological studies linking this class of compounds to the disease, provides additional evidence to support the inclusion of PCB exposure as a risk factor for Parkinson’s disease. Expanded use of the analytical techniques presented here could help further elucidate the role of persistent environmental pollutants in Parkinson’s disease, and potentially, other idiopathic neurodegenerative diseases.
Table 4.
Concentrations of PCB congeners in post-mortem brain tissue from Nun Study subjects exhibiting no, mild, and moderate nigral depigmentation.
| None (n=30) | Mild (n=6) | Moderate (n=4) | |
|---|---|---|---|
| Total PCBs | 2.33 ± 0.16 | 2.11 ± 0.14 | 4.07 ± 0.99* |
| 28 | ND | ND | ND |
| 101 | 0.13 ± 0.02 | 0.11 ± 0.01 | 0.09 ± 0.02 |
| 118 | 0.37 ± 0.04 | 0.53 ± 0.08 | 0.43 ± 0.11 |
| 138 | 0.33 ± 0.03 | 0.21 ± 0.04 | 0.66 ± 0.22*,## |
| 149 | 0.17 ± 0.02 | 0.27 ± 0.09 | 0.18 ± 0.04 |
| 153 | 0.63 ± 0.07 | 0.41 ± 0.11 | 1.23 ± 0.44 |
| 170 | 0.16 ± 0.02 | 0.08 ± 0.01 | 0.27 ± 0.13* |
| 180 | 0.54 ± 0.05 | 0.52 ± 0.14 | 1.21 ± 0.44* |
ND = Not detectable; Values expressed as mean ± S.E.M. (ng/g wet tissue);
p-value ≤ 0.10 moderate vs. none,
p-value < 0.05 moderate vs. mild.
HIGHLIGHTS.
Advanced gas chromatography-mass spectroscopy (GC-MS) methods were employed to identify and quantify eight PCB congeners in post-mortem brain tissue of Parkinson’s and Alzheimer’s disease patients and controls.
PCB congeners 153 and 180 were significantly elevated in post-mortem brain tissue of Parkinson’s disease patients relative to controls.
When stratified by sex, female Parkinson’s disease patients exhibited significantly elevated concentrations of PCB congeners 138, 153, and 180, as well as total PCB levels.
In a separate cohort of women, significantly higher concentrations of total PCBs and congeners 153 and 180 were detected in brain tissue exhibiting moderate nigral depigmentation.
These quantitative body burden data demonstrate an association between brain PCB levels and Parkinson’s disease-related pathology.
Acknowledgments
This work was supported by the National Institutes of Health Mentored Quantitative Research Development Award grant K25 ES014659 (KDP), the Emory Parkinson’s disease Collaborative Environmental Research Center (PD-CERC) under grant P01 ES016731 (GWM, KDP), National Research Service Fellowship Award F30 ES014141 (JMH-M), the Emory Alzheimer’s Disease Research Center grant P50 AG025688 (AIL), and the Emory Neuroscience NINDS Core Facilities grant P30 NS055077 (AIL). Post-mortem brain samples from the Nun Study were generously provided by Dr. David Snowdon (retired) and colleagues from the University of Minnesota, and a preliminary statistical analysis was performed by Joanne Wuu, University of Miami.
