Abstract
A daily body temperature rhythm (BTR) is critical for the maintenance of homeostasis in mammals. While mammals use internal energy to regulate body temperature, ectotherms typically regulate body temperature behaviorally [1]. Some ectotherms maintain homeostasis via a daily temperature preference rhythm (TPR) [2], but the underlying mechanisms are largely unknown. Here, we show that Drosophila exhibit a daily circadian clock dependent TPR that resembles mammalian BTR. Pacemaker neurons critical for locomotor activity are not necessary for TPR; instead, the dorsal neuron 2s (DN2s), whose function was previously unknown, is sufficient. This indicates that TPR, like BTR, is controlled independently from locomotor activity. Therefore, the mechanisms controlling temperature fluctuations in fly TPR and mammalian BTR may share parallel features. Taken together, our results reveal the existence of a novel DN2- based circadian neural circuit that specifically regulates TPR; thus, understanding the mechanisms of TPR will shed new light on the function and neural control of circadian rhythms.
Keywords: circadian rhythm, body temperature rhythm, temperature preference, period, timeless, Drosophila
RESULTS
Mammalian body temperature varies rhythmically throughout the day. This BTR contributes to their homeostasis by regulating sleep and metabolic energy usage [2–4]. While mammals control body temperature by generating heat, ectotherms use behavioral strategies to regulate body temperature [1]. Since ectotherms adapt their body temperature to their surrounding temperature, TPR is believed to be a strategy for ectotherms to achieve homeostasis [2]. For example, slugs, pill bugs, crayfish and goldfish, have been shown to exhibit TPR. Some lizards also exhibit TPR, which causes the temperature of their skin to exhibit a daily rhythm [5]. Although BTR and TPR are well-known phenomena in animal physiology, the mechanisms by which the circadian clock controls rhythms in body temperature are largely unknown in both mammals and ectotherms. Fly-based research has contributed to the discovery of many genes crucial for circadian rhythms, which has subsequently resulted in fundamental discoveries relevant to the workings of molecular clocks in several animals including mammals [6, 7]. Therefore, we sought to understand the molecular and cellular mechanisms that control TPR in Drosophila. As a first step, we determined whether flies exhibit TPR.
Drosophila exhibit a circadian clock dependent temperature preference rhythm
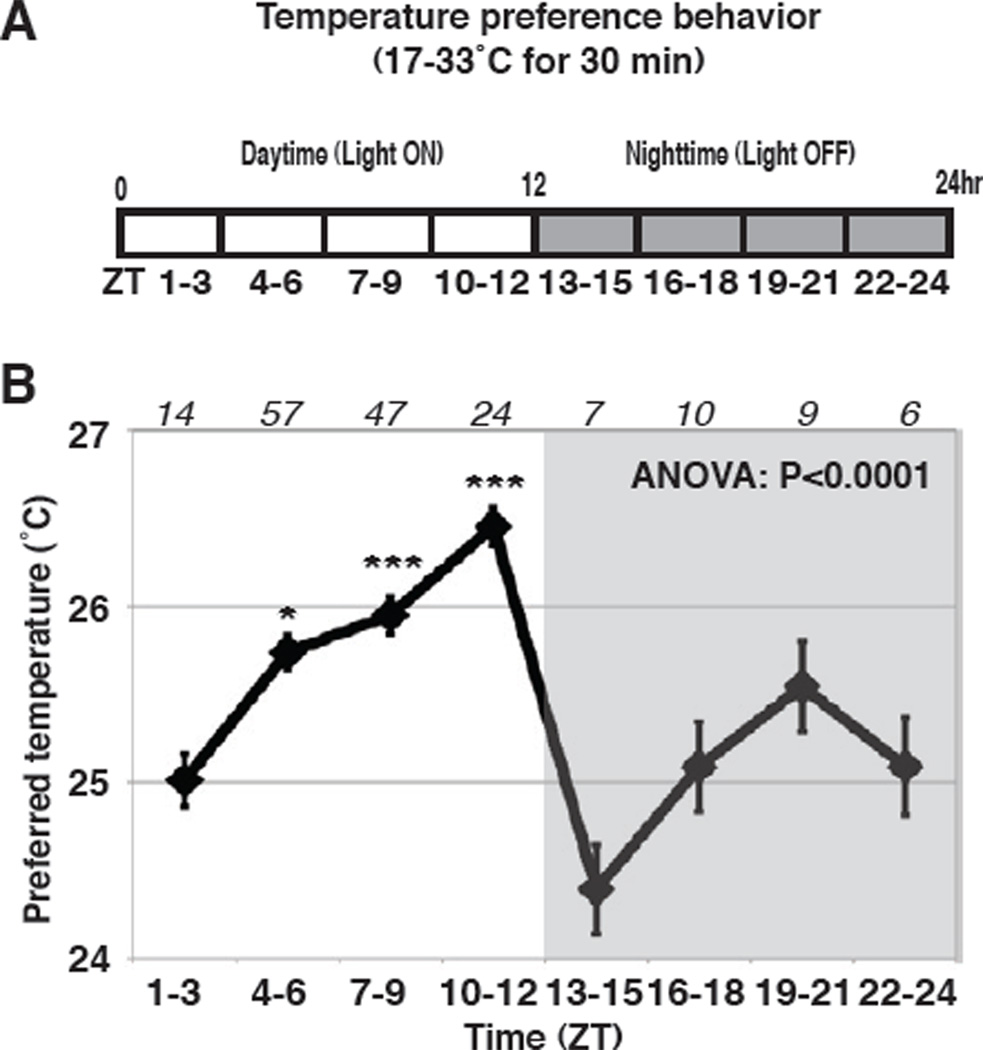
We previously showed that Drosophila exhibit a robust temperature preference behavior [8]. To determine if this preference is rhythmic, behavioral assays were performed at different times of day. Control fly strains w1118, Canton-S (cs) and (yw) were raised under 12h light /12h dark cycles (LD) at 25°C, mimicking natural day and night cycles. During each ZT (Zeitgeber Time) zone (ZT1–3, ZT4–6, ZT7–9, ZT10–12, ZT13–15, ZT16–18, ZT19–21 and ZT22–24), temperature preference behavioral assays were performed for 30 min each using a temperature gradient ranging from ~17–33 °C (Figure 1A). We found that the distribution of preferred temperature shifted from warmer to colder temperatures, and vice versa, depending on the time of day (Figure S1A–D). By plotting their average preferred temperature, we found that their preferred temperature oscillated over the course of 24 hours (ANOVA, P<0.0001) (Figure 1B). The preferred temperature gradually increased from morning (ZT 1–3) to evening (ZT10–12), and reached its peak in the evening at ZT10–12. The preferred temperature was lowest at ZT13–15 and had a second small peak at ZT19–21 (Figure 1B). Thus, we conclude that the fly displays TPR.
