Abstract
Both lead (Pb) exposure and prenatal stress (PS) can produce cognitive deficits, and in a prior study we demonstrated enhanced cognitive deficits in repeated learning of female rats exposed to both of these developmental insults (Cory-Slechta et al., 2010). However, PS can also lead to improved cognitive outcomes that are both gender-and context-dependent. Thus, the current study examined whether Pb ± PS likewise produced repeated learning deficits in males, either after maternal or lifetime Pb exposure. Repeated learning was evaluated using a multiple schedule of repeated learning and performance that required learning 3-response sequences in male offspring that had been subjected to either maternal Pb (0 or 150 ppm) or lifetime Pb exposure (0 or 50 ppm) beginning two mos prior to dam breeding, to prenatal immobilization restraint stress (gestational days 16–17), or to both Pb and PS. Blood Pb, corticosterone, hippocampal glucocorticoid receptor density and brain monoamines were also measured. In contrast to outcomes in females, sequence-specific enhancements of repeated learning accuracy were produced by PS, particularly when combined with Pb, results that appeared to be more robust in combination with lifetime than maternal Pb exposure. A common behavioral mechanism of these improvements appears to be an increase reinforcement density associated with increased response rates and shorter session times seen with PS ± Pb that could both shorten time to reinforcement. Trends towards lower levels of nucleus accumbens dopamine activity seen after both maternal Pb and lifetime Pb combined with PS suggest a possible role for this region/neurotransmitter in enhanced accuracy, whereas PS ± Pb-induced corticosterone changes did not exhibit an obvious systematic relationship to accuracy enhancements. While PS ± Pb-based increases in accuracy appear to be an improved outcome, the benefits of increased response rate are by no means universal, but highly context-dependent and can lead to adverse behavioral effects in other conditions.
Keywords: lead, prenatal stress, reversal learning, sex, corticosterone, nucleus accumbens dopamine
INTRODUCTION
Elevated blood lead (Pb) levels and prenatal stress (PS) are co-occurring risk factors for a variety of diseases and disorders, especially for low socioeconomic status communities (Vivier et al., 2011). These two risk factors also commonly target both the hypothalamic-pituitary-adrenal (HPA) axis and the mesocorticolimbic system of the brain (Barrot et al., 2000; Cory-Slechta et al., 1999; de Kloet et al., 1998; Diorio et al., 1993; Piazza et al., 1996; Rossi-George et al., 2009; Virgolini et al., 2008b), which is of particular note given the extensive interactions that occur between the HPA axis and mesocorticolimbic system in mediation of normal and abnormal behaviors (Belda and Armario, 2009; Locatelli et al., 2010; Spencer et al., 2004; Sullivan and Dufresne, 2006; Walker et al., 2008). Further, both developmental Pb exposure and PS have been associated with common outcomes, including alterations in cognitive functions (Anderson and Armstead, 1995; Bellinger et al., 1994; Bradley and Corwyn, 2002; Canfield et al., 2004; Canfield et al., 2003; Cory-Slechta, 1995; Dietrich et al., 2001; Dohrenwend, 1973; Needleman et al., 1996; Schwartz, 1994). These observations have served as the basis of studies from our laboratory seeking to test the hypothesis that combined exposures to Pb and PS could produce enhanced adverse effects relative to either alone.
Pb exposure has been associated with cognitive deficits, both in human populations and in animal models, with the most recent human cohort studies reporting reductions in IQ down to the lowest blood Pb (PbB) levels that can be reliably measured (Lanphear et al., 2005; Wilhelm et al., 2010). Increasing blood Pb levels have frequently been shown to produce impairments in discrimination learning, repeated learning, reversal learning and in set shifting (transfer of reinforced stimulus parameter) (Canfield et al., 2004; Cohn and Cory-Slechta, 1993; Cory-Slechta et al., 2010; Fan et al., 2010; Guilarte et al., 2003; McGlothan et al., 2008).
In contrast, PS has been associated with dichotomous effects on cognitive function. These contrasting effects likely reflect the growing recognition that early developmental insults such as stress can be highly adaptive, in some cases inducing a resiliency phenotype. Correspondingly, some studies report that early stress can lead to enhancements of cognitive function (Cannizzaro et al., 2006; Fujioka et al., 2001; Oomen et al., 2010; Parker et al., 2005) and to hypo-responsivity of the HPA axis in response to stress, and reduced emotionality and anxiety-like behaviors (Champagne et al., 2009), whereas others report cognitive impairments (Charil et al., 2010; King and Laplante, 2005; Laplante et al., 2004; Lordi et al., 2000). The nature of the outcome appears to depend both upon the magnitude and timing of the stressor, as well as the subject’s developmental status and corresponding capacity to adapt to the stressor (Kapoor et al., 2009; Lyons et al., 2009; Lyons et al., 2010). Moreover, resilient vs. psychopathological outcomes following stressors are both gender and context-dependent and thought to reflect the extent to which early HPA axis reprogramming effects can match requirements in later life environments (Champagne et al., 2008; Weinstock, 2007).
Underlying mechanisms for these differential effects of early stress are as yet incompletely understood, but appear to include dorsal raphe and dorsal striatal serotonin and prefrontal cortex glutamate and dopamine system involvement (Maier and Watkins, 2010; Strong et al., 2011), alterations in brain derived neurotrophic factor (Bergstrom et al., 2008; Cowansage et al., 2010; Feder et al., 2009), alterations in HPA axis function and associated glucocorticoid receptors (Rossi-George et al., 2011) and alterations in sex hormones (Brinton, 2009; Charney, 2004; Frye et al., 2008b; Rossi-George et al., 2011; Weinstock, 2007).
In a prior study, we found that female rats exposed to PS showed sequence-dependent deficits in repeated learning, particularly when combined with lifetime Pb exposure (Cory-Slechta et al., 2010). Given the prominent gender differences in PS outcomes (Weinstock, 2007) and the potential role of sex hormones in resilience (McEwen, 2002), the current study examined the impact of Pb, PS and combined Pb+PS on repeated learning, neurotransmitter levels and HPA axis function in male offspring. Data from both a cohort of rats exposed to maternal Pb (Cory-Slechta et al., 2004) as well as to lifetime Pb were available allowing as well for comparison of the impacts of these different Pb exposure conditions.
MATERIALS AND METHODS
Animals and Experimental Design
Male and female Long-Evans rats used for breeding were obtained from Charles River (Germantown, NY; maternal exposures described in Cory-Slechta et al., 2004) to generate a total of 4 experimental groups of dams: 1) Control: no prenatal stress (NS), no Pb exposure (0 ppm) (0-NS; n=14 and 12 dams for maternal and lifetime Pb exposure, respectively), 2) Prenatal stress (PS) only: prenatal stress (PS), no Pb exposure (0-PS; n=18 and 9 dams, respectively), 3) Pb only: no prenatal stress, Pb exposure (150 (maternal) or 50 (lifetime) ppm;150-NS or 50-NS; n=15 and 8 dams, respectively), and 4) Pb and PS: prenatal stress plus Pb exposure (150-PS or 50-PS; n=23 and 8 dams, respectively).
Dams began exposure to either 0 or 50 (lifetime exposure) or 150 ppm (maternal exposure) Pb acetate in drinking water two months prior to breeding. Maternal Pb exposure ended at offspring weaning, while lifetime Pb exposure was continued post-weaning in offspring. Initiation of Pb exposure two months prior to breeding was used to ensure a significant body burden of Pb as consistent with human environmental exposure. After the two initial two months of Pb exposure, males were placed with females for 5 days. Females were checked daily for the presence of a vaginal plug, and females who evidenced mating (day 1 of pregnancy) were removed and thereafter housed one per cage. Prenatal stress was carried out on gestational days 16 and 17 as described below. Litter sizes were checked at birth, culled to 8 pups per litter, with 4 males and 4 females, if possible, and pup body weights obtained periodically thereafter.
At weaning, offspring were housed one per cage (maternal Pb) or in same gender/treatment pairs (lifetime Pb) for behavioral experiments. This study describes effects in male offspring only. (Female siblings of the male maternal Pb exposure cohorts were utilized in other experiments and not tested here; repeated learning changes in female siblings of the male lifetime Pb exposure conditions have been previously reported (Cory-Slechta et al., 2010).) Male pups were given unrestricted access to food and drinking water until reaching approximately 55 days of age, when caloric intake was regulated until body weights reached 300 gm, at which level they were stabilized for the duration of the experiment through regulated caloric intake.
In the case of maternal Pb exposure, behavioral testing was initiated at approximately 10 months of age. In the case of lifetime Pb exposure, behavioral testing was initiated at approximately 2–3 mos of age. For both maternal and lifetime Pb cohorts, no more than a single offspring/litter was used from any treatment group for any of the outcome measures. Final sample sizes for the offspring groups were: 0-NS: n=9 and 12, 0-PS: n=10 and 12, for maternal and lifetime Pb, respectively, 150-NS and 50-NS: n=7 and 12, respectively, and 150-PS and 50-PS: n=10 and 12, respectively.
Prenatal Stress
Immobilization restraint stress was imposed on days 16 and 17 of gestation. Dams assigned to stress groups were placed in restraining devices (ITTC restrainer model 81 for rodents 250–400 gm; 63 mm (2.5″)) for 45 min 3 times on each of these two days at 900, 1200 and 1500 hours (Ward and Weisz, 1984). This stress paradigm was chosen because it targets development of key brain structures involved in mediating stress and cognition, including hypothalamic nuclei, hippocampus, striatum and frontal cortex (Weinstock et al., 1998), because of its relatively robust effects, its reported association with changes in mesolimbic dopamine systems (Alonso et al., 1994; Henry et al., 1995; Takahashi et al., 1992) and our prior demonstration of its efficacy in elevating corticosterone in dams (Cory-Slechta et al., 2004). Control or Pb only pregnant females (non-stress groups) were left undisturbed in their home cages.
Behavioral Test Apparatus
Behavioral testing was carried out in operant chambers housed in sound-attenuating enclosures ventilated by a fan. Each chamber was equipped with three response levers located on the front wall, i.e., left: (L), right: (R) and center: (C), located 3.5 cm apart 3.8 cm above the grid floor. A pellet trough through which 45 mg food pellets were dispensed was located below the center lever. Continuous white noise was used to mask any extraneous sounds. Behavioral contingencies and data collection for the maternal Pb exposure study were executed in operant chambers controlled using the SKED-11 system (Snapper et al., 1982) and lifetime Pb exposure by SoftCtrl™ Cumulative Record interface and Med-PC Version IV Research Control and Data Acquisition software.
