Abstract
Purpose
We tested the hypothesis that low intensity exercise in mdx mice improves plantarflexor muscle contractile function, resistance to fatigue, and mitochondrial adaptations without exacerbating muscular dystrophy.
Methods
We subjected mdx mice to 12 wk of voluntary, low-resistance wheel running (Run, n=17) or normal cage activities (sedentary; Sed, n=16) followed by in vivo analyses for plantarflexor torque generation and fatigue resistance, or running capacity on a treadmill. Gastrocnemius muscles were further evaluated for exercise-induced mitochondrial adaptations and fiber type distribution and central nuclei. T-tests were used to determine differences between the Sed and Run groups.
Results
Plantarflexor submaximal isometric torques and maximal isometric torque at multiple ankle joint angles, and resistance to fatigue were greater in Run compared to Sed mdx mice (P<0.05). Citrate synthase and β-HAD enzyme activities and COX IV protein expression in gastrocnemius muscles were greater in Run than Sed mdx mice (P≤0.04), along with a trend of fiber type transformation from type IIb to type 2x fibers. Exercise training in mdx mice did not elevate serum creatine kinase levels, but led to a significant reduction of centrally-nucleated myofibers.
Conclusion
Voluntary, low-resistance wheel running in mdx mice can result in skeletal muscle adaptation, leading to improved contractile function and reduced fatigability, with no indication that exercise was detrimental. This study supports the need for further investigation of low intensity exercise as an early therapeutic intervention in ambulatory boys with DMD.
Keywords: Duchenne muscular dystrophy, fatigue, mitochondria, physical activity, skeletal muscle, strength
Exercise is recommended for adults to prevent, reverse, or slow the progression of various chronic diseases including but not limited to cardiovascular disease, diabetes mellitus, osteoporosis, hypertension, and some types of cancer. Similarly, physical activity for children is advised to ward off obesity and diabetes, and exercise has proven beneficial for children diagnosed with specific diseases such as cystic fibrosis (38). Indeed, the American College of Sports Medicine is promoting a global health initiative “Exercise is Medicine”. However, for the muscular dystrophies there is considerable hesitation to prescribe exercise as a therapeutic intervention. The reason for the hesitation, particularly for children with Duchenne muscular dystrophy (DMD), is the concern that exercise or too much physical activity may cause muscle damage and exacerbate the disease. DMD results from mutations in the dystrophin gene and the lack of this protein renders skeletal muscle fibers more susceptible to contraction-induced injury, relative to healthy, dystrophin-positive muscle fibers (32).
Despite the understandable hesitation to prescribe exercise to individuals with DMD, the three clinical studies that have been completed and published on the topic do not report deleterious consequences (10, 34, 36). Patients with DMD who engaged in a resistance type of exercise program realized strength gains over the course of the year-long study, while an age-matched, unexercised DMD cohort continued to have declines in muscle strength (36). Similarly, monthly testing of DMD patients whom unilaterally trained the legs showed that the exercised quadriceps were equal to or stronger than contralateral, unexercised quadriceps (10). Importantly, testing continued for up to two years past the six months of training and no evidence of training or overload weakness was found. Finally, Scott et al published the results of a six month study on boys with DMD, half of whom were randomly assigned exercises with a resistance component and the other half exercises without resistance (34). While there were no differences detected between the two exercise treatment groups in terms of muscle strength or functional abilities, the investigators stated that there was no evidence that increased activity or resistance exercise caused physical deterioration.
Similar to the three DMD-exercise studies, several studies utilizing mdx mice, the mouse model of DMD, have shown that exercise training can yield improved skeletal muscle function without worsening the disease. Months of voluntary running on wheels by mdx mice improved force generation of soleus muscle (6, 19, 39), and increased fatigue resistance of extensor digitorum longus (EDL) muscle (19, 39). Ten to 15 weeks of swimming by mdx mice also resulted in gains of soleus and EDL muscle specific force and fatigue resistance (18, 20). The focus of these previous studies was on small leg muscles and limited to ex vivo isometric contractile analyses. One objective of the current study was to extend those results to functional analyses of a group of leg muscles, specifically the plantarflexors (gastrocnemius, soleus, plantaris muscles). The plantarflexors are particularly important to consider because these muscles are active during ambulation and wheel running in rodents (35, 40) and because the gastrocnemius muscle is severely affected in both patients with DMD and mdx mice (13, 30), making the choice both specific for the intervention being studied (i.e., complying with specificity of exercise training) and clinically relevant. Furthermore, plantarflexor function was comprehensively evaluated for maximal and submaximal torque generation, in relation to ankle angle, concentrically at various velocities, and for fatigue resistance. To our knowledge, this is the first in vivo study of ankle plantarflexor muscle function of mdx mice following exercise training.
Previously we reported that 60 days of voluntary wheel running by mdx mice resulted in shifts in fiber size and myosin heavy chain (MHC) isoform expression and that those beneficial cellular adaptations occurred without any indication of worsened muscle pathology (27). However, we also reported that wheel running by mdx mice did not improve mitochondrial oxidative enzyme capacity in hindlimb muscles as occurred in that of exercised wildtype mice. Because mdx mice voluntary run less per day than wildtype mice (9, 11, 19, 27), it is feasible that the exercise training duration was not sufficient to elicit mitochondrial adaptations. Therefore, a second objective of the current study was to conduct more thorough analyses of mitochondrial biogenesis in response to a longer duration (i.e., 12 vs 8 wk) of voluntary exercise by mdx mice.
In this study, we tested the hypothesis that 12 wk of voluntary, low-resistance wheel running by mdx mice results in greater in vivo plantarflexor muscle contractile function and resistance to fatigue, and that mitochondrial adaptations and fiber type transformation contribute to beneficial skeletal muscle remodeling. Because of the persistent belief that all exercise is deleterious to muscle lacking dystrophin, we continue to test the hypothesis that specific types of exercise by mdx mice, such as wheel running, do not exacerbate the muscle disease. Our primary criterion for disease exacerbation was muscle function because strength and fatigue would worsen if the exercise were causing additional damage to the dystrophin-deficient muscle. Secondary criteria included mdx mouse cage activities and the ability to perform a novel mode of exercise (treadmill running), serum creatine kinase (CK) activity, and the appearance of centrally-nucleated muscle fibers.
Methods
Animals and Study Design
Male and female mdx mice from our colony at the University of Minnesota were used in this study (27). Mice were given commercial rodent chow (2018 Teklad Global 18% Protein Rodent Diet, Harland Teklank, Madison, WI) and water ad libitum and exposed to a 12-h light-dark cycle with the dark cycle starting at 6 p.m. Mice were randomized to a sedentary control group (Sed: n=16 (10 males and 6 females)) or wheel running group (Run: n=17 (11 males and 6 females)) at 4 wk of age. We chose this relatively young mouse age to begin the intervention because muscle function is already impaired at this age in the mdx mouse (28) and it roughly equates to the age at which DMD affects muscle function in young boys (30). For the next 12 wk, each mouse was housed individually in a cage with or without a running wheel. Body mass (BM) was measured weekly.