Figure 1. Fly’s temperature preference is rhythmic over the course of a day.
(A) Schematic of experimental condition. Temperature preference behavior assays were performed for 30 min in each of the eight different time zones (ZT 1–3, 4–6, 7–9, 10–12, 13–15, 16–18, 19–21 and 22–24). Zeitgeber Time (ZT) (12h light/dark cycle; ZT0 is lightson, ZT12 is lights-off). (B) TPR of w1118 flies over 24 hrs. Preferred temperatures were calculated using the distribution of flies in temperature preference behavior (Figure S1). Data are shown as the mean preferred temperature in each time zone. Numbers represent the number of assays. ANOVA, P<0.0001. Tukey-Kramer test compared to ZT1–3, ***P<0.001, **P<0.01 or *P<0.05. By Tukey-Kramer test, compared to ZT13–15, the preferred temperature at ZT4–6, 7–9, 10–12 (P<0.001) and ZT19–21 (P<0.05) were statistically significant (see Table S1).
TPR is under clock control
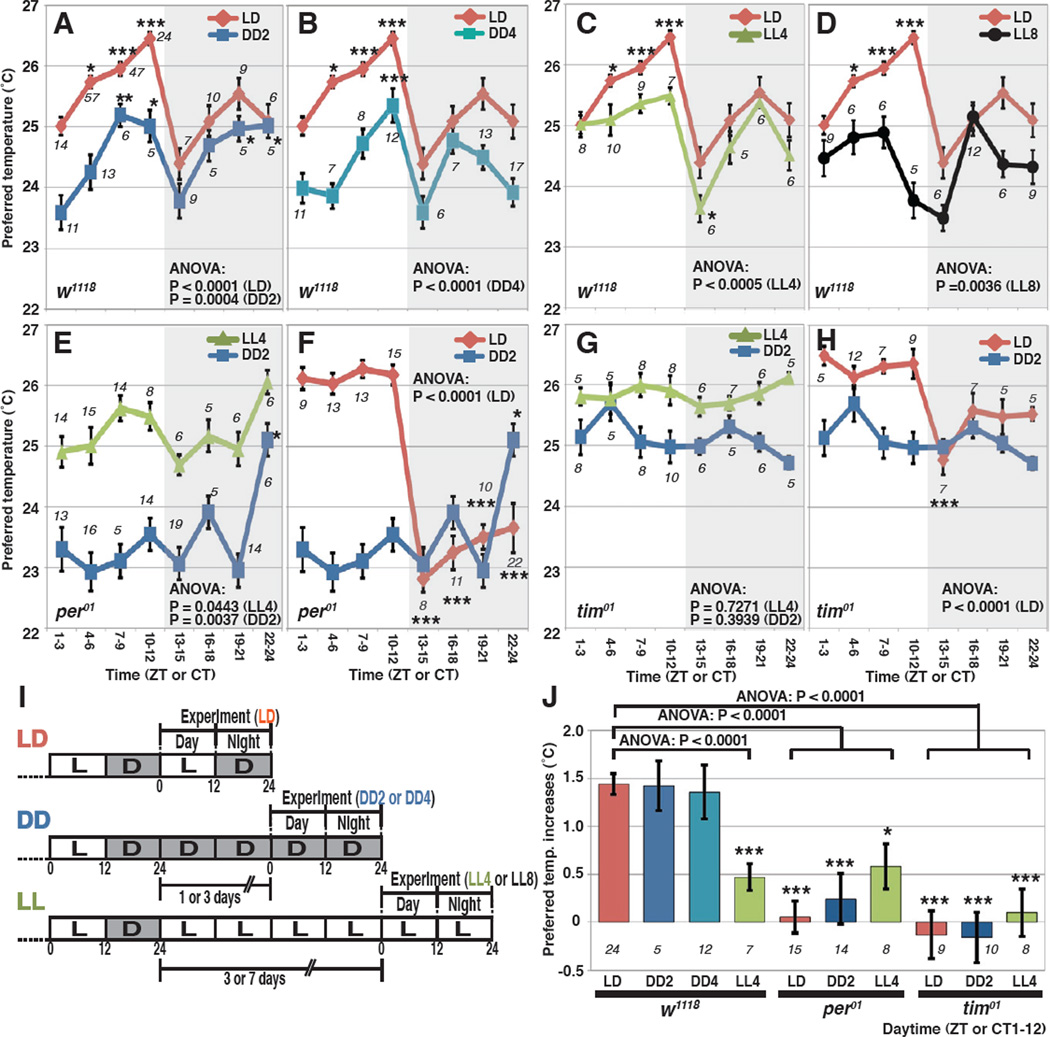
To assess if TPR is clock-regulated or driven by light-dark cycles, we tested flies in “free-running” conditions in DD (constant dark) and LL (constant light) (Figure 2I). We found that w1118 control flies still exhibited TPR during DD day 2 (ANOVA, P=0.0004) (Figure 2A, Table S1) and DD day 4 (ANOVA, P<0.0001) (Figure 2B, Table S1). The phase of these TPR oscillations in DD was the same as under LD condition (Figures 2A and 2B). Thus, TPR is controlled by an endogenous clock. Previous studies using locomotor activity have shown that oscillator functions are abolished by day 4 in LL conditions [9, 10]. Nonetheless, we found that flies kept in LL for 4 days and 8 days still exhibited TPR (Figures 2C and D; Table S1), although the oscillations amplitude was lower on day 8 (Figure 2D and Table S1). Next, we investigated whether the essential circadian clock genes period (per) and timeless (tim) are required for TPR. We found that null mutant, tim01, showed arrhythmic TPR in DD day 2 and in LL day 4 (Figures 2G; Table S1). In the null mutant per01, TPR was also profoundly disrupted under DD and LL. A weak rhythm appeared to be present in LL day 4, but the preferred temperatures in each time zone were not statistically different from ZT1–3 (Figures 2E; Table S1). We therefore conclude that in LL4 also, per01 is arrhythmic as expected. In DD2, per01 showed constant low temperature preference, except at ZT22–24 (Figures 2E; Table S1). The reason for this sudden increase, completely out of phase with the normal peak of TPR in wild-type flies, is unclear. In summary, we conclude that under constant conditions, TPR is profoundly disrupted in both per and tim null mutants, and that TPR is therefore driven by the circadian clock.
Figure 2. The fly’s TPR is circadian clock-dependent.