Multiple Schedule of Repeated Learning (RL) and Performance (P)
As in our prior studies, lever press responding was first shaped in overnight sessions using automated procedures based on a concurrent variable time 20 sec fixed ratio 1 schedule of reinforcement (Cory-Slechta and Weiss, 1985). It produced non-contingent reinforcement deliveries at 20 sec intervals on average during the variable time component or if a response on the designated correct lever occurred. If a total of 10 lever press responses occurred, or 20 min elapsed, the variable time schedule terminated and only the fixed ratio schedule was in effect, wherein food delivery was contingent upon a depression of the designated correct lever. Sessions ended after the delivery of 100 reinforcers. Reinforcement was programmed for only one lever on each of three successive overnight training sessions, although the variable time 20 fixed ratio 1 schedule only operated on the first overnight training session. On the subsequent two overnight sessions, only the fixed ratio 1 schedule was in effect. This procedure ensured comparable reinforcement histories on all three levers prior to the implementation of repeated learning and performance testing to preclude lever bias.
Maternal Pb Exposure Behavioral Training
Following lever press training, a 3-response repeated learning training program was imposed, during which reinforcement was contingent upon completion of a designated sequence of three responses, with the correct sequence changing with each behavioral test session using the following sequences: LRL, LCR, RLR, CLR, CRL, LRC, RLC, CRC, RCL, LCL, CLC and RCR. Sequences were ordered across sessions such that no lever occupied the same position in the sequence from session to session. Sequences requiring a repetitive series of responses, e.g., LL, were excluded to prevent any reinforced history of response perseveration. Daily test sessions were initiated by house-light illumination and lasted 1 hr or until 100 reinforcers had been earned, whichever occurred first. Any error made during the sequence resulted in a time-out period during which the house-light was extinguished until 2 seconds occurred without a response. Errors also required the subject to start the sequence anew (correction procedure). All correct responses were signaled by presentation of a tone stimulus. Correct completion of a sequence produced the tone, the flash of the food cup light, the audible click of the pellet dispenser and food delivery. A total of 36 sessions were carried out during which accuracy across groups rose from 15–20% to 61–71%.
Lifetime Pb Exposure Behavioral Training
Lever press training was followed by two- and then 3-response sequence repeated learning training, as previously detailed (Cohn et al., 1993). Advancement from two- to 3-response sequence training occurred after 22 behavioral test sessions during which time accuracy levels across groups increased from 35–40% to 58–70%. The 3-response sequence training program was switched to the multiple schedule after 23 behavioral test sessions, during which time accuracy levels rose from 35–40% to 60–74%. Sequences used in 3-response training included CRL, RCL, CLR, RLC, LRC and LCR; sequences with duplicative responses, e.g., LLR, were excluded to minimize reinforcement of perseverative responding.
Multiple Schedule of RL and P
Following the 3-response repeated learning training phase, the multiple schedule of repeated learning and performance was imposed in both the maternal and lifetime Pb cohorts. The repeated learning component required completion of a 3-response sequence for each reinforcement delivery, with the correct sequence changing with each successive experimental test session, using the same sequences as in 3-response training described above for each cohort, with the exception of LCR. The performance component likewise required completion of a 3-response sequence for each reinforcement delivery, but in this case the sequence was constant across sessions. The sequence LCR served as the correct sequence for the performance components for both the maternal and lifetime Pb cohorts. This sequence was not used in training for the maternal Pb cohort. For the lifetime Pb cohort, it controlled the highest accuracy levels during training, and was thereby used for the purpose of maximizing the difficulty of the transitions between repeated learning and performance components of the session.
Each session consisted of two presentations each of the repeated learning component and the performance component in the order repeated learning 1, performance 1, repeated learning 2, performance 2. Repeated learning components lasted 10 min and the performance components lasted 5 min (maternal Pb) or 15 min or 25 reinforcer deliveries, whichever occurred first (lifetime Pb). The performance component was signaled by the onset of the stimulus lights above the three levers that remained illuminated during the entire performance component except during error-initiated timeout periods. The repeated learning components were signaled by the offset of the lever lights. A total of 33 (maternal Pb) or 56 (lifetime Pb) sessions were carried out on the multiple repeated learning and performance baseline.
Behavioral Measures of Multiple RL and P Performance
Percent correct was calculated as correct responses/total correct and incorrect responses. Rate of response was determined by total responses divided by total session time including responding during time-out periods. These measures were calculated separately for the 3-response repeated learning training phase and for the repeated learning and performance components of the multiple schedule. In addition, total session length was measured.
Monoamine Levels
Levels of dopamine (DA), the associated metabolites dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA), intracellular DA turnover (DOPAC/DA), NE (except striatum after maternal Pb exposure) and of serotonin (5-HT) and its metabolite 5-HIAA (HIAA) were determined using methods established in our laboratory (Thiruchelvam et al., 2000). Specifically, following rapid decapitation, specified brain regions were dissected out and placed in 0.1N perchloric acid. Tissues were sonicated and centrifuged for 20 min at 10,000 × g. The supernatants were stored at −80°C until analyzed by HPLC-EC. The pellets were digested in 1 ml of 0.5N NaOH for measurements of protein concentration using Bio-Rad assay reagents. Concentrations of neurotransmitters are expressed in terms of ng/mg protein.
Blood Pb Levels
Maternal Pb
Blood Pb levels following maternal Pb exposure were determined only in dams at offspring postnatal days 1 and 21and analyzed using anodic stripping voltammetry according to previously described methods used in our laboratory (Cory-Slechta et al., 1987; Widzowski and Cory-Slechta, 1994) and have been previously reported (Cory-Slechta et al., 2004). The limit of sensitivity of the assay is 5 ug/dl.
Lifetime Pb
For lifetime Pb exposure, blood Pb was measured in dams at 3 time points prior to breeding and again at weaning. Blood was collected from behaviorally-tested offspring at the completion of behavioral testing at approximately 10 mos of age, and from littermates of behaviorally tested offspring at postnatal days 5–6 and 2.5 mos of age. Blood Pb was analyzed by anodic stripping voltammetry using the Lead Care II system with a detection limit of 3.3 μg/dl. All 0-NS and 0-PS values were below detection limits as previously reported (Cory-Slechta et al., 2010).
Corticosterone
Maternal Pb
Dams
Blood was taken by tail nick from dams for determination of stress-induced corticosterone levels immediately after the 3rd restraint stress on the first stress day (gestational day 16). To avoid differences in corticosterone levels due to circadian rhythms, blood samples were collected no later than 1530 hr. Day 16 rather than day 17 was chosen since repeated stress can invoke adaptation that might obscure group differences (Haile et al., 2001; Orsini et al., 2002).
Offspring
Blood was collected via tail nick for determination of basal corticosterone levels in littermates of the subjects used in this experiment at week 9 of their adult behavioral testing, i.e., at approximately 9 mos of age, as previously reported (Cory-Slechta et al., 2004) and from male offspring used in this experiment at the completion of behavioral testing at approximately 14 mos of age.
Corticosterone was measured by competitive enzymeimmunoassay using a rabbit polyclonal corticosterone antibody (Octeia Corticosterone; Alpco Diagnostics, American Laboratory Products). Sensitivity of the assay is 0.23 ng/ml.
Lifetime Pb
Offspring
Trunk blood was collected from pups sacrificed at 5–6 days of age (0-NS=7, 0-PS=3, 50-NS=7, 50-PS=6), and by tail nicks from behaviorally-tested offspring at approximately 4 months of age, again at 8–9 months of age (0-NS=12, 0-PS=9, 50-NS=8 and 50-PS=8), and from a subset of non-behaviorally tested littermates at 10–11 mos of age (0-NS=5, 0-PS=4, 50-NS=3 and 50-PS=2). Approximately 200 μl of blood was collected into pre-chilled tubes and centrifuged at 1685 × G for 10 min, after which serum corticosterone was measured using ImmuChem™ Double Antibody Corticosterone 125I kit according to the manufacturer’s instructions (MP Biomedicals, Orangeburg, NY). All standards and samples were run in duplicate. The minimum detectable level in this assay was 7.7ng/ml as we have previously reported (Rossi-George et al., 2009).
Hippocampal Glucocorticoid Receptor Level Determinations
Maternal Pb Exposure
Tissue preparation and protein extraction
For the determination of cytosolic and nuclear glucocorticoid receptor levels, hippocampus was quickly dissected on an ice plate, immediately snap frozen on dry ice, and stored at −80 °C until use. Tissue was homogenized in a temperature-controlled manner (4°C) using a modification of a previously described procedure (Spencer et al., 2000). Tissue was processed in a solution of 0.5ml buffer/100 mg tissue using a hand-held 2 ml dounce glass-on-glass grinder in homogenization buffer consisting of 6 mM MgCl2, 1 mM EDTA, 10% sucrose, 1 mM phenylmethylsulfonyl fluoride, 3 mM benzamine, 5 ug/ml leupeptin, 1 ug/ml pepstatin A, 1 ug/ml antipain, 1 ug/ml aprotinin, 1 ug/ml soybean trypsin inhibitor, and 50 mM Tris at a final pH of 7.2. The homogenate was centrifuged at 2,000 g for 5 minutes in a refrigerated centrifuge. The supernantant was ultracentrifuged at 105,000 g for 30 minutes yielding the cytosolic fraction. For the nuclear extract, the pellet from low speed centrifugation was re-suspended twice in 0.5 ml homogenizing buffer and centrifuged at 2000 g for 5 minutes. The pellet was then resuspended in 0.25 ml of homogenization buffer containing 0.5 M NaCl, incubated in an ice bath for 1 hour with frequent shaking, and finally centrifuged at 8000 g for 10 min. The resulting supernatant was collected as the nuclear extract. Protein levels were determined in an aliquot of each fraction using a commercially available Bio-Rad kit. Protein concentrations ranged from 4.5 to 7.5 mg/ml for cytosol and 2.0 to 4.0 mg/ml for the nuclear preparation.
Western blotting
Sample loading was counterbalanced across groups and adjusted to a final protein concentration of 40 μg/protein for both cytosol and nuclear assays. Samples were denatured by mixing and boiling with 100 μl of sample buffer containing 10% SDS, 2.5 M sucrose, 0.2 M Tris, 0.02 M EDTA, pH 8.0, 10% 2-mercaptoethanol and bromophenol blue. Proteins were separated by electrophoresis on 3% Bis-polyacrylamide gels and electroblotted overnight onto nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ). Membranes were then blocked for 1 h in a Tris-buffered saline solution containing 5% Carnation dry milk, 50 mM Tris, 0.05% Triton X-100, 2 mM CaCl2, and 0.02% NaN3, at a pH of 7.4. They were subsequently washed with TBS-TX for 25 min and incubated overnight at 4 °C with primary antibody (rabbit polyclonal antibody PA1510A diluted 1:2,000 in TBS-TX with 3% milk for cytosol; mouse monoclonal antibody BuGR2 -MA1-510- diluted 1:6,000 in TBS-TX with 3% milk for nucleus; Affinity Reagents Inc, Golden, CO). After washing for 25 min, membranes were incubated for 2 hr at room temperature with horseradish peroxidase-linked secondary antibody (anti-rabbit antibody diluted 1: 2,000 in TBS-TX with 3% milk, BioRad Laboratories, Hercules, CA for the cytosolic fraction; and anti-mouse antibody 1:10,000, Jackson Immunoresearch Laboratories Inc., West Grove, PA, for the nucleus). The membranes were then washed with TBS-TX for 35 min and proteins visualized on X-ray film (X-OMAT AR Autoradiography film, Kodak, Rochester, NY) using enhanced chemiluminiscence reagents (West-Pico substrate for cytosol and West-Femto for nucleus; Pierce Biotechnology Inc., Rockford, IL). The bands obtained were compared with Kaledoiscope pre-stained standard (BioRad Laboratories, Hercules, CA) to ensure that the most prominent band visualized corresponded to 97 KDa representing glucocorticoid receptor molecular mass. Semi-quantitative densitometric analysis of GR signals was carried out using Scion, a Macintosh-driven-imaging processing software. Relative measurements of glucocorticoid receptor protein levels were determined in the darkest region of the band with the background intensity of the film subtracted-out.