A subset of 11–12 mice in each group were monitored for cage activities during wk 8 of the 12-wk study in order to determine if wheel running caused mdx mice to become inactive in their cages when not on the wheel. At the end of the 12 wk, those same mdx mice were weighed, anesthetized, and assessed for ankle plantarflexor muscle function in vivo. While the mouse was still under anesthesia, serum was collected by retro-orbital bleed for measurement of CK activity (4). Gastrocnemius and tibialis anterior muscles were dissected, weighed, snap frozen in liquid nitrogen and stored at −80 °C for mitochondrial enzyme assays. Mice were sacrificed by excision of the heart while still under anesthesia.
The remaining 10 mdx mice (5 each per Run and Sed groups) were assessed for treadmill running aptitude at the end of the 12-wk study. Immediately before and after the treadmill assessment, acute cage activities were monitored. Following the post-treadmill activity assessment, mice were anesthetized by an intraperitoneal injection of sodium pentobarbital (100 mg/kg BM). The medial gastrocnemius and tibialis anterior muscles, as well as strips of diaphragm muscle, were dissected and mounted in OCT for histological analyses. Mice were sacrificed by excision of the heart while still under pentobarbital anesthesia. For all measurements, the individuals collecting data were blinded as to whether each mouse was from the Sed or Run group. All protocols and animal care procedures were approved by the University of Minnesota’s AAALAC-accredited Institutional Animal Care and Use Committee and complied with guidelines set by the American Physiological Society. Furthermore, the study adhered to the American College of Sports Medicine policy on research with experimental animals.
Wheel Running and 24-Hour Cage Activities
Each Run mouse was housed in a standard mouse cage that contained a low-resistance running wheel (6, 27). The wheels were calibrated such that 1 gram of force was required to initiate wheel movement. This small amount of tension was also important so that the wheel did not freely spin when the mice were not engaged in running. Wheel-running distances were acquired in 24-h intervals for 12 wk. Each Sed mouse was housed in a standard mouse cage without a running wheel.
24-hour cage activities were measured using Activity Monitors (Med Associates Inc., St. Albans, Vermont) (27). Immediately prior to measurement of cage activities, each mouse was familiarized with the activity-monitoring cage by spending 24 h in a mock chamber. Physical activity measurements included distance traveled in the chamber (ambulation), counts of stereotypic and rearing (vertical) activities, and total time spent being active. These data were acquired using Activity Monitor version 5 software (Med Associates).
Treadmill Running and Acute Cage Activities
Exercise-induced inactivity was assessed using a protocol similar to Kobayashi et al (25) with the following modifications. Mice were acclimated to the treadmill each morning for three consecutive mornings in attempt to minimize psychological stress (26). The acclimation consisted of placing mice on the treadmill for 5 min at 0 m/min, followed by 5 min of walking at 8–10 m/min at 0° grade. On the fourth day, voluntary cage activities were assessed using activity chambers (AccuScan Instruments Inc., Columbus, OH) for 30 min beginning at 10 a.m. After assessing this baseline activity, mice were placed on the treadmill which was set at a 15° downhill grade. Mice were encouraged to walk at 5 m/min for 5 min and then at 15 m/min for 10 min, but no electrical shock was used. At completion of the treadmill exercise, mice were placed back into the same activity chamber and activities were measured for another 30 min in order to determine if an acute bout of exercise induced physical inactivity.
In Vivo Plantarflexor Muscle Function
Mice were given a mixture of fentanyl citrate (0.2 mg/kg BM), droperidol (10 mg/kg BM), and diazepam (5 mg/kg BM) in preparation for analysis of in vivo muscle function as previously described (5). Briefly, with the ankle joint positioned at a 90° angle (defined as neutral position 0°), contraction of the plantarflexors was controlled by stimulation of the sciatic nerve after the peroneal nerve had been severed to avoid recruitment of the dorsiflexor muscles. Peak-isometric torque was achieved by varying the voltage delivered to the sciatic nerve at a frequency of 300 Hz and a 0.1-ms square wave pulse. The following physiological torque measurements were adapted from Warren et al. (37). Torque as a function of stimulation frequency was measured during 12 isometric contractions at varying stimulation frequencies (10, 20, 30, 40, 60, 80, 100, 125, 150, 200, 250, 300 Hz). The relationship between torque and ankle angle was determined by the servomotor first passively moving the foot to 20° dorsiflexion, and with the foot held in that position an isometric contraction was performed at 300 Hz. This procedure was repeated with isometric torque being assessed at 5° increments until the last contraction was assessed at 20° plantarflexion. Next, for the torque-velocity relationship, the foot was passively moved to 20° dorsiflexion, and a concentric contraction was performed at 300 Hz with the foot simultaneously moving to 20° plantarflexion at a rate of 1200°/s. This procedure was repeated with concentric contractions performed at 1000°, 800°, 600°, 400°, 200°, 100°, and 0°/s. Power was calculated by multiplying the torque generated at a particular velocity by that velocity. Finally, fatigability of the plantarflexors was assessed by 120 isometric contractions at 60 Hz (300-ms stimulation and 700-ms rest) for a total protocol time of 2 min. Peak isometric torque at the neutral position was assessed pre-fatigue protocol and 10 min post-fatigue protocol. To account for differences in body size among mice, torques (N•mm) were normalized by body mass (kg−1).
Mitochondrial Assays
Gastrocnemius and tibialis anterior muscles were homogenized in 33 mM phosphate buffer (pH 7.4) at a muscle:buffer ratio of 1:20 using a glass tissue grinder on ice. Protein concentration of each homogenate was measured using the BCA protein assay (Thermo Fisher Scientific). Citrate synthase (CS) activity was determined in duplicate and beta-hydroxy acyl-CoA dehydrogenase (β-HAD) activity in triplicate (27). Enzyme activites are normalized to total protein content.
For Western blots, 40 µg of total protein from each gastrocnemius muscle homogenate was separated by SDS-PAGE and transferred onto a PVDF membrane (1). The following antibodies were used for immunoblot analysis: PGC-1α (catalog no. AB3242, Millipore), COX IV (catalog no. 4844, Cell Signalling Technologies), GAPDH (catalog no. AB8245, Millipore), anti-rabbit IRdye800 (catalog no. 926-32211, Li-COR Biosciences), anti-rabbit IRdye680 (catalog no. 926-32221, LI-COR Biosciences). Direct fluorescense was detected and quantified using the Odyssey infrared imaging system (LI-COR Biosciences). Integrated intensity of PGC-1α and COX IV is expressed relative to GAPDH.
Histo-morphological Assessments
Ten micron thick serial cross-sections of gastrocnemius, tibialis anterior, and diaphragm muscles were cut on a microtome cryostat and stained by hematoxylin and eosin (n=3–5 muscles per group). All fibers from gastrocnemius, tibialis anterior, and diaphragm muscle cross sections were evaluated for centrally-nucleated fibers. Additional sections of gastrocnemius muscles were assessed by myosin heavy chain (MHC) immunofluorescence for determining fiber types (n=4 and 5 for Sed and Run, respectively). Antibodies against mouse MHC-I, MHC-IIA, and MHC-IIB (BA-F8, SC-71, and BF-F3, respectively, as originally developed by Schiaffino) were obtained from the Developmental Studies Hybridoma Bank under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. Sections were first fixed in acetone for 10 min at −20 °C, briefly washed in PBS, and then treated with 0.1% Triton-X in PBS for 10 min. Sections were blocked for 30 min and anti-MHC antibodies were added for 15 min at room temperature. A biotinylated anti-mouse IgG antibody was used in conjunction with strepavidin-conjugated fluorochromes to detect the primary antibodies. Biotin and strepavidin solutions were applied to the slides in between each anti-MHC antibody. MHC-I, MHC-IIA, and MHC-IIB were detected with AMCA, Texas Red, and Fluorescein, respectively.