Abbreviations
- AD
Alzheimer’s disease
- ANOVA
analysis of variance
- CERAD
Consortium to establish a registry for Alzheimer’s Disease
- DA
dopamine
- DAT
dopamine transporter
- ECD
electron capture detector
- EI
electron impact ionization
- GC-MS
gas chromatography-mass spectrometry
- MSD
mass selective detector
- MTBE
Methyl tertiary-butyl ether
- PCB
polychlorinated biphenyl
- PD
Parkinson’s disease
- PMI
post-mortem interval
- PTFE
polytetrafluoroethylene
- ROS
reactive oxygen species
- SIM
selective ion monitoring
- SMR
standardized mortality ratio
- TH
tyrosine hydroxylase
- VMAT2
vesicular monoamine transporter 2
Footnotes
Conflict of interest statement
We declare that we have no financial or personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ATSDR (Agency for Toxic Substances and Disease Registry) Toxicological Profile for Polychlorinated Biphenyls (PCBs) [8 September 2007];US Dept of Health and Human Services - Public Health Service. 2000 http://www.atsdr.cdc.gov/toxprofiles/tp17.html. [PubMed]
- Bachour G, Failing K, Georgii S, Elmadfa I, Brunn H. Species and organ dependence of PCB contamination in fish, foxes, roe deer, and humans. Arch Environ Contamination Toxicol. 1998;35:666–73. doi: 10.1007/s002449900429. [DOI] [PubMed] [Google Scholar]
- Bemis JC, Seegal RF. PCB-Induced Inhibition of the Vesicular Monoamine Transporter Predicts Reductions in Synaptosomal Dopamine Content. Toxicological Sciences. 2004;80:288–95. doi: 10.1093/toxsci/kfh153. [DOI] [PubMed] [Google Scholar]
- Bletchly JD. Production, current use and possible rates of future disposal in OECD member countries. In: Barros MC, K H, Visser R, editors. Proceedings of the PCB-Seminar. The Hague, Netherlands: Ministry of Housing, Physical Planning and Environment; 1983. pp. 343–65. [Google Scholar]
- Branchi I, Capone F, Vitalone A, Madia F, Santucci D, Alleva E, et al. Early Developmental Exposure to BDE 99 or Aroclor 1254 Affects Neurobehavioural Profile: Interference from the Administration Route. NeuroToxicology. 2005;26:183–92. doi: 10.1016/j.neuro.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Delea KC, Guillot TS, Wang M, Pennell KD, et al. Polychlorinated biphenyl-induced reduction of dopamine transporter expression as a precursor to Parkinson’s disease-associated dopamine toxicity. Toxicol Sci. 2006;92:490–9. doi: 10.1093/toxsci/kfl018. [DOI] [PubMed] [Google Scholar]
- CFR CoFR. Electronic Code of Federal Regulations. 2011 Sep 8; ed2011. [Google Scholar]
- Chishti MA, Fisher JP, Seegal RF. Aroclors 1254 and 1260 reduce dopamine concentrations in rat striatal slices. Neurotoxicology. 1996;17:653–60. [PubMed] [Google Scholar]
- Corrigan FM, Murray L, Wyatt CL, Shore RF. Diorthosubstituted polychlorinated biphenyls in caudate nucleus in Parkinson’s disease. Exp Neurol. 1998;150:339–42. doi: 10.1006/exnr.1998.6776. [DOI] [PubMed] [Google Scholar]
- Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D. Organochlorine insecticides in substantia nigra in Parkinson’s disease. J Toxicol Environ Health A. 2000;59:229–34. doi: 10.1080/009841000156907. [DOI] [PubMed] [Google Scholar]
- Dewailly E, Mulvad G, Pedersen HS, Ayotte P, Demers A, Weber JP, et al. Concentration of organochlorines in human brain, liver, and adipose tissue autopsy samples from Greenland. Environ Health Perspect. 1999;107:823–8. doi: 10.1289/ehp.99107823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA. [March 14, 2011];EPA Method 8082A. 2000 http://www.caslab.com/EPA-Methods/PDF/EPA-Method-8082A.pdf.
- Erickson MD. Analytical chemistry of PCBs. 2. Boca Raton: CRC Press LLC; 1997. [Google Scholar]
- Fon EA, Pothos EN, Sun BC, Killeen N, Sulzer D, Edwards RH. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997;19:1271–83. doi: 10.1016/s0896-6273(00)80418-3. [DOI] [PubMed] [Google Scholar]
- Frame GM, Cochran JW, Boewadt SS. Complete PCB congener distributions for 17 Aroclor mixtures determined by 3 HRGC systems optimized for comprehensive, quantitative, congener-specific analysis. J High Res Chromatogr. 1996;19:657–68. [Google Scholar]
- Gainetdinov RR, Jones SR, Fumagalli F, Wightman RM, Caron MG. Re-evaluation of the role of the dopamine transporter in dopamine system homeostasis. Brain research. 1998;26:148–53. doi: 10.1016/s0165-0173(97)00063-5. [DOI] [PubMed] [Google Scholar]
- Grandjean P, White RF, Sullivan K, Debes F, Murata K, Otto DA, et al. Impact of contrast sensitivity performance on visually presented neurobehavioral tests in mercury-exposed children. Neurotoxicol Teratol. 2001;23:141–6. doi: 10.1016/s0892-0362(01)00134-9. [DOI] [PubMed] [Google Scholar]