(A–H) Comparison of TPR over the course of 24 hours in w1118, per01 and tim01 flies kept in LD (red lines), DD2 (blue lines), DD4 (light blue lines), LL4 (green lines) and LL8 (black lines). w1118 flies kept in LD and DD2 (A), LD and DD4 (B), LD and LL4 (C) and LD and LL8 (D). The same LD data from Figure 1B were used in A-D. per01 flies kept in LL4 and DD2 (E), and LD and DD2 (F). The same DD2 data for per01 were used for the comparison in E–F. tim01 flies kept in LL4 and DD2 (G), and LD and DD2 (H). The same DD2 data for tim01 were used for the comparison in G–H. Numbers represent the number of assays. CT is Circadian Time in DD or LL. Tukey-Kramer test comparison to ZT1–3, ***P<0.001, **P<0.01 or *P<0.05. More detailed statistics are shown in Table S1.
(I) Schematic of experimental conditions. w1118 flies were raised under LD conditions and were transferred to DD or LL conditions. Then, TPR assays were performed over the course of 24 hours in LD, DD2, DD4, LL4 or LL8.
(J) The bar graph shows daytime temperature increases (ZT or CT 1–12) for w1118, per01 and tim01 in the daytime TPR in LD (red), DD2 (blue), DD4 (light blue) and LL4 (green) conditions. The value of temperature increases during the daytime was calculated by subtracting the preferred temperature at ZT or CT1-3 from the preferred temperature at ZT or CT10–12. ANOVA was performed on the following groups; w1118 (LD, DD2, DD4 and LL4); per01 (LD, DD2 and LL4) and w1118 (LD); and tim01 (LD, DD2 and LL4) and w1118 (LD). Tukey-Kramer test comparison to w1118 in LD.
Interestingly, we also observed a “masking” effect of the LD cycle, in which per01 and tim01 flies preferred a higher temperature during the day (ZT1–12) and a lower temperature during the night (ZT13–24) (Figures 2F and H; Table S1) [11]. This shows that when the flies are exposed to light they choose a higher temperature. It is consistent with the fact that w1118 flies kept in LD prefer higher temperatures during the daytime than those kept in DD2 (Figure 2A) and that per01 and tim01 flies kept in LL4 prefer a higher temperature than those kept in DD2 (Figures 2E and G). Therefore, light affects the fly’s temperature preference independent of the circadian clock. Importantly however, in per01 and tim01 flies, time-dependent changes in temperature preferences are completely lost during the course the day and during the course of the night, even in LD (Figures 2F and H).
The ~1–1.5 °C increase in temperature preference observed during the course of the day – which we will refer to as “daytime TPR” - is the most salient, robust and reproducible feature of clock-controlled TPR (Figures 1B and 2J). Particularly important for the studies described below, daytime TPR is highly consistent in various genetic backgrounds in LD (Figures S1E and F). In order to understand the neural mechanisms controlling circadian TPR, we therefore focused on daytime TPR.