All images were acquired on a Leica DM2000 microscope with a Qimaging Micropublisher RTV 5.0 digital camera. Cross-sectional areas (CSA) were determined by measuring fiber circumferences using ImageJ software (National Institutes of Health, Bethesda, MD). Gastrocnemius fiber types and CSA were assessed in three fields per section for a total of ~600 fibers per muscle. These analyses were done on a fiber-by-fiber basis so that CSA could be evaluated for each fiber type. Fibers that were not labeled by any of the anti-MHC antibodies were classified as type 2x fibers. Mean data on CSA for each fiber type and a mean fiber type percentage were calculated for each muscle analyzed. These muscle means were then used for statistical analyses.
Statistical Analyses
T-tests were initially used to show that wheel running distance, cage activities, body mass-normalized plantarflexor torques, and CK values did not significantly differ between male and female mdx mice (P≥0.19), and therefore data from male and female mice were collapsed. T-tests were used to determine if there were differences between Run and Sed mdx mice for 24-h cage activities, treadmill running and associated acute cage activities, plantarflexor muscle functions, CK activity, body and muscle masses, mitochondrial enzyme activities and protein contents, and histological parameters. Statistical analyses were done using SigmaStat version 3.5 (Systat Software Inc; Point Richmond, CA). Values are reported as means (SE) and significance was accepted with an α level of 0.05.
Results
Wheel Running and 24-Hour Cage Activities
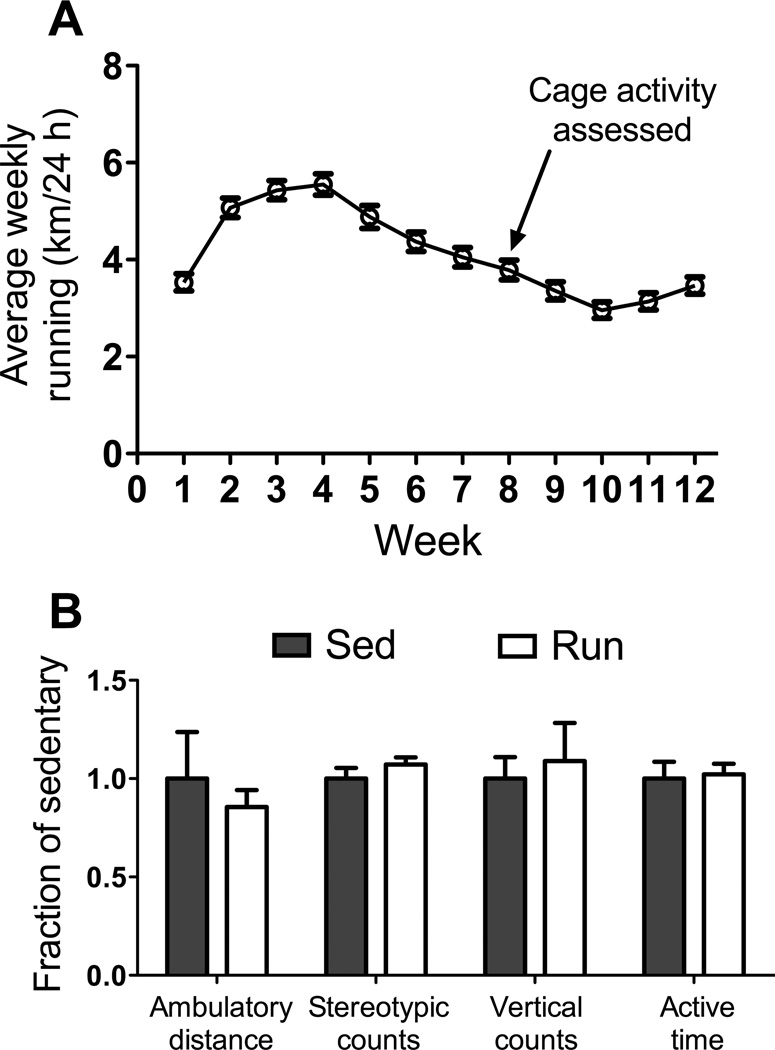
Run mice averaged ~3.4 km per day of voluntary-wheel running across the 12-wk study (Figure 1A). To determine if the daily running influenced physical activities off the wheel, cage activities of Sed and Run mice were monitored for a 24-h period during wk 8. There were no differences between Sed and Run groups for any parameter of cage activity assessed (Figure 1B): ambulatory distance (453±108 vs. 388±39 m/24 h, respectively; P=0.576); stereotypic counts (41.9±2.3 vs. 44.9±1.5 thousand counts/24 h, respectively; P=0.283); vertical counts (3427±376 vs. 3732±664 counts/24 h, respectively; P=0.695); active time (208±56 vs. 213±11 min/24 h, respectively; P=0.828). These results indicate that voluntary-wheel running exercise does not induce physical inactivity in mdx mice.
Figure 1.
Wheel running and cage activities by mdx mice. A: Distances run on wheels by mdx mice per 24 h shown as weekly averages over the course of the 12-wk study. B: Cage activities and time spent being active per 24 h by mdx mice that wheel ran (Run) were not different relative to those of mdx mice that were housed in traditional mouse cages without wheels (sedentary, Sed) indicating that chronic exercise did not induce physical inactivity. Data are mean, SE.
Treadmill Running and Acute Cage Activities
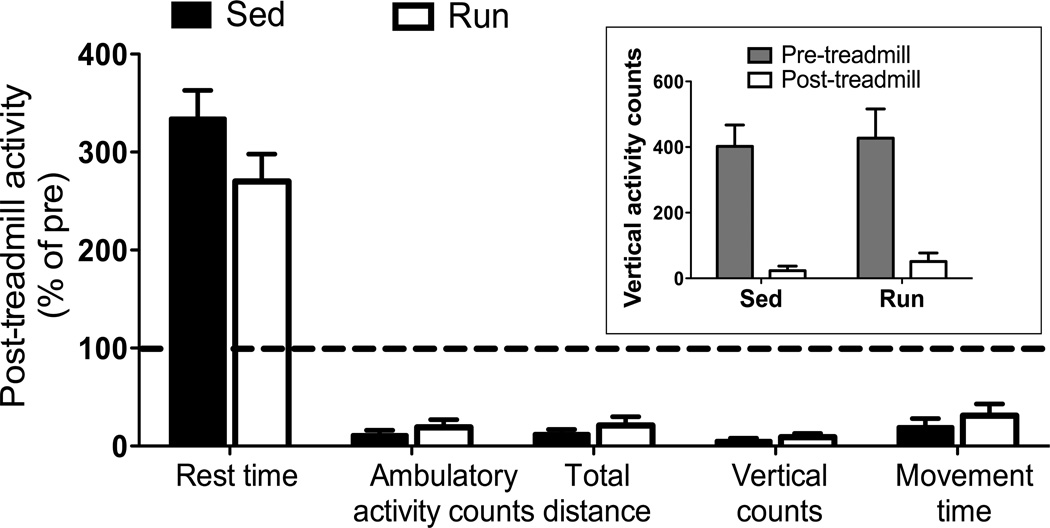
We used a previously-described exercise test for mdx mice to determine if chronic exercise training would affect acute physical activity immediately following a novel bout of forced-treadmill running. All mice regardless of group substantially decreased their voluntary activity during the 30 min following forced running on a treadmill. For example, vertical activity counts were only 5 and 9% of pre-treadmill levels in both Sed and Run groups, respectively (Figure 2 inset; P=0.424). Likewise, there were no differences between Sed and Run mdx mice for any other measurement of post-treadmill physical activity (Figure 2; P≥0.155).