Lifetime Pb Exposure
Hippocampal cytosolic and nuclear glucocorticoid receptor density values were reported for littermates of males from the current study that had undergone Fixed Interval behavioral testing (Rossi-George et al., 2011).
Statistical Analyses
Blood Pb, Corticosterone and Neurotransmitter Levels
Blood Pb levels, corticosterone and glucocorticoid receptor levels were analyzed using ANOVAs with time point and stress (NS, PS) as between group factors as appropriate to experimental conditions. Post-hoc tests were carried out using Fishers Protected Least Significant Difference tests.
Neurotransmitter levels of offspring were analyzed using ANOVA with both Pb and stress as between group factors. In the case of significant interactions of Pb by stress, one-way ANOVAs were carried out comparing the 4 treatment groups (0-NS, 0-PS, 150-NS and 150-PS). For neurotransmitter levels, ANOVAs were carried out separately for each neurotransmitter in each brain region and for maternal and lifetime Pb exposures.
Behavioral Performance
Changes in percent correct and response rate for 3-response training (sequence effects) and for the multiple repeated learning and performance schedule were analyzed using repeated measures ANOVAs with both Pb and stress as between group factors and sessions as a within group factor. Outcomes were examined separately by sequence, by component (repeated learning and performance) for the multiple schedule, and separately for maternal vs. lifetime Pb exposure. In the event of main effects or interactions, subsequent ANOVAs were carried out comparing the 4 groups at specific time points. Changes in accuracy from repeated learning component 1 to repeated learning component 2 were analyzed using repeated measures ANOVAs.
RESULTS
Blood Pb (PbB) Levels
Maternal Pb
As previously reported (Cory-Slechta et al., 2004), group mean ± SE blood Pb values of dams averaged <5 μg/dl for the 0-NS and 0-PS groups, 32.6 ± 4.4 for the 150-NS group and 40.3 ± 2.1 μg/dl for the 150-PS group at postnatal day 1, and < 5 μg/dl for the 0-NS and 0-PS groups, 42.7 ± 4.0 μg/dl for the 150-NS group and 38 ± 3.3 μg/dl for the 150-PS group at postnatal day 21. While Pb significantly increased blood Pb levels at both time points (p<0.0001), values were not influenced by PS.
Lifetime Pb
As previously reported (Cory-Slechta et al., 2010), blood Pb levels of dams averaged 5–7 μg/dl prior to breeding, but had increased to 13 μg/dl by the end of weaning (time: F=23.26, p<0.0001). Offspring blood Pb levels for 50-NS and 50-PS groups were: postnatal day 5–6: 12.5 ± 1.46 and 12.89 ± 1.07; 2.5 months of age: 6.43 ± .38 and 6.75 ± .849; final: 8.98 ± .632 and 9.13 ± .75μg/dl, respectively, and were not influenced by PS.
Multiple Schedule of Repeated Learning and Performance
Overall Accuracy
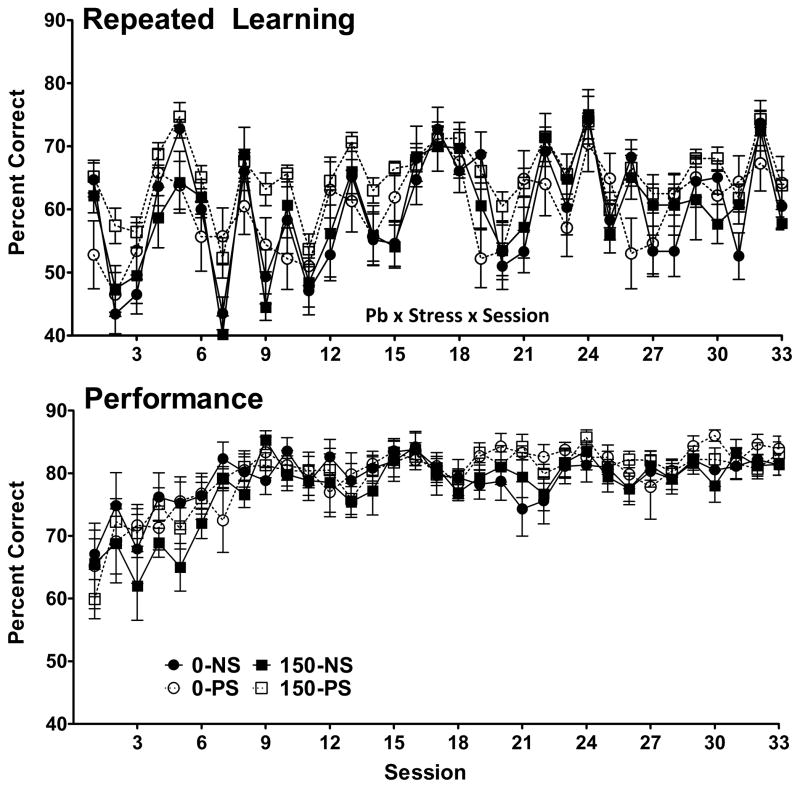
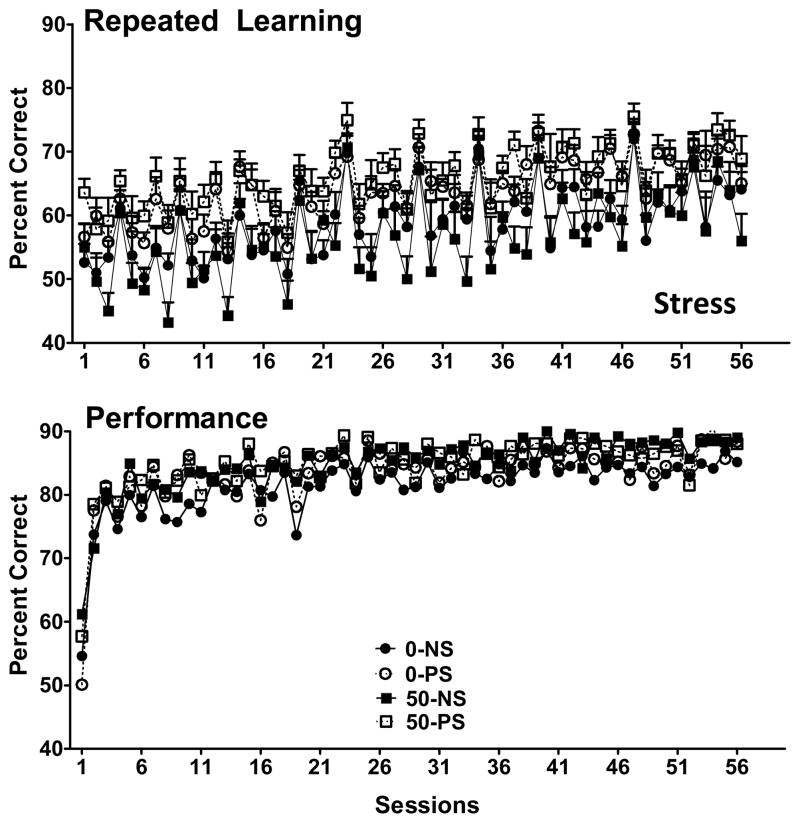
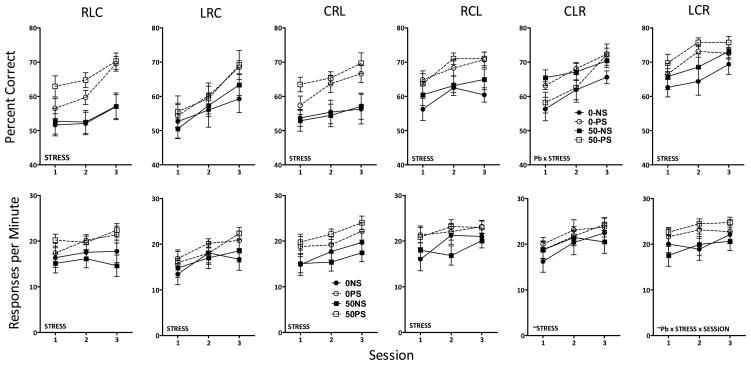
Changes in accuracy (percent correct) in the repeated learning (top) and performance (bottom) components of the multiple schedule are shown in Figure 1 for maternal Pb and Figure 2 for lifetime Pb. As expected, accuracy in the repeated learning component was lower than in the performance component, both with maternal and lifetime Pb cohorts. In the performance component, neither Pb nor PS influenced accuracy either for maternal or lifetime Pb cohorts, which rose over the first 10 sessions to approximately 80–90% where they remained for the duration of testing. In contrast, for the repeated learning component, a Pb x PS x session interaction (F(32,992)=1.87, p=0.0025) was found after maternal Pb exposure, as was a main effect of PS for lifetime Pb (F(1,2420)=14.7, p=0.0004), the latter based on modest but significantly higher accuracy values across sessions in the 0-PS and 50-PS groups. Repeated learning accuracy was characterized by substantial variability from session to session (i.e., from sequence to sequence). Indeed, an ANOVA examining accuracy based on first session data across sequences during the repeated learning 1 component of the multiple repeated learning and performance schedule in the maternal Pb cohort confirmed a highly significant main effect of sequence after maternal Pb (F(10,351)=15.95, p<0.0001).
Figure 1.
Group mean ± S.E. values for percent correct (accuracy) during the repeated leaning (top) and performance (bottom) components of the multiple schedule of RL and P schedule across sessions for groups exposed to maternal Pb and PS. Values represent averages of RL1 and RL2 and P1 and P2. Group sample sizes were 9, 10, 7 and 10 for the 0-NS, 0-PS, 150-NS and 150-PS groups, respectively. Pb x stress x session = interaction from the repeated measures ANOVA.
Figure 2.
Group mean ± S.E. values for percent correct (accuracy) during the repeated leaning (top; RL1) and performance (bottom; P1) components of the multiple schedule of RL and P schedule across sessions for groups exposed to lifetime Pb and PS. Group sample sizes were 12 for all groups. Stress = main effect of PS from the repeated measures ANOVA.