The Morning and Evening oscillators are neither necessary nor sufficient for daytime TPR
There are ~150 central pacemaker cells in the fly brain, which are functional homologs of mammalian SCN neurons [12]. The pacemaker cells are divided into groups of lateral neurons (s-LNv, l-LNv, 5th-sLNv, LNd and LPN) and dorsal neurons (DN1, DN2 and DN3) based on their brain location (Figure S2B) [13]. Drosophila’s locomotor activity has two peaks in the morning and evening. While the morning activity is controlled by M oscillators (s-LNv), the evening activity is controlled by E oscillators (5th-sLNv, LNd). The function of the DNs and LPNs is not yet well understood, but they appear to play a particularly important role in the detection of environmental inputs such as temperature and light [13–18]. In TPR, we found that the preferred temperature started to rise in the morning (dawn) and reached its peak in the evening (dusk) when locomotor activity is high (Figure 1), indicating that TPR is synchronized with locomotor activity and might thus be controlled by the same pacemaker neurons. Therefore, we first determined whether the Morning and Evening oscillators are necessary and/or sufficient for daytime TPR by targeting subsets of pacemaker cells with well-characterized Gal4 lines with specific spatial expressions (Figure S2A).
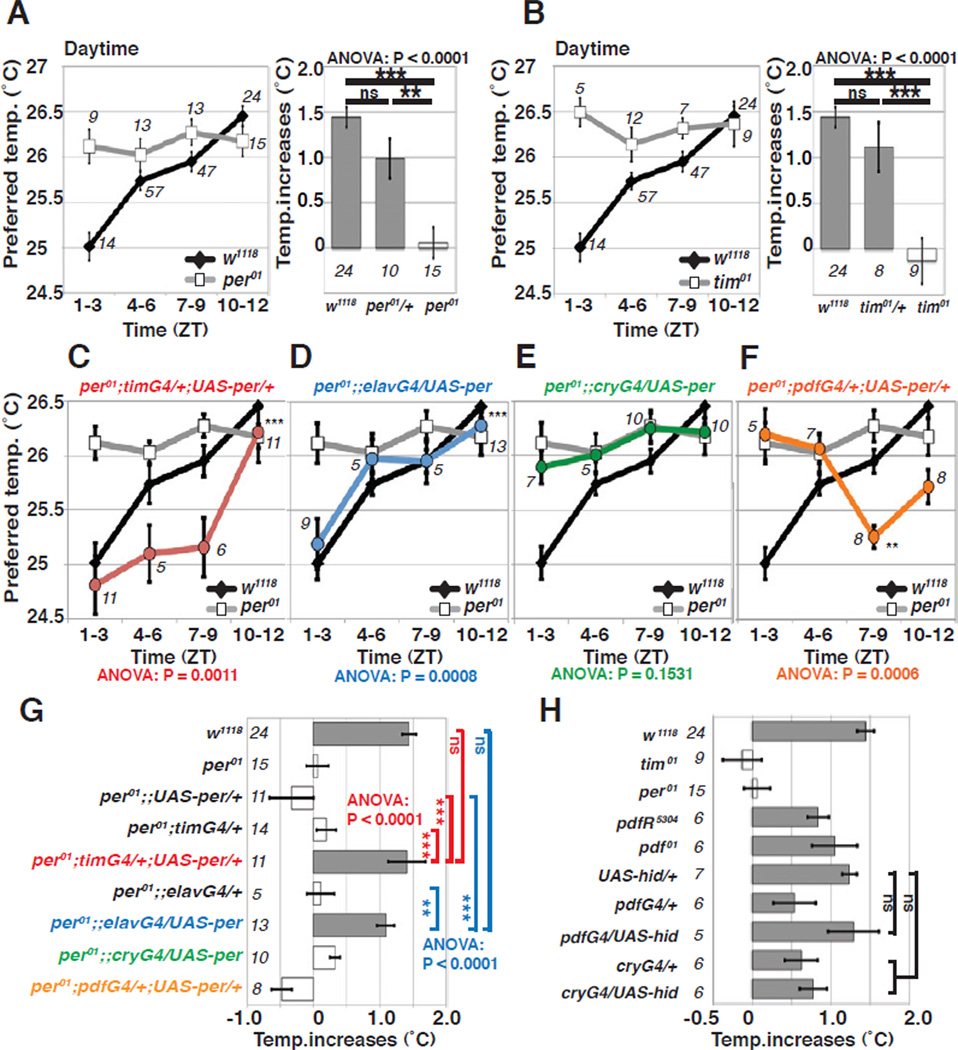
During the daytime, per01 and tim01 flies constantly preferred ~26 and 26.5 °C respectively and did not exhibit daytime TPR in LD (Figures 3A and B). For locomotor activity, tissue-specific expression of PER by either elav-Gal4 (a pan-neuronal driver) or tim-Gal4 (a driver for all circadian cells) is known to rescue the per01 arrhythmic phenotype in LD and DD [19, 20]. For TPR, we found that both the elav- and tim-GAL4 drivers were able to rescue daytime TPR in per01 under LD conditions (Figures 3C, D and G). This result indicates that PER expression in circadian neurons is sufficient for daytime TPR.
Figure 3. The Morning and Evening oscillators are not necessary for daytime TPR in LD.
(A–B) The line graphs show preferred temperatures of w1118 and null mutants per01 (A), and w1118 and null mutants tim01 (B) during the daytime (ANOVA, P=0.77 (per01), P=0.67 (tim01)). The bar graphs show daytime TPR (ZT1–12) in LD condition for each genotype. The same LD daytime data from Figures 1B, 2F and 2H were used for w1118, per01 and tim0, respectively. Tukey-Kramer test comparison to w1118, ***P<0.001, **P<0.01 or *P<0.05.