Figure 2.
Severe inactivity displayed by mdx mice following an acute bout of downhill treadmill exposure regardless of exercise group (P≥0.283). Relative to ambulation (activity counts or distance), vertical counts, and movement time immediately before the treadmill episode, all mdx mice reduced these activities drastically immediately after being removed from the treadmill. Inset: Absolute counts of vertical activity during the 30 min before and after the treadmill episode. Data are mean, SE (P≥0.155 for Sed-Run comparison).
Serum CK, and Body and Muscle Masses
At the end of the 12-wk study, CK activity in the serum of Sed and Run mdx mice was not different (Table 1). Body masses did not differ between Sed and Run groups at the end of the study (Table 1) or at wk 0 through 11 (P≥0.168). Heart mass trended toward being greater in the Run mice (Table 1) and was significantly greater than Sed mice when expressed relative to body mass (4.6±0.2 and 3.9±0.1 mg/g, respectively; P=0.001). Gastrocnemius and tibialis anterior muscle masses did not differ between Sed and Run mdx mice (Table 1).
Table 1.
Creatine kinase activity and body and muscle masses of mdx mice that had been sedentary or voluntarily ran on wheels for 12 weeks.
| Sed (n=11)* | Run (n=12)* | P-values | |
|---|---|---|---|
| Serum creatine kinase activity (U/l) | 1286 ± 82 | 1162 ± 92 | 0.338 |
| Body mass (g) | 33.2 ± 1.5 | 31.6 ± 0.7 | 0.318 |
| Heart mass (mg) | 128.1 ± 5.5 | 144.1 ± 6.5 | 0.075 |
| Gastrocnemius muscle mass (mg) | 175.0 ± 7.2 | 173.6 ± 10.3 | 0.913 |
| Tibialis anterior muscle mass (mg) | 65.8 ± 2.7 | 65.2 ± 1.9 | 0.867 |
Values are means ± SE.
n’s for creatine kinase activity were 7 and 8, respectively.
In Vivo Plantarflexor Muscle Function
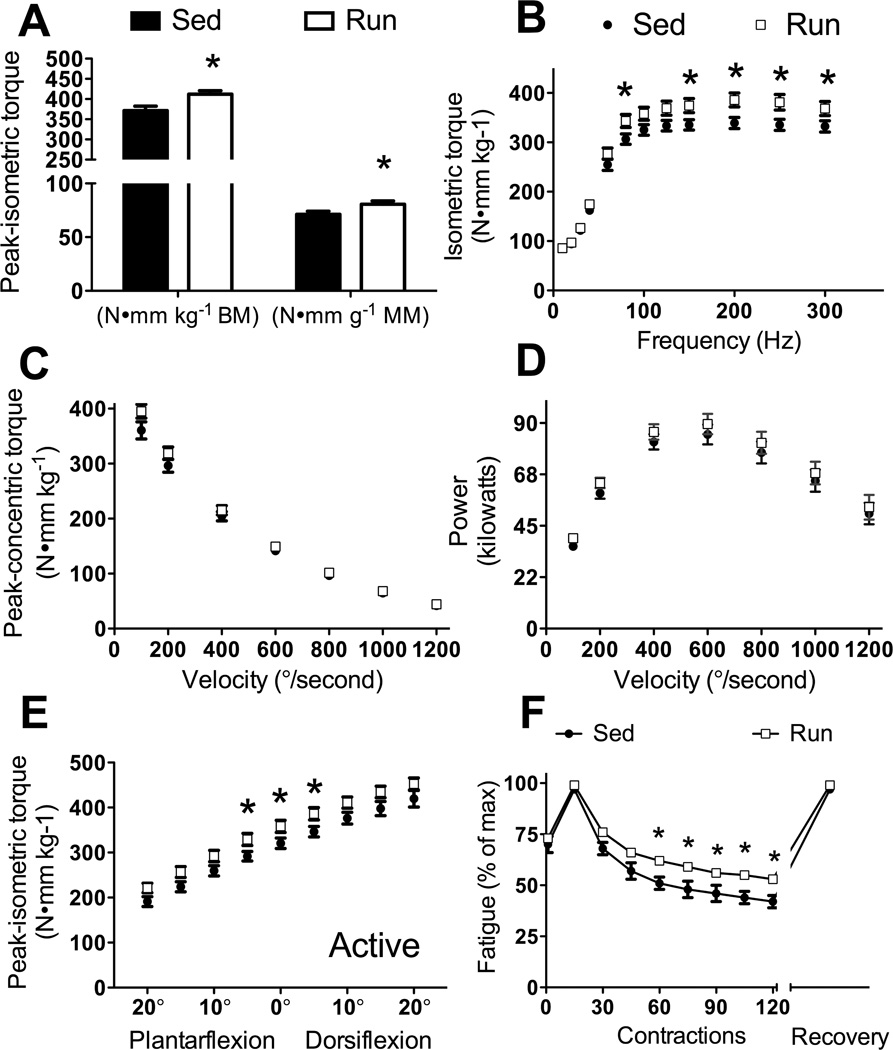
Run mice had 11% greater maximal isometric torque compared to Sed mice (P=0.010; Figure 3A, left bars). Additionally, maximal-isometric torque normalized by gastrocnemius muscle mass was 13% greater in Run mice compared to Sed mice (P=0.048; Figure 3A, right bars) indicating that plantarflexor muscles beneficially adapted to the daily running. During ambulation, rarely are muscles performing maximal isometric contractions at an ankle-joint angle of 90°, therefore muscle function at submaximal activation frequencies, as well as at different velocities and various joint angles was tested. Corresponding with maximal-isometric torque, sub-maximal torque at many frequencies were significantly greater in Run compared to Sed mice (80, 150, 200, 250, 300 Hz, P≤0.047; Figure 3B).
Figure 3.