Overall Response Rates
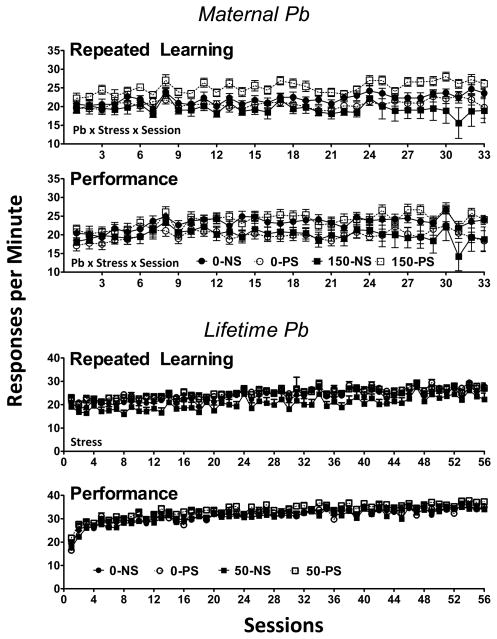
Response rates across sessions are shown for the repeated learning and performance components for maternal (top two rows) and lifetime Pb (bottom two rows) in Figure 3. Repeated learning and performance response rates were generally comparable with maternal Pb, with mean values ranging from 15–25 responses per minute. A Pb x PS x session interaction was found in both the repeated learning (F(32,992)=1.76, p=0.006) and performance (F(32,992)=1.85, p=0.003) components. In repeated learning, 150-PS produced the highest responses rates, and 150-NS the lowest response rates. ANOVAs carried out separately at each session confirmed generally elevated levels of the 150-PS group beginning at session 3 relative to all other groups (depending upon sequence, see below), whereas few consistent differences were found among the other 3 groups (0-NS, 0-PS, 150-NS). Group differences were less systematic in the performance component, and generally attributable, when they occurred, to increases of the 150-PS group relative to 0-PS and/or 150-NS groups.
Figure 3.
Group mean ± S.E. response rates (responses per minute) values during the repeated leaning and performance components of the multiple schedule across sessions for maternal Pb and PS (Top two panels) and lifetime Pb and PS (bottom two panels). Sample sizes and conditions are as described for Figures 1 and 2. Pb x stress x session = interaction from the repeated measures ANOVA. Stress = main effect of PS from the repeated measures ANOVA. Pb x stress x session = interaction from the repeated measures ANOVA.
For the lifetime Pb cohort, response rates ranged from 15–30 responses per minute in the repeated learning component and 25–30 responses per minute in the performance component. Whereas no effects of Pb ± PS on response rates in the performance component were seen, a main effect of PS was found during the repeated learning component (F(1,2464)=4.4, p=0.042) based primarily on generally lower levels of the 50-NS group relative to all other groups.
Sequence Dependent Effects of Pb ± PS on RL Accuracy
Maternal Pb Exposure and 3-Response Repeated Learning Training
No PS ± Pb-induced changes in accuracy were seen during 3-response training.
Maternal Pb Exposure and Multiple Repeated Learning and Performance Schedule
Accuracy
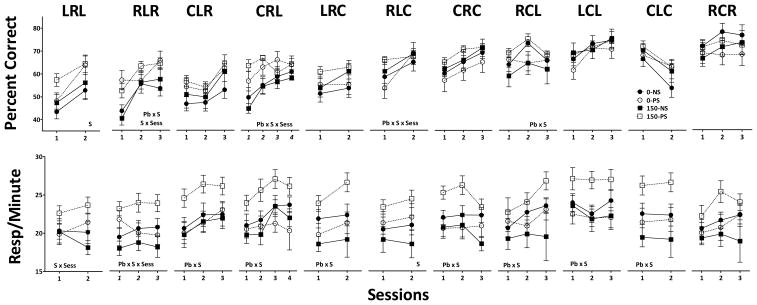
Accuracy values for individual sequences are presented in Figure 4 (top row) in order of increasing 0-NS accuracy levels (worst to best) in session one from left to right. Accuracy levels during session one of control (0-NS) subjects were essentially tri-modal, with 5 of the 11 sequences (LRL, RLR, CLR, CRL, LRC) producing values ranging from 43–52%, 2 sequences (RLC, CRC) generating intermediate accuracy levels (58–60%), and the final 4 sequences (RCL, LCL, CLC, RCR) associated with initial higher accuracy levels ranging from 63–66%.
Figure 4.
Group mean ± S.E. values for percent correct (top) and for response per minute values averaged over RL1 and RL2 over the presentations of each of the RL sequences, as identified, over the course of testing on the multiple RL and P schedule for maternal Pb and PS. Sequences are arranged in order from left to right of ascending percent correct levels (hardest to easiest) of the 0-NS group on session one. Corresponding response rate values are shown in the bottom row. Sample sizes are as described in Figure 1. S=main effect of PS, Pb x S=interaction of Pb by PS, S x Session=interaction of PS by session; Pb x S x session= interaction of Pb by PS by session in repeated measures ANOVA.
PS ± Pb increased repeated learning accuracy for some sequences, particularly those controlling lower initial accuracy levels (i.e., the ‘harder sequences’). Specifically, PS increased LRL accuracy across the two sessions during which it was presented (F(1,32)=6.03, p=0.0197), although this increase was most evident for the 150-PS group at session 1 (+33% relative to 0-NS), but seen in both the 0-PS and 150-PS groups at session 2 (+23% relative to 0-NS). RLR accuracy was modified by both Pb (Pb x session (F(2,64)=5.76, p=0.005) and PS (PS x session (F(2,64)=3.875, p=0.026). These outcomes were primarily due to PS-related increases in accuracy in session 1 (p=0.0008), but selectively to a 150-PS increase in session 2 relative to 0-NS and 0-PS (p=0.035 and 0.024, respectively); the 150-NS group did not differ from any other group. For CRL, a Pb x PS x session interaction (F(3,96)=2.70, p=0.0497) reflected increased 150-PS accuracy relative to 0-NS and 150-NS groups (p=0.009 and 0.003, respectively) and increased 0-PS accuracy relative to 150-NS (p=0.035) in session 1, and higher 150-PS accuracy relative to 0-NS and 150-NS (p=0.008 and 0.017, respectively), and 0-PS increases relative to 0-NS (p=0.039) in session 2. Additionally, a Pb x PS x session interaction for RLC (F(1,32)=4.18, p=0.049) was due to the increased 150-PS accuracy relative to 0-PS (p=0.0396) at session one.
Response Rates
PS ± Pb-associated response rate changes were found for all sequences except RCR (bottom row Figure 4) that included significant, or near significant, Pb x PS interactions (CLR: (F(1,30)=4.03, p=0.0537); CRL: (F(1,31)=3.387, p=0.075); LRC: (F(1,32)=9.24, p=0.0047); CRC: (F(1,32)=7.69, p=0.009); RCL: (F(1,32)=3.876, p=0.057); LCL: (F(1,32)=5.45, p=0.026); CLC: (F(1,32)=7.17, p=0.0116) or Pb x PS x session interactions (RLR: (F(2,64)=3.51, p=0.036). Post hoc analyses confirmed that these Pb x PS interactions were almost invariably due to increased rates in the 150-PS group relative to all 3 other groups. For sequence LRL, a PS x session interaction (F(1,32)=11.32, p=0.002) was due to elevated rates of the PS groups (0-PS and 150-PS) during session 2. Additionally, PS groups exhibited higher response rates in the presence of sequence RLC (PS: F(1,32)=4.37, p=0.045).
Lifetime Pb Exposure and 3-Response Repeated Learning Training
Accuracy
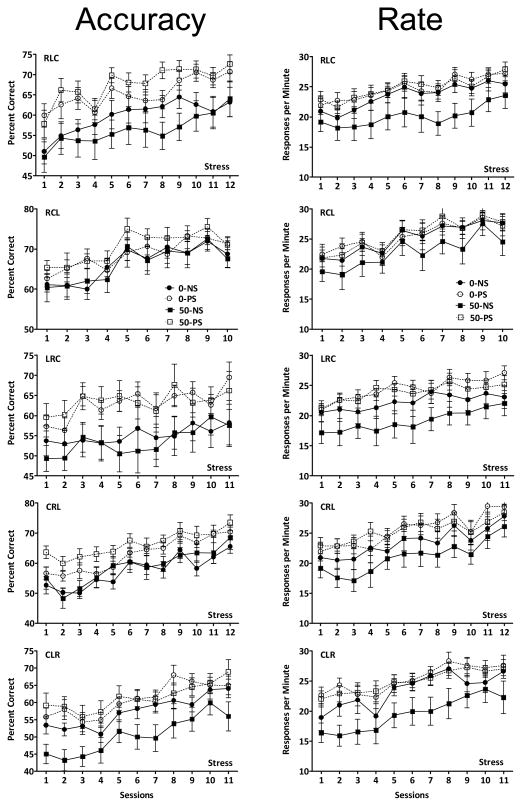
PS-related increases in accuracy were found for 5 of the 6 sequences for the lifetime Pb exposure group as shown in Figure 5 (top row) arranged in order of increasing 0-NS accuracy values (worst to best) in session 1 from left to right. PS increased accuracy levels for sequences RLC (F(1,88)=15.16, p=0.0003), CRL (F(1,88)=13.20, p=0.0007), CLR (F(1,88)=11.03, p=0.0018) and LCR (F(1,88)=5.01, p=0.03). For 3 of these 5 sequences (RLC, CRL and LCR), mean accuracy levels of the 50-PS group were higher, albeit not statistically significantly, than those of the 0-PS group. For sequence CLR, a Pb x PS interaction (F(1,88)= 5.35, p=0.0255) reflected higher initial 0-PS and 50-NS accuracy levels relative to 0-NS and 50-PS during the initial two sessions.
Figure 5.
Group mean ± S.E. values for percent correct (top) and for response per minute values in RL1 over the presentations of each of the RL sequences, as identified, over the course of three lever training for lifetime Pb and PS. Sequences are arranged in order from left to right of ascending percent correct levels (hardest to easiest) of the 0-NS group on session one. Corresponding response rate values are shown in the bottom row. Sample sizes are as described in Figure 2. S=main effect of PS, Pb x S=interaction of Pb by PS, Pb x S x session= interaction of Pb by PS by session in repeated measures ANOVA.
Response Rate
PS-based increases in response rates were found for all 6 sequences with modest but significant or marginally significant effects (main effect of PS: RLC: F(1,88)= 5.34, p=0.0255; CRL: F(1,88)= 5.48, p=0.0238; CLR: (F(1,88)=4.73, p=0.035; LCR; F(1,88)=3.58, p=0.065; RCL: Pb x PS x session (F(2,88)=2.64, p=0.0767).