(C–F) The line graphs show preferred temperatures of w1118 and rescue fly lines in the null mutant per01 background during the daytime in LD. PER expression using tim-Gal4 (C, red) and elav-Gal4 (D, blue) are sufficient to rescue the per01 mutant phenotype for daytime TPR (ZT1–12). PER expression using cry13-Gal4 (E, green) and pdf-Gal4 (F, orange) are not sufficient to rescue the per01 mutant phenotype for daytime TPR in LD.
(G) The bar graph shows the daytime TPR (ZT1–12) in LD for each genotype shown in Figure 3A, C–F. The Morning and Evening oscillators are not sufficient. ANOVA was performed on the following groups; w1118, per01;;UAS-per/+, per01;timG4/+, and per01;timG4/+;UAS-per/+ (red); as well as w1118, per01;;UAS-per/+, per01;;elavG4/+ and per01;;elavG4/UAS-per (blue). Tukey-Kramer test comparison to each control fly line.
(H) The Morning and Evening oscillators are not necessary. The bar graph shows the daytime TPR (ZT1–12) in LD of flies with ablated Morning and/or Evening oscillator cells. The flies were still able to increase their preferred temperature to similar levels as the control flies during LD. The patterns of the TPR of pdf01 and pdfRhan5304 are shown in Figures S2C–D.
We then tried to rescue per01 flies only in the M cells with pdf-Gal4 and then in both the M and E cells with cry13-Gal4 [19, 21–23]. pdf-Gal4 is well known to rescue morning activity, whereas cry13-Gal4 restores both morning and evening activities [21]. We reexamined the locomotor activity in LD and reached the same conclusion (Figure S3A, B, E and F). Surprisingly, however, neither driver was able to rescue daytime TPR under LD conditions, although pdf-Gal4 was able to drive a weaker-amplitude rhythm with an abnormal phase (Figures 3E, F and G). Thus, the M and E cells are not sufficient for daytime TPR. Furthermore, to determine whether Morning and Evening oscillators are necessary, we ablated them by expressing the proapoptic gene hid with pdf-Gal4 and cry13-Gal4. We confirmed that pdf-neuron ablated flies exhibited loss of the morning peak of locomotor activity and advanced evening activity, whereas cry-neuron ablated flies exhibited loss of both the morning and evening peak of locomotor activity (Figure S3G-K), as previously reported [19, 22]. However, interestingly, both Morning oscillators ablated (pdf-Gal4/UAS-hid) and Morning/ Evening oscillators ablated (cry-Gal4/UAS-hid) flies were still able to increase their preferred temperature to similar levels as in the control flies during LD (Figure 3H). This data indicates that Morning and Evening oscillators are neither necessary nor sufficient for daytime TPR in LD.
The neuropeptide PDF is a key molecule expressed in s-LNv and l-LNv cells. Both PDF and its receptor PDF-Receptor (PDFR) are required for normal circadian locomotor behavior in LD and DD [22, 24, 25]. However, the null mutants of these genes, pdf01 and pdfrHan5304, exhibited daytime TPR similar to those of wild type flies during LD (Figures 3H and S2C-D), which suggests that PDF and PDF-R are also dispensable for daytime TPR in LD. This result is consistent with the data in Figures 3E-H that suggested s-LNvs and l-LNvs cells are not required for daytime TPR.
DN2 neurons are sufficient for daytime TPR
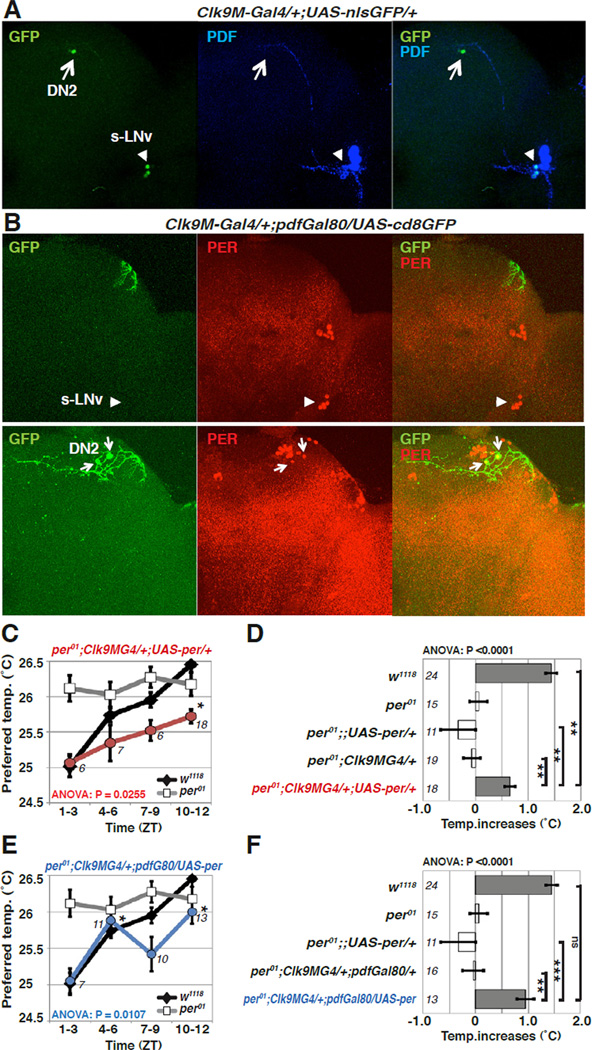
We then determined whether a subset of dorsal pacemaker neurons might control daytime TPR. DN2 neurons are suspected to be important for temperature entrainment of circadian neurons in larvae [15]. However, the functions of the DN2s in the adult are largely unknown, although they still seem particularly sensitive to temperature cycles [16]. To analyze the functions of DN2 cells in daytime TPR, we created new Gal4 lines by using a DNA fragment containing the first three Clk introns and exons 2 and 3. We found that Clk9M-Gal4 is selectively expressed in both s-LNvs and DN2s (Figures 4A and S2A). Since pdf-Gal80 can eliminate Gal4-dependent expression in the s-LNvs, expression in Clk9M-Gal4::pdf-Gal80 flies is limited to the DN2 neurons (Figures 4B and S2A). By using these flies (per01; Clk9M-Gal4/+; UAS-per/+ and per01; Clk9MGal4/+;pdf-Gal80/UAS-per), we found that PER expression in DN2 cells rescues ~46% and ~66% of the per01 mutant phenotypes for daytime TPR, respectively (Figures 4C–F). This result is consistent with the data in Figures 3E–H that cry13-Gal4 expressing neurons are not required for daytime TPR, because cry13-Gal4 is not expressed in the DN2 neurons (Figure S2A) [19, 23]. Thus, this result indicates that PER expression in DN2 neurons is sufficient for daytime TPR.