In vivo contractility of the plantarflexors (gastrocnemius, plantaris, soleus muscles) improved with 12 wk of voluntary wheel running by mdx mice (Run) compared to mdx mice that did not wheel run (Sed). A. Peak isometric torque normalized to body mass (left bars) and gastrocnemius muscle mass (right bars). B. Isometric torque as a function of stimulation frequency. C. Concentric torque production as a function of ankle angular velocity. D. Power generated by the plantarflexors as a function of ankle angular velocity. E. Peak isometric torque at nine ankle positions with 0° defined as the foot being perpendicular to the tibia. F. Torque loss from a fatiguing bout of contractions. Fatigue was calculated as a percentage of the greatest torque generated during the 120-contraction protocol. Relative torques of 1st and every 15th contraction during the protocol and a recovery torque 10 min following the 120th contraction are plotted. Data are mean, SE and error bars not seen are contained within the symbol. *, Significantly different from Sed.
During ambulation, plantarflexion torque is required for forward motion changes depending on velocity of movement and load being moved. To determine whether wheel running altered plantarflexion contraction velocity or power, concentric torque was tested at different angular velocities. There were no significant differences in concentric torques between Sed and Run mice at any velocity, although torque at 100°/s was trending toward significance (P=0.09; Figure 3C). Collectively, maximal velocity was calculated to be 1537°/s for both Sed and Run mice. Power was not affected by wheel running (P≥0.096) and collectively maximal power was achieved at 39% of maximal torque and 40% of maximal velocity at an angular velocity of 600°/s (Figure 3D).
Isometric torque was tested at different ankle-joint angles to determine if wheel running induced changes in the isometric torque-angle relationship. Run mice generated ~12% greater isometric torques at 5° dorsiflexion, 0°, and 5° plantarflexion compared to Sed mice (P≤0.041; Figure 3E). Other joint angles did not reach statistical significance, but all showed trends for isometric torque being greater for Run compared to Sed mice (P≤0.072). Passive torque was tested at different ankle-joint angles to determine if wheel running induced changes in muscle compliance. Passive torque about the ankle joint was not significantly affected by wheel running at any ankle-joint angle (P≥0.056).
Finally, a fatiguing protocol was designed to sufficiently stress the metabolic capacity of the plantarflexors without inducing muscle damage. Run mice had greater fatigue resistance than Sed mice (Figure 3F). By the end of the fatiguing protocol, Run mice were generating 53% of peak torque while Sed mice were only generating 42% (P≤0.05; Figure 3F). Ten min after the fatiguing protocol, isometric torque returned to pre-fatigue values validating fatigue and the absence of muscle injury.
Mitochondrial Analyses
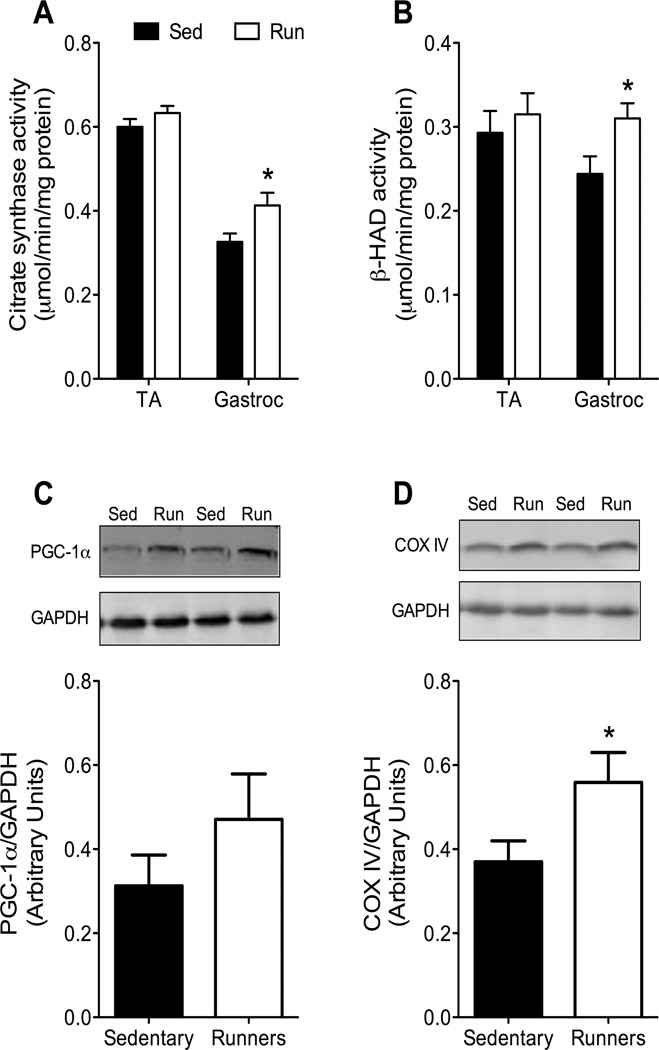
Citrate synthase and β-HAD specific activities in tibialis anterior muscle were not different between Sed and Run mice (P≥0.224; Figure 4A–B). However, specific enzyme activities in gastrocnemius muscle were 27% higher in Run than Sed mdx mice (P≤0.025; Figure 4A–B). Although the abundance of PGC-1α protein was up to 50% higher in gastrocnemius muscles of Run than Sed mice, this did not reach statistical significance (P=0.243; Figure 4C). COX IV protein expression in gastrocnemius muscle was significantly higher in Run compared with Sed mice (P=0.044; Figure 4D).
Figure 4.
Mitochondrial adaptations in gastrocnemius muscles of mdx mice what wheel ran for 12 wk. A–B. Specific activities of mitochondrial enzymes in gastrocnemius muscles, but not tibialis anterior (TA) muscles, were greater in Run than Sed mdx. β-HAD, beta-hydroxy acyl-CoA dehydrogenase. C–D. Expression of proteins involved in mitochondrial biogenesis in gastrocnemius muscles of mdx mice. Top: Western immunoblots of peroxisome proliferator-activated receptor γ-coactivator 1α (PGC-1α) and cytochrome c oxidase IV (COX IV) proteins from Sed and Run mdx mice. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was measured as a loading control. Bottom: Quantification of the relative amounts of proteins after normalizing to GAPDH. *, Significantly different from Sed.
Histo-morphological Assessments
There was no effect of wheel running on the percentage of tibialis anterior or diaphragm fibers that had central nuclei (P≥0.405). Tibialis anterior muscles from Sed and Run mdx mice contained 74±2 and 72±1% centrally-nucleated fibers, respectively, and diaphragm 34±1 and 32±2%, respectively. Gastrocnemius muscles, however, from Run mice had significantly fewer centrally-nucleated fibers than those from Sed mice (Table 2). Fiber type distribution for the medial gastrocnemius muscle was not significantly affected by wheel running, although a trend existed for muscles from Run mice to contain a higher percentage of type 2x fibers relative to those from Sed mice (Table 2). The CSA of type 2 fibers were not different between gastrocnemius muscles from Sed and Run mice (Table 2).
Table 2.