Lifetime Pb Exposure and Multiple RL and P Schedule
Accuracy
PS also increased RL accuracy in a sequence-specific manner in the multiple schedule (Figure 6; left column), with main effects of PS for 4 of the 5 sequences (RLC: F(1,484)=11.86, p=0.0013; LRC: F(1,440)=7.09, p=0.01; CLR: F(1,484)=12.01, p=0.0012; CRL: F(1,440)=9.07, p=0.0043). Interestingly, however, sequences associated with PS enhancements here differed somewhat from those observed during 3-response training. Specifically, PS-related increased RLC accuracy was sustained, but the PS-related increase in RCL accuracy during 3-lever training was no longer evident. PS did not increase LRC or CLR accuracy during 3-lever training, whereas significant PS enhancements were observed during the multiple schedule, particularly for LRC. As during 3-response training, mean values of the 50-PS group were often higher, although not statistically significantly, than those of the 0-PS group.
Figure 6.
Group mean ± S.E. values for percent correct (left) and for response per minute values (rate; right) in RL1 over the presentations of each of the RL sequences, as identified, over the course of the multiple schedule for lifetime Pb and PS. Sequences are arranged in order from left to right of ascending percent correct levels (hardest to easiest) of the 0-NS group on session one. Sample sizes are as described in Figure 2. S=main effect of stress in repeated measures ANOVA.
Response Rates
Sequences showing PS-based increased accuracy likewise showed PS-based response rate alterations (RLC: F(1,484)=4.44, p=0.0408; LRC: F(1,440)=5.05, p=0.0297; CLR: F(1,484)=4.32, p=0.0435; CRL: F(1,440)=5.65, p=0.0218). However, as can be seen from Figure 6 (right column), although PS-based in statistical analysis, these effects appear to derive primarily from the generally lower response rate of the 50-NS group across sequences during the multiple schedule.
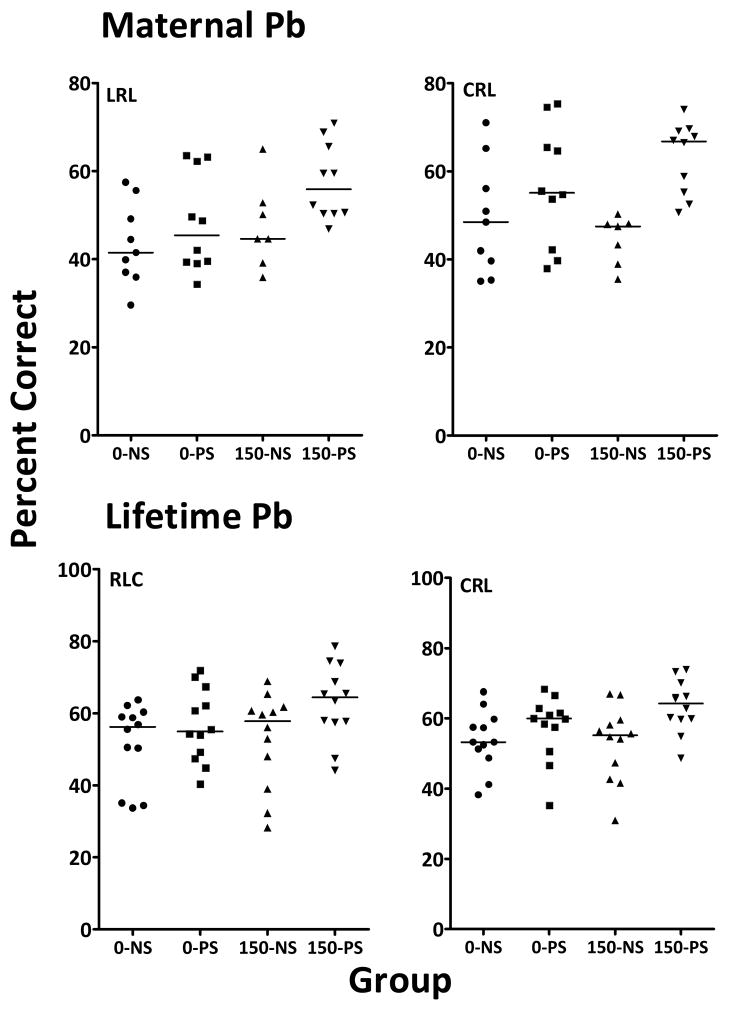
Individual Animal Data
Accuracy values of individual animals within each treatment group for session one of identified sequences associated with PS-based enhancement are shown in Figure 7 for maternal (top) and lifetime (bottom) Pb exposure conditions. Accuracy values of 150-PS subjects (maternal Pb) were uniformly shifted to the high end of the range of values shown by controls (0-NS) for both sequences, and this effect was also evident for 0-PS subjects for sequence CRL. Similarly, accuracy values of 50-PS subjects (lifetime Pb) were shifted towards or even above the range of control values, effects likewise seen with the 0-PS group for sequence CRL.
Figure 7.
Individual percent correct values for each group from session 1 for identified sequences for maternal Pb (top row) during the multiple schedule of repeated learning and performance, and from session 1 for identified sequences for lifetime Pb and PS during 3-lever training. Dashed line indicates group median value.
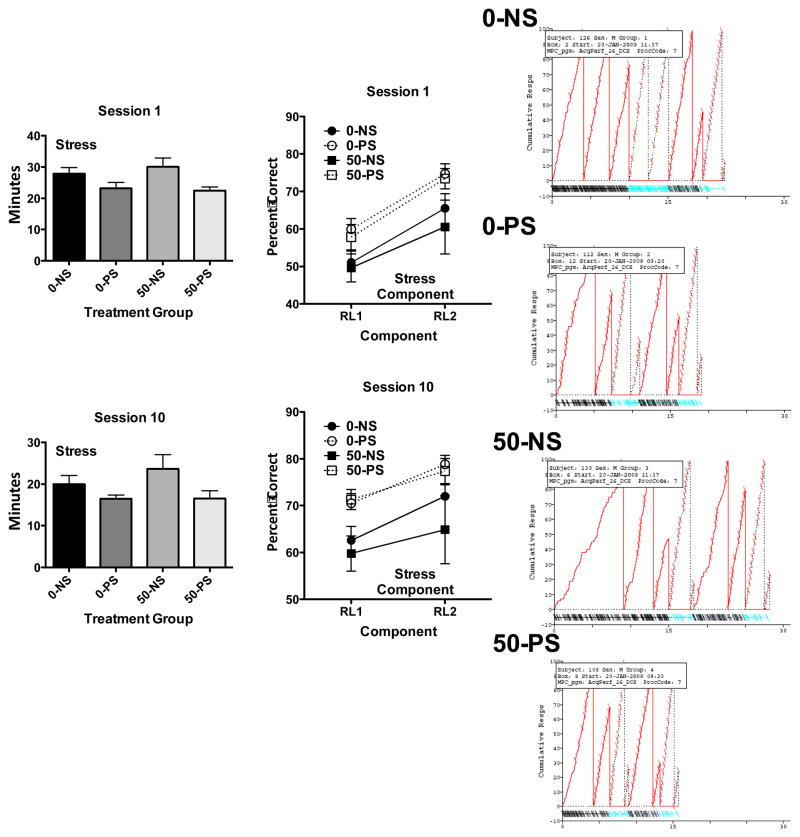
Session Length and Within-Session Learning
Session length (minutes) for sessions 1 and sessions 10 of the sequence RLC during the multiple schedule of repeated learning and performance after lifetime Pb exposure were significantly reduced by PS (F(1,44)=9.44, p=0.0037 and F(1,44)=5.67, p=0.022, respectively) by levels ranging from 17–19% (Figure 8, left column). The shorter session lengths were also evident from cumulative records of performance (right column from session 10) here depicting the subject with the man value from each treatment group. That within-session learning was occurring is evident from the significant increases in within-session percent correct from repeated learning component 1 to repeated learning component 2 in both sessions 1 and 10 (middle column; F(1,44)=65.68, p<0.0001 and F(1,44)=22.65, p<0.0001, respectively). Consistent with the outcomes depicted in Figure 6 (top left graph), PS was also associated with higher accuracy levels in both sessions (F(1,44)=7.64, p=0.0083 and F(1,44)=9.09, p=0.0042, respectively).
Figure 8.
Left: Group mean ± S.E. values for session length (min) for session 1 (top) and session 10 (bottom) of sequence RCL during the multiple schedule for lifetime Pb-exposed treatment groups. Middle: Group mean ± S.E. values for percent correct from RL1 to RL2 for session 1 (top) and session 10 (bottom) for sequence RCL during the multiple schedule for lifetime Pb-exposed treatment groups. Right: Representative cumulative records from session 10 of sequence RCL. Responses cumulate vertically to 100 at which point the pen resets. RL components are indicated by solid line resets; P components by dashed line resets. Pips indicate each reinforcement delivery for correct completion of a sequence. The bottom tracing shows errors during each of the components.
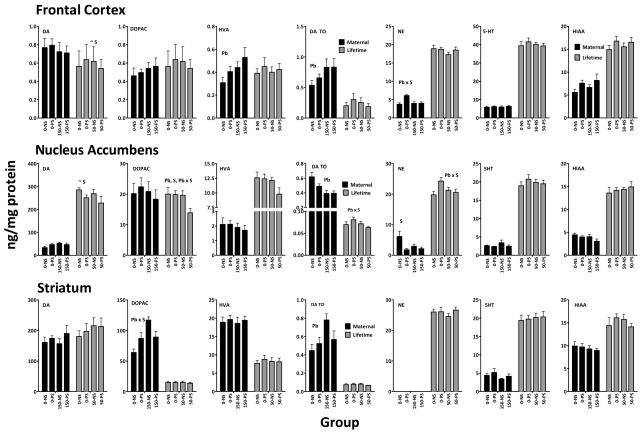
Neurotransmitter Changes
Monoaminergic neurotransmitter changes determined at the completion of behavioral testing were compared primarily for the purpose of assessing any patterns of or profiles of changes which might overlap and thus contribute to the corresponding accuracy enhancements observed with both maternal and lifetime Pb (Figure 9). The only suggestive pattern of correspondence was seen in nucleus accumbens, particularly in levels of metabolites and dopamine turnover. For DOPAC, HVA and DA turnover, lowest values, albeit not necessarily statistically significant, were observed in the Pb+PS groups after both maternal and lifetime Pb. Additionally, group mean values of frontal cortex 5-HIAA were higher in PS and Pb+PS groups in both maternal and lifetime Pb conditions. No other corresponding patterns were observed.
Figure 9.
Group mean ± S.E. levels (ng/mg/protein) of frontal cortex, nucleus accumbens and striatal DA, DOPAC, HVA and DA turnover (DA TO), NE and 5-HT and 5-HIAA at the completion of behavioral testing for the treatment groups, as indicated from the maternal and lifetime Pb and PS conditions. Group sample sizes as described in Figure 1 and 2, respectively Pb=main effect of Pb; S=main effect of PS; Pb x S=interaction of Pb with PS in the two factor ANOVA.