Figure 4. DN2 neurons are sufficient to generate daytime TPR in LD.
(A-B) Clk9M-Gal4; pdf-Gal80 is selectively expressed in DN2s. (A)Clk9M-Gal4::UAS-nlsGFP (nuclear GFP) flies were stained with anti-GFP (green) and anti-PDF (blue). The PDF antibody labels the s-LNv (arrowhead) and l-LNvs neurons. Clk9M-Gal4 labels both DN2 (arrow) and s-LNv neurons (arrowhead). 2 DN2 cells are observed in Clk9MGal4::UAS-nGFP flies, but GFP staining was frequently weaker in one of them.
(B) Clk9M-Gal4, pdf-Gal80/ UAS-cd8GFP flies are stained with anti-GFP and anti-PER. The PER antibody labels circadian pacemaker cells in the brain. pdf-Gal80 eliminates the expression in s-LNv neurons. Clk9M-Gal4, pdf-Gal80/ UAS-cd8GFP is selectively expressed in the DN2 neurons. Upper and lower pictures show different Z-stacks of the same brain.
(C–F) PER expression in DN2 neurons is sufficient to rescue daytime TPR of per01 in LD. The line graphs (C, E) show preferred temperatures of w1118 and rescue fly lines in the null mutant per01 background during the daytime. The same LD daytime data from Figures 1B, and 2F were used for w1118 and per01, respectively. PER expression in Clk9M-Gal4, pdf-Gal80 (E, blue) is sufficient to rescue the per01 mutant phenotype for daytime TPR (ZT1–12), while PER expression in Clk9M-Gal4 (C, red) partially rescues it. The bar graphs (D, F) show the daytime TPR (ZT1–12) for each genotype. ANOVA was performed on the following groups; w1118, per01;;UAS-per/+, per01; Clk9M-Gal4/+, and per01; Clk9M-Gal4/+;UAS-per/+ (D), as well as w1118, per01;;UAS-per/+, per01; Clk9MGal4/+; pdf-Gal80/+, and per01; Clk9M-Gal4/+; pdf-Gal80/UAS-per (F), Tukey-Kramer test comparison to ZT1–3 (C, E) and each control fly line (D, F). ***P<0.001, **P<0.01 or *P<0.05.
DN2s regulate TPR independently from circadian locomotor behavior
To determine whether the DN2s also influence circadian locomotor behavior, we tested locomotor activity using per01; Clk9M-Gal4/+; pdf-Gal80/UAS-per flies. We found that these flies are not able to rescue the per01 locomotor activity phenotypes under either light-dark (LD) or temperature (TC) cycles (Figures S3A–D and S3L–O). These flies were also arrhythmic in DD (data not shown). Therefore, our data indicates that adult DN2 only regulates TPR, not locomotor activity. Taken together, our data indicate that locomotor activity and TPR are controlled by distinct pacemaker cells in adult flies.
DISCUSSION
Here, we show that Drosophila exhibit a daily TPR– low in the morning, high in the evening - that follows a similar pattern as in humans [26]. This study is not only the first demonstration of fly TPR, but also the first systematic analysis of the molecular and neural mechanisms underlying TPR. We found that TPR is controlled by the DN2s, which might explain why temperature preference remains rhythmic in LL in Figure 2C and D. The DN2s do not express CRYPTOCHROME (CRY), a blue-light sensor crucial for circadian photoreception [27–29]. Arrhythmicity in LL is caused by constant activation of CRY and thus constant degradation of TIM [23, 30]. Therefore, CRY negative DN2 neurons may maintain residual rhythms in LL for a longer period of time than CRY positive circadian neurons. To explore this possibility, we performed immunostaining of brains with TIM-antibody and analyzed the staining levels of DN2 cells in LL 4 days. Although we found TIM signal to be weakly rhythmic in DN2 neurons, these oscillations were not statistically significant. Further studies will thus be needed to verify that DN2 neurons maintain residual rhythm in LL. Since locomotor activity is controlled by CRY positive circadian neurons and rapidly becomes arrhythmic in LL, the maintenance of TPR rhythms in LL also supports the conclusion that locomotor activity and TPR are controlled by independent circadian neural pathways.