Histo-morphology of the medial head of gastrocnemius muscles from mdx mice that were sedentary or voluntarily ran on wheels for 12 weeks.
| Sed (n=4) | Run (n=5) | P-values | |
|---|---|---|---|
| Centrally-nucleated fibers (%) | 68.5 ± 3.2 | 57.2 ± 2.1 | 0.018 |
| Type 1 fibers (%) | 0.9 ± 0.2 | 2.0 ± 1.3 | 0.506 |
| Type 2a fibers (%) | 5.2 ± 1.3 | 8.4 ± 4.3 | 0.540 |
| Type 2x fibers (%) | 6.2 ± 1.4 | 18.0 ± 4.7 | 0.068 |
| Type 2b fibers (%) | 87.7 ± 2.6 | 71.7 ± 9.7 | 0.197 |
| Type 2a CSA (µm2) | 887 ± 379 | 708 ± 103 | 0.627 |
| Type 2x CSA (µm2) | 1597 ± 227 | 1385 ± 180 | 0.483 |
| Type 2b CSA (µm2) | 2428 ± 85 | 1975 ± 265 | 0.187 |
Values are means ± SE.
CSA, cross-sectional area.
Discussion
To help determine if exercise might be a therapeutic strategy to preserve functional abilities of individuals with DMD, we used young mdx mice and investigated skeletal muscle outcomes following 12 wk of voluntary wheel running. Our results support the hypothesis that in vivo muscle strength and fatigue resistance are greater in exercised compared to sedentary mdx mice and that mitochondrial adaptations contribute to the beneficial, exercise-induced skeletal muscle remodeling. Furthermore, there was no indication of exercise-induced injury or disease exacerbation. While there are clear limitations of translating findings from mdx mice to patients with DMD, our results corroborate and extend many other reports that exercise can be favorable to muscle lacking dystrophin.
The results of the present study show that maximal torque of the ankle plantarflexors was greater in mdx mice that engaged in 12 wk of exercise compared to sedentary mdx mice, and this occurred at multiple ankle joint angles (Figure 3). Furthermore, these strength gains occurred at functionally-relevant, sub-maximal frequencies (15). The ~12% gains in strength were apparent when torque was normalized to gastrocnemius muscle mass indicating that exercise induced some intrinsic improvement in that muscle. Our in vivo plantarflexor functional results are in line with previous results showing that low-intensity types of exercise improved soleus muscle maximal isometric specific force (18–20). The mechanism(s) by which exercise improves specific strength (i.e., force or torque normalized for muscle size) in mdx mice has not been elucidated but theoretically may involve improved acto-myosin function or some enhanced ability to transmit the force that is generated.
Exercise-induced shifts in fiber types have previously been reported in mdx muscle (18, 19, 21, 27), and in the present study fiber type transformation was also apparent as there were trends for more IIx and less IIb fibers in the gastrocnemius muscle (Table 2). Shifts in fiber MHC isoform expression are often associated with changes in oxidative capacity, which in turn can be related to fatigue. Previous studies have shown that isolated mdx soleus and EDL muscles are more resistant to fatiguing contractions as a result of chronic exercise training (18–20, 39). In the present study we extend those results to show that plantarflexors have improved fatigue-resistance in vivo as a result of 12 wk of low-intensity exercise. Fatigue resistance was directly shown in our study as torque loss was less in muscles from Run compared to Sed mdx mice (Figure 4). Also, the reduction in torque that resulted from the fatiguing protocol was completely recovered 10 min later. It is essential to show this recovery index, particularly for dystrophic muscle, to assure that fatigue and not contraction-induced injury had occurred. These results on the plantarflexors are an important finding because ankle plantarflexion is critical for ambulation and wheel running (35, 40) and because the major plantarflexor muscle, the gastrocnemius, is severely affected by the loss of dystrophin (13, 30).
Fatigue resistance following chronic exercise training by mdx mice is also important to evaluate because recently it was suggested that mdx mice exhibit an exaggerated fatigue response (25). We also found that mdx mice become extremely inactive immediately following a brief exposure to downhill treadmill running, regardless of whether or not the mice had been wheel-run exercising the past 12 wk (Figure 2). These results would seem to imply that fatigue resistance was not improved by chronic exercise in our study, but several observations argue against this conclusion. Among the 10 mdx mice that were subjected to the treadmill experiment there was a wide range of compliance, as has been reported previously (17). Some mice were easily able to complete the 15 min protocol with little hand prodding, while other mice found this exercise protocol extremely difficult and resisted running; regardless, all mdx mice exhibited the severe inactivity when placed back in a cage (Figure 2). Also, it seems unlikely that walking/running 175 m on the treadmill was a fatiguing event when some of these same mice voluntarily ran on a wheel 3000–5000 m per night and had the same 24-h cage activities as mice that did not wheel run (Figure 1). Though results of the present study cannot explain the exaggerated inactivity displayed by mdx following exposure to downhill treadmill running, we offer that it does not reflect muscle fatigue. Rather we suggest that mdx mice exhibit some stress response that manifests as physical inactivity reflecting the animal’s willingness to move, not so much its physical ability to move, and as shown by Kobayashi and co-workers is related to nNOS biology (25).
Comparable with the plantarflexor fatigue resistance that resulted from chronic exercise training, mitochondrial adaptations occurred in gastrocnemius muscle of mdx mice that wheel ran. Previously we reported that mitochondrial enzyme activities did not improve with exercise in muscles of mdx mice as they did in C57BL mice (27). However, in our previous study enzyme activities were normalized to muscle wet masses and because mdx muscles contain relatively more fluid and less non-collagen protein than corresponding muscles from C57BL mice (13, 14), normalizing enzyme activities to protein content and determining specific enzyme activity is more appropriate. To re-address the issue, and to determine if a longer duration of low intensity exercise would better induce mitochondrial adaptations, mitochondrial enzyme activities and proteins known to be key in exercise-induced mitochondrial biogenesis were analyzed. Citrate synthase and β-HAD enzyme activities, as well as COX IV protein, were ~25% greater in gastrocnemius muscle from Run compared to Sed mdx mice (Figure 4). PGC-1α, a transcriptional co-activator of COX IV, was not significantly different between groups, perhaps due to a time-of-sampling effect and/or due to larger biological variation between samples within each group. In sum, these data indicate that exercise training adaptations in mitochondrial are intact, especially considering that the adaptations occurred in muscle of mdx mice that wheel ran on average only 3.4 km/24 h over the course of the study (Figure 1A), which is significantly less than what healthy, wildtype mice run (11, 19, 27, 39).
The improvements in strength, fatigue resistance, and oxidative capacity were realized after 12 wk of exercise by mdx mice with no indication of muscle injury or disease exacerbation. Foremost, in this study we show that all functional parameters of the plantarflexors were better or not different in Run compared to Sed mice (Figure 3), indicating that chronic muscle injury had not occurred in those muscles. Similar results have been reported for soleus and EDL muscle function following exercise training by mdx mice (11, 18–20, 39). If major muscle groups other than the plantarflexors had been detrimentally affected by wheel running, we would have expected that 24-h cage activities would have been lower and serum CK activity higher in Run than Sed mice, and those differences did not occur (Figure 1B and Table 1). Our findings on cage activities are novel, while others have reported similar results for CK activity post-exercise training in mdx mice (7, 9). Centrally-nucleated fibers are another indicator of muscle damage/regeneration that is frequently monitored in dystrophic mice and that parameter was not worsened by chronic exercise in gastrocnemius, tibialis anterior, or diaphragm muscle. In fact, a smaller percentage of gastrocnemius fibers from Run mdx mice had central nuclei compared to those from Sed mice (Table 2). How this occurred is intriguing to consider. Previously we showed that the percentage of abnormally large fibers in soleus and EDL muscles of mdx mice were significantly reduced after exercise training (27), and those very large fibers typically had central nuclei (unpublished observations). Thus, it is conceivable that exercise expedites loss of abnormally large, centrally-nucleated, perhaps low force-generating fibers; this speculation needs to be addressed in future studies. Collectively, we have complied with the suggested standard outcome measures for DMD pre-clinical experiments using mdx mice (17) and show that appropriate exercise can be designed to benefit skeletal muscle without worsening disease pathology.