- Hardin ED, Meehan PW, Melton MA, Shore FL. A critical evaluation of EPA methods 680 and 8080 for PCB analysis. In: Addis G, editor. Proceedings of the 1989 PCB Seminar. Vol. 7. 1990. pp. 441–4. Report No. EL/GS/EN-6792. [Google Scholar]
- Hatcher JM, Pennell KD, Miller GW. Parkinson’s disease and pesticides: A toxicological perspective. Trends Pharmacol Sci. 2008;29:322–9. doi: 10.1016/j.tips.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher JM, Richardson JR, Guillot TS, McCormack AI, Di Monte DA, Jones DP, et al. Dieldrin exposure induces oxidative damage in the mouse nigrostriatal dopamine system. Exp Neurol. 2007;204:619–30. doi: 10.1016/j.expneurol.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Humphrey HEB. Effects of exposure to PCBs and related compounds on growth and activity in children. Neurotoxicology and Teratology. 1990;12:319–26. doi: 10.1016/0892-0362(90)90050-m. [DOI] [PubMed] [Google Scholar]
- Lee DW, Gelein RM, Opanashuk LA. Heme-Oxygenase-1 promotes polychlorinated biphenyl mixture aroclor 1254-induced oxidative stress and dopaminergic cell injury. Toxicol Sci. 2006;90:159–67. doi: 10.1093/toxsci/kfj052. [DOI] [PubMed] [Google Scholar]
- Malkiewicz K, Mohammed R, Folkesson R, Winblad B, Szutowski M, Benedikz E. Polychlorinated biphenyls alter expression of alpha-synuclein, synaptophysin and parkin in the rat brain. Toxicol Lett. 2006;161:152–8. doi: 10.1016/j.toxlet.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Mariussen E, Fonnum F. The effect of polychlorinated biphenyls on the high affinity uptake of the neurotransmitters, dopamine, serotonin, glutamate and GABA, into rat brain synaptosomes. Toxicol. 2001;159:11–21. doi: 10.1016/s0300-483x(00)00374-7. [DOI] [PubMed] [Google Scholar]
- Miller GW, Erickson JD, Perez JT, Penland SN, Mash DC, Rye DB, Levey AI. Immunochemical analysis of vesicular monoamine transporter (VMAT2) protein in Parkinson’s disease. Exp Neurol. 1999a;156:138–148. doi: 10.1006/exnr.1998.7008. [DOI] [PubMed] [Google Scholar]
- Miller GW, Gainetdinov RR, Levey AI, Caron MG. Dopamine transporters and neuronal injury. Trends Pharmacol Sci. 1999b;20:424–9. doi: 10.1016/s0165-6147(99)01379-6. [DOI] [PubMed] [Google Scholar]
- Miller GW, Staley JK, Heilman CJ, Perez JT, Mash DC, Rye DB, et al. Immunochemical analysis of dopamine transporter protein in Parkinson’s disease. Ann Neurol. 1997;41:530–9. doi: 10.1002/ana.410410417. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurol. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mortimer JA, Goshe KM, Riley KP, Markesberry WR, Snowdon DA. Delayed recall, hippocampal volume and Alzheimer neuropathology: Findings from the Nun Study. 2004;62:428–432. doi: 10.1212/01.wnl.0000106463.66966.65. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Miller GW. Acute exposure to aroclor 1016 or 1260 differentially affects dopamine transporter and vesicular monoamine transporter 2 levels. Toxicol Lett. 2004;148:29–40. doi: 10.1016/j.toxlet.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ, Rice DC. Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect. 2003;111:357–576. doi: 10.1289/ehp.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegal RF, Bush B, Brosch KO. Sub-chronic exposure of the adult rat to Aroclor 1254 yields regionally-specific changes in central dopaminergic function. Neurotoxicol. 1991;12:55–65. [PubMed] [Google Scholar]
- Seegal RF, Okoniewski RJ, Brosch KO, Bemis JC. Polychlorinated biphenyls alter extraneuronal but not tissue dopamine concentrations in adult rat striatum: an in vivo microdialysis study. Environ Health Perspect. 2002;110:1113–7. doi: 10.1289/ehp.021101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life. Findings from the Nun Study. JAMA. 1996;275:528–32. [PubMed] [Google Scholar]
- Snowdon DA, Tully CL, Smith CD, Riley KP, Markesbery WR. Serum folate and the severity of atrophy of the neocortex in Alzheimer disease: findings from the Nun Study. Am J Clinical Nutr. 2000;71:993–998. doi: 10.1093/ajcn/71.4.993. [DOI] [PubMed] [Google Scholar]
- Steenland K, Hein MJ, Cassinelli RT, 2nd, Prince MM, Nilsen NB, Whelan EA, et al. Polychlorinated biphenyls and neurodegenerative disease mortality in an occupational cohort. Epidemiol. 2006;17:8–13. doi: 10.1097/01.ede.0000190707.51536.2b. [DOI] [PubMed] [Google Scholar]