Corticosterone and Hippocampal Glucocorticoid Receptors
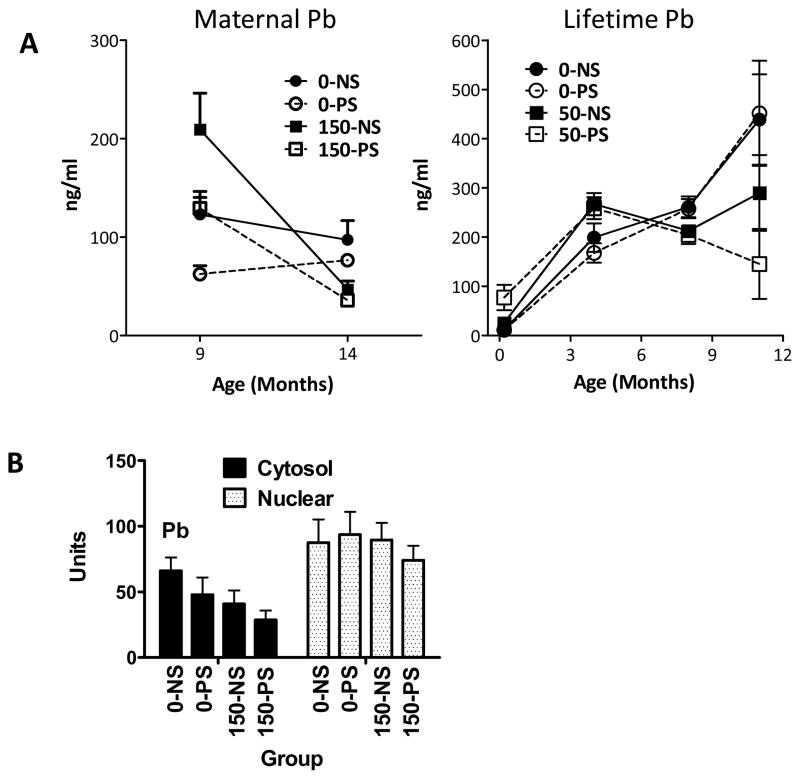
Maternal Pb
Corticosterone levels determined at the completion of behavioral testing (approximately 14 mos) in subjects are shown in Figure 10 (panel A, left) along with values from littermates of approximately 9 mos of age, collected following 9 weeks (males) of behavioral testing using a fixed interval schedule of food reinforcement (Cory-Slechta et al., 2004) for comparative purposes. As previously reported (Cory-Slechta et al., 2004), Pb exposure significantly increased corticosterone levels at 9 mos of age, effects not modified by PS. However, at 14 mos of age, Pb significantly decreased corticosterone levels by 42–53% (F(1,32)=24.334, p<0.0001), but PS again did not influence levels.
Figure 10.
A: Group mean ± S.E. levels (ng/ml) of corticosterone following maternal and lifetime Pb exposure for groups as indicated. Sample sizes ranged from 7–10 for maternal Pb and 3–7 (PND 5–6), 8–12 for 4 and 8–9 mos and 2–5 for 10–11 mos of age (post-behavioral testing) in lifetime Pb exposure groups. B: Group mean ± S.E. hippocampal GR cytosolic and nuclear GR density in response to maternal Pb exposure. Sample sizes range from 6–8. Pb indicates main effect in the two factor ANOVA.
Levels of hippocampal cytosolic glucocorticoid receptors determined at the completion of behavioral testing (14 mos) were decreased by maternal Pb exposure (F(1,25)=4.74, p=0.039) particularly when combined with PS (−57%), whereas nuclear GR levels were comparable across groups.
Lifetime Pb
Corticosterone values were available from males at several time points in the lifetime Pb cohort (Figure 10, panel A, right). Pb marginally increased corticosterone at postnatal days 5–6 (F(1,16)=4.07, p=0.0607), effects that were more pronounced (+35%) at 4 mos of age (F(1,43)=11.3, p=0.0016). However, by the completion of behavioral testing (8–9 mos of age), Pb produced modest (−22%) but significant reductions in corticosterone (F(1,44)=8.07, p=0.0068), which were even more pronounced (up to −68%) at 10–11 mos of age (F(1,12)=5.56, p=0.036).
DISCUSSION
This study sought to determine whether Pb exposure when combined with PS would enhance deficits in repeated learning compared to either alone, as previously observed in female rats (Cory-Slechta et al., 2010). However, in contrast to outcomes with females, PS tended to improve accuracy of repeated learning in males, with some additional evidence of further enhancement of this effect under conditions of Pb +PS, effects that were sequence-specific and not seen in the performance component where no learning was involved after initial multiple schedule training. These enhancements emerged during the multiple repeated learning and performance schedule following maternal Pb, whereas they emerged earlier during the 3-response training that precedes imposition of the multiple repeated learning and performance schedule with lifetime Pb exposure. For maternal Pb, enhancement of accuracy was generally seen during the earliest sessions in which the sequence occurred, after which the control (0-NS) and Pb only groups achieved comparable levels of accuracy (Figure 4), suggesting that this effect was not an enhancement of accuracy levels per se, but more rapid attainment of maximal percent correct levels. For lifetime Pb, however, there appeared to be a sustained PS and particularly PS+Pb enhancement that persisted even for the duration of multiple RL and P schedule testing (Figure 6), suggesting a more pronounced impact of lifetime Pb relative to maternal Pb.
The similarity of outcomes in males is particularly striking given the differences in many significant parameters of these cohorts, including Pb exposure levels and timing of exposure, housing conditions, age of animals at the time of behavioral testing, and differences in training and sequences used, among others. The findings are similar to other studies reporting PS-related cognitive enhancement. Cannizzaro et al. (Cannizzaro et al., 2006), using a can test that required rats to identify a single rewarded can, identified by white tape rather than white paint among 7 cans, found enhanced accuracy levels in adolescent male offspring whose dams had been subjected to a protracted immobilization on gestational day 16. Notably, learning was even further enhanced when a subsequent stressor followed the prenatal stressor, daily brief maternal separation from days 2–21; these enhancements did not reflect associated changes in corticosterone. Both stressors alone also increased the time spent in the open arms on an elevated plus maze, interpreted as reducing anxiety. Similarly, Fujioka et al. (Fujioka et al., 2001) reported enhanced 8 arm radial maze learning in male offspring following exposure of dams to single 30 min restraint stress sessions on gestational days 15–17, effects likewise not attributable to corticosterone. The ability of glucocorticoids and prenatal stress to either impair or enhance learning and memory (Champagne et al., 2008) appear to depend upon the magnitude of the prenatal stress, with enhanced effects said to arise from milder prenatal stressors, and to reflect the timing of the prenatal stressor (Kapoor et al., 2009), gender (Mueller and Bale, 2007) and prior behavioral experience (Yang et al., 2003).
While the direction of PS ± Pb effects on repeated learning in males was opposite to that seen with females (Cory-Slechta et al., 2010), they were similar to females in their sequence- dependence. The gender-specific outcomes show a striking parallel to sex specific effects in reversal learning in juvenile baboons following late second-early third trimester administration of betamethasone, a synthetic glucocorticoid (Rodriguez et al., 2011), in which males tended to show reductions in errors while females showed enhanced error levels, effects the authors attribute to altered programming. Moreover, these sex-specific changes were also highly discrimination problem specific. While there were some differences in the specific sequences enhanced by maternal vs. lifetime Pb, likely reflecting differences both in training conditions and sequences used in testing (i.e., sequences with repeats on one lever, e.g., RLR, were used in the maternal Pb study, but not with lifetime Pb) in the two cohorts, they tended to share the feature of occurring in sequences which required the moving from one side of the chamber to the other (e.g., R to L or L to R sequences).
Mechanisms by which such enhancements occur, both behavioral and biochemical, are of clear interest both for understanding brain-behavior relationships and for the development of therapeutic strategies. One likely behavioral mechanism of these effects is a higher reinforcement density arising from the increased response rates seen with both maternal and lifetime PS and PS+Pb groups (Figure 3–5). Increased response rates can shorten the time to reward delivery and thereby generate a higher reinforcement density. Indeed, session lengths were significantly shortened in lifetime PS and PS+Pb groups (Figure 8) and since the paradigm involved fixed numbers of reinforcers per component, would also increase reinforcement density and time to reward It is well established that delayed reward retards learning (Saltzman, 1951; Wolfe, 1934) and that learning is highly sensitive to reinforcement density (Ito and Nakamura, 1998). In support of this potential behavioral mechanism, response rates of lifetime Pb+PS females who exhibited enhanced cognitive deficits did not differ relative to controls. Furthermore, consistent and strong correlations between accuracy levels and response rates for both for the multiple repeated learning and performance schedule for maternal Pb exposure and for both 3-response training and multiple repeated learning and performance sessions for lifetime Pb in males were found in exploratory linear regression analyses (not shown).
To determine whether maternal and lifetime Pb ± PS treatment revealed any shared neurotransmitter changes that might provide some suggestive pathways/regions of involvement, neurotransmitter levels were examined at the completion of behavioral testing. The only shared effect, and one minor in magnitude, was a reduction in nucleus accumbens DA function particularly with Pb+PS in response to lifetime Pb exposure conditions and similar but non-significant trends with maternal Pb. Several caveats are attendant to such an interpretation, however. We have previously demonstrated that behavioral testing per se modifies neurotransmitter levels (Rossi-George et al., 2011), with behavioral testing generally attenuating Pb ± PS effects. Therefore, the post-behavioral neurotransmitter measured changes shown here may actually reflect adaptations rather than the initial changes induced by Pb and or PS. Further, these data reflect a single point in time, and not necessarily dynamic changes, in addition to the other experimental differences between the maternal and lifetime Pb conditions. Despite the caveats, however, it is notable that females with lifetime Pb+PS associated cognitive deficits actually showed the opposite change, i.e., increases in nucleus accumbens DA turnover that were significantly correlated with the corresponding deficits in behavioral performance (Cory-Slechta et al., 2010). Determining the role of neurotransmitter changes in these behavioral changes would be significantly assisted by time course data comparing neurotransmitter changes in behaviorally-tested vs. non-behaviorally tested littermates.
Corticosterone levels were ultimately decreased by Pb and Pb+PS after both maternal and lifetime exposures. Glucocorticoids facilitate long-term memory in a biphasic capacity, e.g., reductions in accuracy in a radial arm maze were seen when levels were either increased or decreased below control conditions (Park et al., 2006). However, current findings are at odds with this interpretation, given that lower corticosterone levels were herein associated with increased accuracy. However, it is important to note that both maternal Pb exposure and PS permanently alter HPA axis function (Rossi-George et al., 2009) and may consequently alter the magnitude/shape of this biphasic dose-effect curve for glucocorticoid effects on behavior as well.