The appropriate design of an exercise intervention is an important consideration. The exercise-induced benefits for mdx mice in terms of strength gains and fatigue resistance reported here and by others were realized following 10–16 wk of wheel running or swimming exercise (18–20, 39), while 3–4 wk of wheel running may (7) or may not (9) elicit improved muscle function. These results suggest that duration is an important exercise design parameter. Indeed, acute exercise of short duration can cause injury to untrained skeletal muscle in dystrophic (e.g., (33)) as well as wildtype mice (22). Mode and intensity are also important exercise parameters to be considered. For example, moderate to high intensity treadmill exercise is recommended to exacerbate pathology in mdx mice (17), while lower intensity, chronic treadmill training has been shown to be beneficial (24). Because the two types of exercise that are used to elicit contraction-induced injury in mdx mice are forced and relatively high intensity and/or are predominated by eccentric contractions, it is possible that beneficial outcomes were realized in the present study because the exercise was low intensity and was voluntary whereby permitting intermittent bouts of activity as mdx mice engaging and disengaging in wheel running at will (27). Several reviews and workshop reports have been devoted to the topic of DMD and exercise or physical activity over the past decade (2, 3, 8, 12, 16, 29, 31) and a deficiency noted in most of those articles is that the optimal exercise parameters for DMD are not known. Recently, a large clinical interventional study called No Use is Disuse (NUD) has been described and the outcomes of that study will likely extend our knowledge regarding exercise parameters for patients with DMD (23).
In conclusion, our study shows that 12 wk of a voluntary, low-intensity exercise regimen can improve strength, fatigue resistance, and mitochondrial oxidative capacity of ankle plantarflexion muscles of mdx mice. In addition, there was no indication that muscle was detrimentally affected by the exercise. These findings in mdx mice indicate that exercise should be further studied as a potential therapy to help slow the progression of functional declines in DMD.
Acknowledgements
This research was supported by a grant from the Muscular Dystrophy Association (DAL) and grants from the NIH; K02-AG036827 (DAL), P30-AR0507220 (University of Minnesota Muscular Dystrophy Center), R01-AR050429 (ZY), and T32-AR07612 provided support to KAB and JAC. The authors thank Gordon Warren for suggesting and modifying the in vivo plantarflexor contractility analyses for our study. We also thank the following University of Minnesota DPT students for their contributions: Danelle Dommer, Tara Kelly, Rachel Larson, Wyatt LaVigne, Megan Liebaert, Devin Ludowese, Paige Neeser, Alison Pagliaccetti, Joseph Schoess, and Kaitlin Wagner.
Footnotes
Results of the present study do not constitute endorsement by ACSM and the authors disclose no conflict of interest.
References
- 1.Akimoto T, Ribar TJ, Williams RS, Yan Z. Skeletal muscle adaptation in response to voluntary running in Ca2+/dependent protein kinase IV-deficient mice. Am J Physiol Cell Physiol. 2004;287:C1311–C1319. doi: 10.1152/ajpcell.00248.2004. [DOI] [PubMed] [Google Scholar]
- 2.Ansved T. Muscle training in muscular dystrophies. Acta Physiol Scand. 2001;171:359–366. doi: 10.1046/j.1365-201x.2001.00839.x. [DOI] [PubMed] [Google Scholar]
- 3.Ansved T. Muscular dystrophies: influence of physical conditioning on the disease evolution. Curr Opin Clin Nutr Metab Care. 2003;6:435–439. doi: 10.1097/01.mco.0000078987.18774.D9. [DOI] [PubMed] [Google Scholar]
- 4.Baltgalvis KA, Call JA, Nikas JB, Lowe DA. Effects of prednisolone on skeletal muscle contractility in mdx mice. Muscle Nerve. 2009;40:443–454. doi: 10.1002/mus.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Call JA, Ervasti JM, Lowe DA. TAT-muUtrophin mitigates the pathophysiology of dystrophin and utrophin double-knockout mice. J Appl Physiol. 2011;111:200–205. doi: 10.1152/japplphysiol.00248.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Call JA, McKeehen JN, Novotny SA, Lowe DA. Progressive resistance voluntary wheel running in the mdx mouse. Muscle Nerve. 2010;42:871–880. doi: 10.1002/mus.21764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Call JA, Voelker KA, Wolff AV, McMillan RP, Evans NP, Hulver MW, Talmadge RJ, Grange RW. Endurance capacity in maturing mdx mice is markedly enhanced by combined voluntary wheel running and green tea extract. J Appl Physiol. 2008;105:923–932. doi: 10.1152/japplphysiol.00028.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter GT, Abresch RT, Fowler WM., Jr Adaptations to exercise training and contraction-induced muscle injury in animal models of muscular dystrophy. Am J Phys Med Rehabil. 2002;81:S151–S161. doi: 10.1097/00002060-200211001-00016. [DOI] [PubMed] [Google Scholar]
- 9.Carter GT, Wineinger MA, Walsh SA, Horasek SJ, Abresch RT, Fowler WM., Jr Effect of voluntary wheel-running exercise on muscles of the mdx mouse. Neuromuscul Disord. 1995;5:323–332. doi: 10.1016/0960-8966(94)00063-f. [DOI] [PubMed] [Google Scholar]
- 10.de Lateur BJ, Giaconi RM. Effect on maximal strength of submaximal exercise in Duchenne muscular dystrophy. Am J Phys Med. 1979;58:26–36. [PubMed] [Google Scholar]
- 11.Dupont-Versteegden EE, McCarter RJ, Katz MS. Voluntary exercise decreases progression of muscular dystrophy in diaphragm of mdx mice. J Appl Physiol. 1994;77:1736–1741. doi: 10.1152/jappl.1994.77.4.1736. [DOI] [PubMed] [Google Scholar]
- 12.Eagle M. Report on the muscular dystrophy campaign workshop: exercise in neuromuscular diseases Newcastle, January 2002. Neuromuscul Disord. 2002;12:975–983. doi: 10.1016/s0960-8966(02)00136-0. [DOI] [PubMed] [Google Scholar]
- 13.Garlich MW, Baltgalvis KA, Call JA, Dorsey LL, Lowe DA. Plantarflexion contracture in the mdx mouse. Am J Phys Med Rehabil. 2010;89:976–985. doi: 10.1097/PHM.0b013e3181fc7c9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glesby MJ, Rosenmann E, Nylen EG, Wrogemann K, Serum CK. calcium, magnesium, and oxidative phosphorylation in mdx mouse muscular dystrophy. Muscle Nerve. 1988;11:852–856. doi: 10.1002/mus.880110809. [DOI] [PubMed] [Google Scholar]