Our data reveals striking parallel features between fly TPR and mammalian BTR, although the modes of heat production are not the same. We demonstrate here that flies exhibit robust temperature increases during the daytime, which is the same phenomenon as diurnal mammalian BTR. Furthermore, ablation studies in rats show that BTR is controlled by SCN neurons that target a different subset of subparaventricular zone neurons than those that control locomotor activity [31]. Thus, both fly TPR and mammalian BTR exhibit circadian clock dependent temperature fluctuations, independently regulated from locomotor activity. Taken together, our data raises the possibility that mammalian BTR and fly TPR are evolutionally conserved, which may be because temperature fluctuation in an animal’s body is fundamental for maintenance of its homeostasis.
Why do flies exhibit TPR? Flies probably exhibit TPR primarily to maintain homeostasis, like mammals [2]. Mammalian BTR has been shown to have a clear interaction with sleep [4], and it has been reported that mechanisms controlling fly sleep are analogous to those controlling mammalian sleep [6, 32–34]. Therefore, fly TPR may also have a relationship with sleep. Intriguingly, we did observe that PER expression limited to pdf neurons can generate a weak TPR with an abnormal phase in per01 mutants (Figure 3F), suggesting that pdf neurons may have a role in the TPR circuits. pdf positive (sLNv and lLNv) neurons regulate sleep [34] and sLNvs are known to project near the DN2s [35]. Therefore, pdf neurons may be able to modulate DN2 activity even when these neurons are arrhythmic, and may represent the neural basis for an interaction between TPR and sleep.
Additionally TPR may provide feedback to circadian pacemakers. Ambient temperature fluctuations can entrain not only peripheral clocks in mammals [36, 37] and flies [38] but also circadian pacemaker neurons in the fly brain, which contribute to Morning and Evening locomotor activity [14, 16, 39, 40]. Since TPR can generate temperature fluctuations in the fly body, the output of TPR may thus reinforce or refine circadian rhythm entrainment. For circadian locomotor behavior, the DN2s could actually participate in the reinforcement, since in the larval brain the DN2s help the sLNvs entraining to temperature cycles [15]. Therefore, by further exploring this newly discovered circadian rhythm, Drosophila TPR might not only help understanding the mechanisms underlying body temperature control in animals, but also contribute to a greater understanding of circadian rhythm’s plasticity.
Supplementary Material
HIGHLIGHTS.
We identify a novel circadian behavior in flies, temperature preference rhythm (TPR).
Drosophila TPR follows a similar pattern as body temperature rhythm in humans.
Drosophila TPR is regulated independently from circadian locomotor activity.
TPR is controlled by a newly identified DN2- based pacemaker circuit in the brain
Acknowledgements
We are grateful to Dr. Paul Taghert for the per01, tim01 and cry-Gal413 flies, Dr. Michael Rosbash for the anti-PDF and anti-PER, and Bloomington Drosophila fly stock center. We thank Drs. Paul A. Garrity, Christian I. Hong, Emi Nagoshi, Richard A. Lang, Tiffany A. Cook, Satoshi H. Namekawa, Tadahiro Goda, Ms. Jennifer R. Leslie, and Mr. Michael Platt for comments and advice on the manuscript. This research was supported by Trustee Grant from Cincinnati Children’s Hospital, JST/PRESTO and March of Dimes to F. N. H, and by NIH R01 grant GM079182 to P. E.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
F.N.H and H.K designed research. F.N.H, H.K, L.M.H and X.T performed TPR behavioral experiments. J.L and P.E performed locomotor activity behavioral experiments and Clk9M-Gal4 staining. Y.L and P.E.H made Clk9M-Gal4 fly lines. F.N.H, H.K and L.M.H wrote the manuscript.
REFERENCES
- 1.Stevenson RD. The relative importance of behavioral and physiological adjustments controlling body temperature in terrestrial ectotherms. The American Naturalist. 1985;126 [Google Scholar]
- 2.Refinetti R, Menaker M. The circadian rhythm of body temperature. Physiol Behav. 1992;51:613–637. doi: 10.1016/0031-9384(92)90188-8. [DOI] [PubMed] [Google Scholar]
- 3.Weinert D. Circadian temperature variation and ageing. Ageing Res Rev. 2010;9:51–60. doi: 10.1016/j.arr.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Krauchi K. The thermophysiological cascade leading to sleep initiation in relation to phase of entrainment. Sleep Med Rev. 2007;11:439–451. doi: 10.1016/j.smrv.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Ellis DJ, Firth BT, Belan I. Circadian rhythms of locomotor activity and temperature selection in sleepy lizards, Tiliqua rugosa. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2007;193:695–701. doi: 10.1007/s00359-007-0224-z. [DOI] [PubMed] [Google Scholar]
- 6.Crocker A, Sehgal A. Genetic analysis of sleep. Genes Dev. 2010;24:1220–1235. doi: 10.1101/gad.1913110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allada R, Chung BY. Circadian organization of behavior and physiology in Drosophila. Annu Rev Physiol. 2010;72:605–624. doi: 10.1146/annurev-physiol-021909-135815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konopka RJ, Pittendrigh C, Orr D. Reciprocal behaviour associated with altered homeostasis and photosensitivity of Drosophila clock mutants. J Neurogenet. 1989;6:1–10. doi: 10.3109/01677068909107096. [DOI] [PubMed] [Google Scholar]