As observed after maternal Pb exposure (Figure 10; not measured with lifetime Pb exposure), hippocampal glucocorticoid receptor levels declined in response to Pb, with a slightly (albeit not significantly) greater drop with Pb+PS, findings consistent with the corresponding decrements in corticosterone in the maternal Pb cohort. After lifetime Pb exposure, however, 50-PS was associated with significantly increased hippocampal cytosolic glucocorticoid receptor density, and Pb per se with increases in nuclear glucocorticoid receptor density (Rossi-George et al., 2011), suggesting that the differential glucocorticoid receptor changes produced maternal vs. lifetime Pb are not a likely mechanistic explanation for the common learning enhancements seen with PS ± Pb. Moreover, like brain neurotransmitter levels (Cory-Slechta et al., 2009), corticosterone levels and corresponding profiles of Pb ± PS-associated changes are modified by behavioral experience (Virgolini et al., 2008a), and thus could reflect adaptations rather than initial Pb ± PS-induced effects.
Androgens have also been shown to enhance performance (Cherrier et al., 2001; Osborne et al., 2009). Testosterone enhanced water maze performance of male rats, an effect that may be mediated by intracellular androgen receptors in dorsal hippocampus (Edinger and Frye, 2007). Other studies suggest that androgen metabolites may enhance cognition via their impact on intracellular estrogen receptor beta (Frye et al., 2008a; Frye et al., 2008c; Osborne et al., 2009). Given that lifetime Pb and PS impaired rather than enhanced learning in female offspring (Cory-Slechta et al., 2010), whereas acceleration of learning was observed here in males exposed to Pb + PS, sex hormone differences in mediation of behavior represent a potentially significant mechanism to be explored for contributions to these effects.
Unlike chronic postweaning Pb exposure in males (Cohn and Cory-Slechta, 1993; Cory-Slechta et al., 2010), neither lifetime nor maternal Pb alone in males significantly impaired learning, a finding that could reflect differences in the developmental period of exposure, and/or the use of different training and procedures and the addition of sequences (maternal Pb) in this study that had multiple responses on one lever.
In summary, PS, particularly when combined with Pb, can produce a sequence-dependent acceleration (maternal) or enhancement of repeated learning in male offspring, effects that may reflect their corresponding increased rates of responding. While at first pass, this might be viewed as a positive effect, rates of responding are beneficial only in specific behavioral contexts, whereas in other cases they may impair behavioral function. For example, PS markedly increased fixed interval response rates in male offspring (Rossi-George et al., 2011), as can Pb exposure (Cory-Slechta et al., 1998), alterations which represent behavioral inefficiency and sub-optimal or even dysfunctional resource/energy use as rate increases cannot accelerate reinforcement availability. Moreover, increased fixed interval response rates have been reported to be a surrogate for impulsivity in children (Darcheville et al., 1992; 1993). Increased response rates in memory or delay of reward tasks can decrease reward and reinforcement density by causing premature responding. Underlying biochemical and neurochemical mechanisms of the accelerated learning seen here requires additional examination, but could include changes in the glucocorticoid dose-effect function, and/or alterations in mesocorticolimbic dopamine function.
Highlights.
PS exposure with exposure can enhance repeated learning in male offspring.
These effects are specific for some response sequences but not others.
Increases in reinforcement density due to increased response rates may underlie these effects.
Nucleus accumbens dopamine function is a suggestive mechanistic avenue for these effects.
Acknowledgments
Grants ES012712 (D. Cory-Slechta) and ES001247 (T. Gasiewicz).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso SJ, Navarro E, Rodriguez M. Permanent dopamine alterations in the N. Accumbens after prenatal stress. Pharmacol Biochem Behav. 1994;49:353–8. doi: 10.1016/0091-3057(94)90433-2. [DOI] [PubMed] [Google Scholar]
- Anderson NB, Armstead CA. Toward understanding the asociation of socioeconomic status and health: A new challenge for the biopsychosocial approach. Psychosom Med. 1995;57:213–25. doi: 10.1097/00006842-199505000-00003. [DOI] [PubMed] [Google Scholar]
- Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci. 2000;12:973–9. doi: 10.1046/j.1460-9568.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- Belda X, Armario A. Dopamine D1 and D2 dopamine receptors regulate immobilization stress-induced activation of the hypothalamus-pituitary-adrenal axis. Psychopharmacology (Berl) 2009;206:355–65. doi: 10.1007/s00213-009-1613-5. [DOI] [PubMed] [Google Scholar]
- Bellinger D, Hu H, Titlebaum L, Needleman HL. Attentional correlates of dentin and bone lead levels in adolescents. Arch Environ Health. 1994;49:98–105. doi: 10.1080/00039896.1994.9937461. [DOI] [PubMed] [Google Scholar]
- Bergstrom A, Jayatissa MN, Mork A, Wiborg O. Stress sensitivity and resilience in the chronic mild stress rat model of depression; an in situ hybridization study. Brain Res. 2008;1196:41–52. doi: 10.1016/j.brainres.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. 2002;53:371–99. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci. 2009;30:212–22. doi: 10.1016/j.tips.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield RL, Gendle MH, Cory-Slechta DA. Impaired neuropsychological functioning in lead-exposed children. Dev Neuropsychol. 2004;26:513–40. doi: 10.1207/s15326942dn2601_8. [DOI] [PubMed] [Google Scholar]
- Canfield RL, Henderson CR, Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003;348:1517–26. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannizzaro C, Plescia F, Martire M, Gagliano M, Cannizzaro G, Mantia G, et al. Single, intense prenatal stress decreases emotionality and enhances learning performance in the adolescent rat offspring: interaction with a brief, daily maternal separation. Behav Brain Res. 2006;169:128–36. doi: 10.1016/j.bbr.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, et al. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28:6037–45. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne DL, de Kloet ER, Joels M. Fundamental aspects of the impact of glucocorticoids on the (immature) brain. Semin Fetal Neonatal Med. 2009;14:136–42. doi: 10.1016/j.siny.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Charil A, Laplante DP, Vaillancourt C, King S. Prenatal stress and brain development. Brain Res Rev. 2010;65:56–79. doi: 10.1016/j.brainresrev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. The American journal of psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Asthana S, Plymate S, Baker L, Matsumoto AM, Peskind E, et al. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology. 2001;57:80–8. doi: 10.1212/wnl.57.1.80. [DOI] [PubMed] [Google Scholar]
- Cohn J, Cory-Slechta DA. Subsensitivity of lead-exposed rats to the accuracy-impairing and rate-altering effects of MK-801 on a multiple schedule of repeated learning and performance. Brain Res. 1993;600:208–18. doi: 10.1016/0006-8993(93)91375-3. [DOI] [PubMed] [Google Scholar]
- Cohn J, Cox C, Cory-Slechta DA. The effects of lead exposure on learning in a multiple repeated acquisition and performance schedule. Neurotoxicology. 1993;14:329–46. [PubMed] [Google Scholar]
- Cory-Slechta DA. Relationships between lead-induced learning impairments and changes in dopaminergic, cholinergic, and glutamatergic neurotransmitter system functions. Annu Rev Pharmacol Toxicol. 1995;35:391–415. doi: 10.1146/annurev.pa.35.040195.002135. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, O’Mara DJ, Brockel BJ. Nucleus accumbens dopaminergic mediation of fixed interval schedule-controlled behavior and its modulation by low-level lead exposure. J Pharmacol Exp Ther. 1998;286:794–805. [PubMed] [Google Scholar]
- Cory-Slechta DA, O’Mara DJ, Brockel BJ. Learning versus performance impairments following regional administration of MK-801 into nucleus accumbens and dorsomedial striatum. Behav Brain Res. 1999;102:181–94. doi: 10.1016/s0166-4328(99)00015-7. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Stern S, Weston D, Allen JL, Liu S. Enhanced learning deficits in female rats following lifetime Pb exposure combined with prenatal stress. Toxicol Sci. 2010;117:427–38. doi: 10.1093/toxsci/kfq221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Virgolini MB, Rossi-George A, Weston D, Thiruchelvam M. Experimental manipulations blunt time-induced changes in brain monoamine levels and completely reverse stress, but not Pb+/− stress-related modifications to these trajectories. Behav Brain Res. 2009;205:76–87. doi: 10.1016/j.bbr.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Virgolini MB, Thiruchelvam M, Weston DD, Bauter MR. Maternal stress modulates the effects of developmental lead exposure. Environ Health Perspect. 2004;112:717–30. doi: 10.1289/ehp.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Weiss B. Alterations in schedule-controlled behavior of rodents correlated with prolonged lead exposure. In: Balster RL, editor. Behavioral Pharmacology: The Current Status. New York: Alan R. Liss; 1985. pp. 487–501. [Google Scholar]
- Cory-Slechta DA, Weiss B, Cox C. Mobilization and redistribution of lead over the course of calcium disodium ethylenediamine tetracetate chelation therapy. Journal of Pharmacology and Experimental Therapeutics. 1987;243:804–13. [PubMed] [Google Scholar]
- Cowansage KK, LeDoux JE, Monfils MH. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Current molecular pharmacology. 2010;3:12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- Darcheville JC, Riviere V, Wearden JH. Fixed-interval performance and self-control in children. J Exp Anal Behav. 1992;57:187–99. doi: 10.1901/jeab.1992.57-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcheville JC, Riviere V, Wearden JH. Fixed-interval performance and self-control in infants. J Exp Anal Behav. 1993;60:239–54. doi: 10.1901/jeab.1993.60-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Dietrich KN, Ris MD, Succop PA, Berger OG, Bornschein R. Early exposure to lead and juvenile delinquency. Neurotoxicol Teratol. 2001;23:511–8. doi: 10.1016/s0892-0362(01)00184-2. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–47. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend BP. Social status and stressful life events. J Pers Soc Psychol. 1973;28:225–35. doi: 10.1037/h0035718. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Androgens’ performance-enhancing effects in the inhibitory avoidance and water maze tasks may involve actions at intracellular androgen receptors in the dorsal hippocampus. Neurobiol Learn Mem. 2007;87:201–8. doi: 10.1016/j.nlm.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Fan G, Feng C, Wu F, Ye W, Lin F, Wang C, et al. Methionine choline reverses lead-induced cognitive and N-methyl-d-aspartate receptor subunit 1 deficits. Toxicology. 2010;272:23–31. doi: 10.1016/j.tox.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nature reviews Neuroscience. 2009;10:446–57. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Edinger K, Sumida K. Androgen administration to aged male mice increases anti-anxiety behavior and enhances cognitive performance. Neuropsychopharmacology. 2008a;33:1049–61. doi: 10.1038/sj.npp.1301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Edinger K, Sumida K. Androgen administration to aged male mice increases anti-anxiety behavior and enhances cognitive performance. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008b;33:1049–61. doi: 10.1038/sj.npp.1301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Koonce CJ, Edinger KL, Osborne DM, Walf AA. Androgens with activity at estrogen receptor beta have anxiolytic and cognitive-enhancing effects in male rats and mice. Horm Behav. 2008c;54:726–34. doi: 10.1016/j.yhbeh.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka T, Fujioka A, Tan N, Chowdhury GM, Mouri H, Sakata Y, et al. Mild prenatal stress enhances learning performance in the non-adopted rat offspring. Neuroscience. 2001;103:301–7. doi: 10.1016/s0306-4522(00)00582-0. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Toscano CD, McGlothan JL, Weaver SA. Environmental enrichment reverses cognitive and molecular deficits induced by developmental lead exposure. Ann Neurol. 2003;53:50–6. doi: 10.1002/ana.10399. [DOI] [PubMed] [Google Scholar]