- 15.Gorassini M, Eken T, Bennett DJ, Kiehn O, Hultborn H. Activity of hindlimb motor units during locomotion in the conscious rat. J Neurophysiol. 2000;83:2002–2011. doi: 10.1152/jn.2000.83.4.2002. [DOI] [PubMed] [Google Scholar]
- 16.Grange RW, Call JA. Recommendations to define exercise prescription for Duchenne muscular dystrophy. Exerc Sport Sci Rev. 2007;35:12–17. doi: 10.1249/01.jes.0000240020.84630.9d. [DOI] [PubMed] [Google Scholar]
- 17.Grounds MD, Radley HG, Lynch GS, Nagaraju K, De Luca A. Towards developing standard operating procedures for pre-clinical testing in the mdx mouse model of Duchenne muscular dystrophy. Neurobiol Dis. 2008;31:1–19. doi: 10.1016/j.nbd.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes A, Lynch GS, Williams DA. The effects of endurance exercise on dystrophic mdx mice. I. Contractile and histochemical properties of intact muscles. Proc R Soc Lond B Biol Sci. 1993;253:19–25. doi: 10.1098/rspb.1993.0077. [DOI] [PubMed] [Google Scholar]
- 19.Hayes A, Williams DA. Beneficial effects of voluntary wheel running on the properties of dystrophic mouse muscle. J Appl Physiol. 1996;80:670–679. doi: 10.1152/jappl.1996.80.2.670. [DOI] [PubMed] [Google Scholar]
- 20.Hayes A, Williams DA. Contractile function and low-intensity exercise effects of old dystrophic (mdx) mice. Am J Physiol. 1998;274:C1138–C1144. doi: 10.1152/ajpcell.1998.274.4.C1138. [DOI] [PubMed] [Google Scholar]
- 21.Hayes A, Williams DA. Contractile properties of clenbuterol-treated mdx muscle are enhanced by low-intensity swimming. J Appl Physiol. 1997;82:435–439. doi: 10.1152/jappl.1997.82.2.435. [DOI] [PubMed] [Google Scholar]
- 22.Irintchev A, Wernig A. Muscle damage and repair in voluntarily running mice: strain and muscle differences. Cell Tissue Res. 1987;249:509–521. doi: 10.1007/BF00217322. [DOI] [PubMed] [Google Scholar]
- 23.Jansen M, de Groot IJ, van Alfen N, Geurts A. Physical training in boys with Duchenne Muscular Dystrophy: the protocol of the No Use is Disuse study. BMC Pediatr. 2010;10:55. doi: 10.1186/1471-2431-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaczor JJ, Hall JE, Payne E, Tarnopolsky MA. Low intensity training decreases markers of oxidative stress in skeletal muscle of mdx mice. Free Radic Biol Med. 2007;43:145–154. doi: 10.1016/j.freeradbiomed.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi YM, Rader EP, Crawford RW, Iyengar NK, Thedens DR, Faulkner JA, Parikh SV, Weiss RM, Chamberlain JS, Moore SA, Campbell KP. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456:511–515. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kregel KC, Allen DL, Booth FW, Fleshner MR, Henriksen EJ, Musch TI, O'Leary DS, Parks CM, Poole DC, Ra'anan AW, Sheriff DD, Sturek MS, Toth LA. Resource Book for the Design of Animal Exercise Protocols. Vol. 152. American Physiological Society; 2006. [Google Scholar]
- 27.Landisch RM, Kosir AM, Nelson SA, Baltgalvis KA, Lowe DA. Adaptive and nonadaptive responses to voluntary wheel running by mdx mice. Muscle Nerve. 2008;38:1290–1303. doi: 10.1002/mus.21141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowe DA, Williams BO, Thomas DD, Grange RW. Molecular and cellular contractile dysfunction of dystrophic muscle from young mice. Muscle Nerve. 2006;34:92–100. doi: 10.1002/mus.20562. [DOI] [PubMed] [Google Scholar]
- 29.Markert CD, Ambrosio F, Call JA, Grange RW. Exercise and Duchenne muscular dystrophy: toward evidence-based exercise prescription. Muscle Nerve. 2011;43:464–478. doi: 10.1002/mus.21987. [DOI] [PubMed] [Google Scholar]
- 30.Mathur S, Lott DJ, Senesac C, Germain SA, Vohra RS, Sweeney HL, Walter GA, Vandenborne K. Age-related differences in lower-limb muscle cross-sectional area and torque production in boys with Duchenne muscular dystrophy. Arch Phys Med Rehabil. 2010;91:1051–1058. doi: 10.1016/j.apmr.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald CM. Physical activity, health impairments, and disability in neuromuscular disease. Am J Phys Med Rehabil. 2002;81:S108–S120. doi: 10.1097/00002060-200211001-00012. [DOI] [PubMed] [Google Scholar]
- 32.Petrof BJ. The molecular basis of activity-induced muscle injury in Duchenne muscular dystrophy. Mol Cell Biochem. 1998;179:111–123. doi: 10.1023/a:1006812004945. [DOI] [PubMed] [Google Scholar]
- 33.Radley-Crabb H, Terrilln J, Shavlakadze T, Tonkin J, Arthur P, Grounds M. A single 30min treadmill exercise session is suitable for 'proof-of concept studies' in adult mdx mice: A comparison of the early consequences of two different treadmill protocols. Neuromuscul Disord. 2011;22:170–182. doi: 10.1016/j.nmd.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Scott OM, Hyde SA, Goddard C, Jones R, Dubowitz V. Effect of exercise in Duchenne muscular dystrophy. Physiotherapy. 1981;67:174–176. [PubMed] [Google Scholar]
- 35.Thota AK, Watson SC, Knapp E, Thompson B, Jung R. Neuromechanical control of locomotion in the rat. J Neurotrauma. 2005;22:442–465. doi: 10.1089/neu.2005.22.442. [DOI] [PubMed] [Google Scholar]
- 36.Vignos PJ, Jr, Watkins MP. The effect of exercise in muscular dystrophy. Jama. 1966;197:843–848. [PubMed] [Google Scholar]
- 37.Warren GL, Stallone JL, Allen MR, Bloomfield SA. Functional recovery of the plantarflexor muscle group after hindlimb unloading in the rat. Eur J Appl Physiol. 2004;93:130–138. doi: 10.1007/s00421-004-1185-3. [DOI] [PubMed] [Google Scholar]
- 38.Wheatley CM, Wilkins BW, Snyder EM. Exercise is medicine in cystic fibrosis. Exerc Sport Sci Rev. 2011;39:155–160. doi: 10.1097/JES.0b013e3182172a5a. [DOI] [PubMed] [Google Scholar]
- 39.Wineinger MA, Abresch RT, Walsh SA, Carter GT. Effects of aging and voluntary exercise on the function of dystrophic muscle from mdx mice. Am J Phys Med Rehabil. 1998;77:20–27. doi: 10.1097/00002060-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Wu H, Rothermel B, Kanatous S, Rosenberg P, Naya FJ, Shelton JM, Hutcheson KA, DiMaio JM, Olson EN, Bassel-Duby R, Williams RS. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. Embo J. 2001;20:6414–6423. doi: 10.1093/emboj/20.22.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]