- 10.Qiu J, Hardin PE. per mRNA cycling is locked to lights-off under photoperiodic conditions that support circadian feedback loop function. Mol Cell Biol. 1996;16:4182–4188. doi: 10.1128/mcb.16.8.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page TL. Masking in invertebrates. Chronobiol Int. 1989;6:3–11. doi: 10.3109/07420528909059137. [DOI] [PubMed] [Google Scholar]
- 12.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubruille R, Emery P. A plastic clock: how circadian rhythms respond to environmental cues in Drosophila. Mol Neurobiol. 2008;38:129–145. doi: 10.1007/s12035-008-8035-y. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Liu Y, Bilodeau-Wentworth D, Hardin PE, Emery P. Light and temperature control the contribution of specific DN1 neurons to Drosophila circadian behavior. Curr Biol. 2010;20:600–605. doi: 10.1016/j.cub.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Picot M, Klarsfeld A, Chelot E, Malpel S, Rouyer F. A role for blind DN2 clock neurons in temperature entrainment of the Drosophila larval brain. J Neurosci. 2009;29:8312–8320. doi: 10.1523/JNEUROSCI.0279-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyasako Y, Umezaki Y, Tomioka K. Separate sets of cerebral clock neurons are responsible for light and temperature entrainment of Drosophila circadian locomotor rhythms. J Biol Rhythms. 2007;22:115–126. doi: 10.1177/0748730407299344. [DOI] [PubMed] [Google Scholar]
- 17.Tang CH, Hinteregger E, Shang Y, Rosbash M. Light-mediated TIM degradation within Drosophila pacemaker neurons (s-LNvs) is neither necessary nor sufficient for delay zone phase shifts. Neuron. 2010;66:378–385. doi: 10.1016/j.neuron.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshii T, Hermann C, Helfrich-Forster C. Cryptochrome-positive and -negative clock neurons in Drosophila entrain differentially to light and temperature. J Biol Rhythms. 2010;25:387–398. doi: 10.1177/0748730410381962. [DOI] [PubMed] [Google Scholar]
- 19.Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko M, Park JH, Cheng Y, Hardin PE, Hall JC. Disruption of synaptic transmission or clock-gene-product oscillations in circadian pacemaker cells of Drosophila cause abnormal behavioral rhythms. J Neurobiol. 2000;43:207–233. doi: 10.1002/(sici)1097-4695(20000605)43:3<207::aid-neu1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- 22.Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- 23.Emery P, Stanewsky R, Helfrich-Forster C, Emery-Le M, Hall JC, Rosbash M. Drosophila CRY is a deep brain circadian photoreceptor. Neuron. 2000;26:493–504. doi: 10.1016/s0896-6273(00)81181-2. [DOI] [PubMed] [Google Scholar]
- 24.Mertens I, Vandingenen A, Johnson EC, Shafer OT, Li W, Trigg JS, De Loof A, Schoofs L, Taghert PH. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron. 2005;48:213–219. doi: 10.1016/j.neuron.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Hyun S, Lee Y, Hong ST, Bang S, Paik D, Kang J, Shin J, Lee J, Jeon K, Hwang S, et al. Drosophila GPCR Han is a receptor for the circadian clock neuropeptide PDF. Neuron. 2005;48:267–278. doi: 10.1016/j.neuron.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol. 1998;275:R1478–R1487. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- 27.Yoshii T, Todo T, Wulbeck C, Stanewsky R, Helfrich-Forster C. Cryptochrome is present in the compound eyes and a subset of Drosophila's clock neurons. J Comp Neurol. 2008;508:952–966. doi: 10.1002/cne.21702. [DOI] [PubMed] [Google Scholar]
- 28.Benito J, Houl JH, Roman GW, Hardin PE. The blue-light photoreceptor CRYPTOCHROME is expressed in a subset of circadian oscillator neurons in the Drosophila CNS. J Biol Rhythms. 2008;23:296–307. doi: 10.1177/0748730408318588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klarsfeld A, Malpel S, Michard-Vanhee C, Picot M, Chelot E, Rouyer F. Novel features of cryptochrome-mediated photoreception in the brain circadian clock of Drosophila. J Neurosci. 2004;24:1468–1477. doi: 10.1523/JNEUROSCI.3661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay SA, Rosbash M, Hall JC. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- 31.Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005;28:152–157. doi: 10.1016/j.tins.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 33.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 34.Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, Kang K, Liu X, Garrity PA, Rosbash M, et al. PDF cells are a GABAresponsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helfrich-Forster C. The neuroarchitecture of the circadian clock in the brain of Drosophila melanogaster. Microsc Res Tech. 2003;62:94–102. doi: 10.1002/jemt.10357. [DOI] [PubMed] [Google Scholar]
- 36.Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330:379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown SA, Zumbrunn G, Fleury-Olela F, Preitner N, Schibler U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol. 2002;12:1574–1583. doi: 10.1016/s0960-9822(02)01145-4. [DOI] [PubMed] [Google Scholar]
- 38.Glaser FT, Stanewsky R. Temperature synchronization of the Drosophila circadian clock. Curr Biol. 2005;15:1352–1363. doi: 10.1016/j.cub.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 39.Busza A, Murad A, Emery P. Interactions between circadian neurons control temperature synchronization of Drosophila behavior. J Neurosci. 2007;27:10722–10733. doi: 10.1523/JNEUROSCI.2479-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshii T, Heshiki Y, Ibuki-Ishibashi T, Matsumoto A, Tanimura T, Tomioka K. Temperature cycles drive Drosophila circadian oscillation in constant light that otherwise induces behavioural arrhythmicity. Eur J Neurosci. 2005;22:1176–1184. doi: 10.1111/j.1460-9568.2005.04295.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.