- Haile CN, GrandPre T, Kosten TA. Chronic unpredictable stress, but not chronic predictable stress, enhances the sensitivity to the behavioral effects of cocaine in rats. Psychopharmacology (Berl) 2001;154:213–20. doi: 10.1007/s002130000650. [DOI] [PubMed] [Google Scholar]
- Henry C, Guegant G, Cador M, Abrnauld e, Arsaut J, Le Moal M, et al. Prenatal stress facilitates amphetamine-induced sensitization and induces long-lasting changes in dopamine receptors in the nucleus accumbens. Brain Res. 1995;685:179–86. doi: 10.1016/0006-8993(95)00430-x. [DOI] [PubMed] [Google Scholar]
- Ito M, Nakamura K. Humans’ choice in a self-control choice situation: sensitivity to reinforcer amount, reinforcer delay, and overall reinforcement density. J Exp Anal Behav. 1998;69:87–102. doi: 10.1901/jeab.1998.69-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Kostaki A, Janus C, Matthews SG. The effects of prenatal stress on learning in adult offspring is dependent on the timing of the stressor. Behav Brain Res. 2009;197:144–9. doi: 10.1016/j.bbr.2008.08.018. [DOI] [PubMed] [Google Scholar]
- King S, Laplante DP. The effects of prenatal maternal stress on children’s cognitive development: Project Ice Storm. Stress. 2005;8:35–45. doi: 10.1080/10253890500108391. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–9. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante DP, Barr Rg, Brunet A, DuFort GG, Meaney ML, Saucier J-F, et al. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatr Res. 2004;56:400–10. doi: 10.1203/01.PDR.0000136281.34035.44. [DOI] [PubMed] [Google Scholar]
- Locatelli V, Bresciani E, Tamiazzo L, Torsello A. Central nervous system-acting drugs influencing hypothalamic-pituitary-adrenal axis function. Endocrine development. 2010;17:108–20. doi: 10.1159/000262533. [DOI] [PubMed] [Google Scholar]
- Lordi B, Patin V, Protais P, Mellier D, Caston J. Chronic stress in pregnant rats: effects on growth rate, anxiety and memory capabilities of the offspring. Int J Psychophysiol. 2000;37:195–205. doi: 10.1016/s0167-8760(00)00100-8. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ, Katz M, Schatzberg AF. Developmental cascades linking stress inoculation, arousal regulation, and resilience. Front Behav Neurosci. 2009;3:32. doi: 10.3389/neuro.08.032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ, Schatzberg AF. Animal models of early life stress: implications for understanding resilience. Dev Psychobiol. 2010;52:616–24. doi: 10.1002/dev.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Role of the medial prefrontal cortex in coping and resilience. Brain Res. 2010;1355:52–60. doi: 10.1016/j.brainres.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23:921–39. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- McGlothan JL, Karcz-Kubicha M, Guilarte TR. Developmental lead exposure impairs extinction of conditioned fear in young adult rats. Neurotoxicology. 2008;29:1127–30. doi: 10.1016/j.neuro.2008.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol Behav. 2007;91:55–65. doi: 10.1016/j.physbeh.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Needleman HL, Riess JA, Tobin MJ, Biesecker GE, Greenhouse JB. Bone lead levels and delinquent behavior. Journal of the American Medical Association. 1996;275:363–9. [PubMed] [Google Scholar]
- Oomen CA, Soeters H, Audureau N, Vermunt L, van Hasselt FN, Manders EM, et al. Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. J Neurosci. 2010;30:6635–45. doi: 10.1523/JNEUROSCI.0247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini C, Ventura R, Lucchese F, Puglisi-Allegra S, Cabib S. Predictable stress promotes place preference and low mesoaccumbens dopamine response. Physiol Behav. 2002;75:135–41. doi: 10.1016/s0031-9384(01)00629-1. [DOI] [PubMed] [Google Scholar]
- Osborne DM, Edinger K, Frye CA. Chronic administration of androgens with actions at estrogen receptor beta have anti-anxiety and cognitive-enhancing effects in male rats. Age (Dordr) 2009;31:191–8. doi: 10.1007/s11357-009-9114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CR, Campbell AM, Woodson JC, Smith TP, Fleshner M, Diamond DM. Permissive influence of stress in the expression of a u-shaped relationship between serum corticosterone levels and spatial memory errors in rats. Dose Response. 2006;4:55–74. doi: 10.2203/dose-response.004.01.005.Park. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Justus KR, Schatzberg AF, Lyons DM. Mild early life stress enhances prefrontal-dependent response inhibition in monkeys. Biol Psychiatry. 2005;57:848–55. doi: 10.1016/j.biopsych.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deroche V, Maccari S, Simon H, Le Moal M. glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc Natl Acad Sci U S A. 1996;93:8716–20. doi: 10.1073/pnas.93.16.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JS, Zurcher NR, Keenan KE, Bartlett TQ, Nathanielsz PW, Nijland MJ. Prenatal betamethasone exposure has sex specific effects in reversal learning and attention in juvenile baboons. Am J Obstet Gynecol. 2011;204:545, e1–10. doi: 10.1016/j.ajog.2011.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-George A, Virgolini MB, Weston D, Cory-Slechta DA. Alterations in glucocorticoid negative feedback following maternal Pb, prenatal stress and the combination: a potential biological unifying mechanism for their corresponding disease profiles. Toxicol Appl Pharmacol. 2009;234:117–27. doi: 10.1016/j.taap.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-George A, Virgolini MB, Weston D, Thiruchelvam M, Cory-Slechta DA. Interactions of lifetime lead exposure and stress: behavioral, neurochemical and HPA axis effects. Neurotoxicology. 2011;32:83–99. doi: 10.1016/j.neuro.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman IJ. Delay of reward and human verbal learning. J Exp Psychol. 1951;41:437–9. doi: 10.1037/h0063205. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Low-level lead exposure and children’s IQ: a meta-analysis and search for a threshold. Environ Res. 1994;65:42–55. doi: 10.1006/enrs.1994.1020. [DOI] [PubMed] [Google Scholar]
- Snapper AG, Kadden RM, Inglis GB. State notation of behavioral procedures. Behavior Research Methods and Instrumentation. 1982;14:329–42. [Google Scholar]
- Spencer RL, Kalman BA, Cotter CS, Deak T. Discrimination between changes in glucocorticoid receptor expression and activation in rat brain using western blot analysis. Brain Res. 2000;868:275–86. doi: 10.1016/s0006-8993(00)02341-6. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Ebner K, Day TA. Differential involvement of rat medial prefrontal cortex dopamine receptors in modulation of hypothalamic-pituitary-adrenal axis responses to different stressors. The European journal of neuroscience. 2004;20:1008–16. doi: 10.1111/j.1460-9568.2004.03569.x. [DOI] [PubMed] [Google Scholar]
- Strong PV, Christianson JP, Loughridge AB, Amat J, Maier SF, Fleshner M, et al. 5-hydroxytryptamine 2C receptors in the dorsal striatum mediate stress-induced interference with negatively reinforced instrumental escape behavior. Neuroscience. 2011;197:132–44. doi: 10.1016/j.neuroscience.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Dufresne MM. Mesocortical dopamine and HPA axis regulation: role of laterality and early environment. Brain Res. 2006;1076:49–59. doi: 10.1016/j.brainres.2005.12.100. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Turner JG, Kalin NH. Prenatal stress alters brain catecholaminergic activity and potentiates stress-induced behavior in adult rats. Brain Res. 1992;574:131–7. doi: 10.1016/0006-8993(92)90809-n. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson’s disease. J Neurosci. 2000;20:9207–14. doi: 10.1523/JNEUROSCI.20-24-09207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgolini MB, Rossi-George A, Lisek R, Weston DD, Thiruchelvam M, Cory-Slechta DA. CNS effects of developmental Pb exposure are enhanced by combined maternal and offspring stress. Neurotoxicology. 2008a;29:812–27. doi: 10.1016/j.neuro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgolini MB, Rossi-George A, Weston D, Cory-Slechta DA. Influence of low level maternal Pb exposure and prenatal stress on offspring stress challenge responsivity. Neurotoxicology. 2008b;29:928–39. doi: 10.1016/j.neuro.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier PM, Hauptman M, Weitzen SH, Bell S, Quilliam DN, Logan JR. The important health impact of where a child lives: neighborhood characteristics and the burden of lead poisoning. Maternal and child health journal. 2011;15:1195–202. doi: 10.1007/s10995-010-0692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annual review of clinical psychology. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Ward IL, Weisz J. Differential effects of maternal stress on circulating levels of corticosterone, progesterone and testosterone in male and female rat fetuses and their mothers. Endocrinology. 1984;114:1635–44. doi: 10.1210/endo-114-5-1635. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Gender Differences in the Effects of Prenatal Stress on Brain Development and Behaviour. Neurochem Res. 2007 doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Poltyrev T, Schorer-Apelbaum D, Men D, McCarty R. Effect of prenatal stress on plasma corticosterone and catecholamines in response to footshock in rats. Physiol Behav. 1998;64:439–44. doi: 10.1016/s0031-9384(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Widzowski DV, Cory-Slechta DA. Homogeneity of regional brain lead concentrations. Neurotoxicology. 1994;15:295–308. [PubMed] [Google Scholar]
- Wilhelm M, Heinzow B, Angerer J, Schulz C. Reassessment of critical lead effects by the German Human Biomonitoring Commission results in suspension of the human biomonitoring values (HBM I and HBM II) for lead in blood of children and adults. Int J Hyg Environ Health. 2010;213:265–9. doi: 10.1016/j.ijheh.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Wolfe JB. The effect of delayed reward upon learning in the white rat. J Comp Psychol. 1934;17:1–21. [Google Scholar]
- Yang Y, Cao J, Xiong W, Zhang J, Zhou Q, Wei H, et al. Both stress experience and age determine the impairment or enhancement effect of stress on spatial memory retrieval. J Endocrinol. 2003;178:45–54. doi: 10.1677/joe.0.1780045. [DOI] [PubMed] [Google Scholar]