SUMMARY
Background
During epithelial morphogenesis, a complex comprised of the βPIX (PAK-interacting exchange factor β) and class I PAKs (p21-activated kinases) is recruited to adherens junctions. Scrib, the mammalian orthologue of the Drosophila polarity determinant and tumor suppressor Scribble, binds βPIX directly. Scrib is also targeted to adherens junctions by E-cadherin, where Scrib strengthens cadherin-mediated cell-cell adhesion. While a role for the Scrib-βPIX-PAK signaling complex in promoting membrane protrusion at wound edges has been elucidated, a function for this complex at adherens junctions remains unknown.
Results
Here, we establish that Scrib targets βPIX and PAK2 to adherens junctions where a βPIX-PAK2 complex counterbalances apoptotic stimuli transduced by Scrib and elicited by cadherin-mediated cell-cell adhesion. Moreover, we show that this signaling pathway regulates cell survival in response to osmotic stress. Finally, we determine that in suspension cultures, the Scrib-βPIX-PAK2 complex functions to regulate anoikis elicited by cadherin engagement, with Scrib promoting and the βPIX-PAK2 complex suppressing anoikis, respectively.
Conclusions
Our findings demonstrate that the Scrib-βPIX-PAK2 signaling complex functions as an essential modulator of cell survival when localized to adherens junctions of polarized epithelia. The activity of this complex at adherens junctions is thereby essential for normal epithelial morphogenesis and tolerance of physiological stress. Furthermore, when localized to adherens junctions, the Scrib-βPIX-PAK2 signaling complex serves as a key determinant of anoikis sensitivity, a pivotal mechanism in tumor suppression. Thus, this work also reveals the need to expand the definition of anoikis to include a central role for adherens junctions.
INTRODUCTION
The importance of epithelial architecture in the suppression of tumorigenesis is highlighted by recent work, but many of the underlying mechanisms remain elusive [1]. Genetic screens in Drosophila have identified several protein complexes that serve not only as essential regulators of epithelial polarity but also as potent tumor suppressors [2]. Loss of the Drosophila polarity protein Scribble (Scrib) facilitates metastatic dissemination of tumors initiated by oncogenes such as Ras or Notch. Surprisingly, in mammals, Scrib has not been found to play an essential role in polarity establishment. In polarizing epithelial cells, Scrib is recruited to adherens junctions in response to E-cadherin engagement where it promotes junctional stability and inhibits motility [3, 4]. Scrib associates directly with the Rho family guanine nucleotide exchange factor βPIX [5]. The best-characterized role of the Scrib-βPIX complex is in promoting actin polymerization and membrane protrusion at the leading edge of motile cells [2]. However, in polarized epithelial cells, βPIX localizes to adherens junctions [6]. Here, we demonstrate that when localized to adherens junctions, Scrib and βPIX, together with the p21-activated kinase 2 (PAK2), play essential roles in modulating cell-cell contact-dependent survival signaling, which impact our current understanding of Scrib and cadherins as epithelial tumor suppressors.
RESULTS
βPIX is an essential epithelial survival factor
To elucidate the role of βPIX and associated molecules in cell-cell contact-dependent signaling, we utilized inducible expression of short hairpin RNAs (shRNAs). Madin-Darby Canine Kidney (MDCK) cells expressing a previously validated βPIX shRNA [7], exhibited robust knockdown of βPIX (βPIX-kd) in the presence of doxycycline (dox), but no appreciable knockdown in the absence of dox (Figure S1A). As no cell line showed knockdown in the absence of dox, all subsequent experiments were performed under induced conditions with vector transduced MDCK cells serving as negative control (Figure S1B).
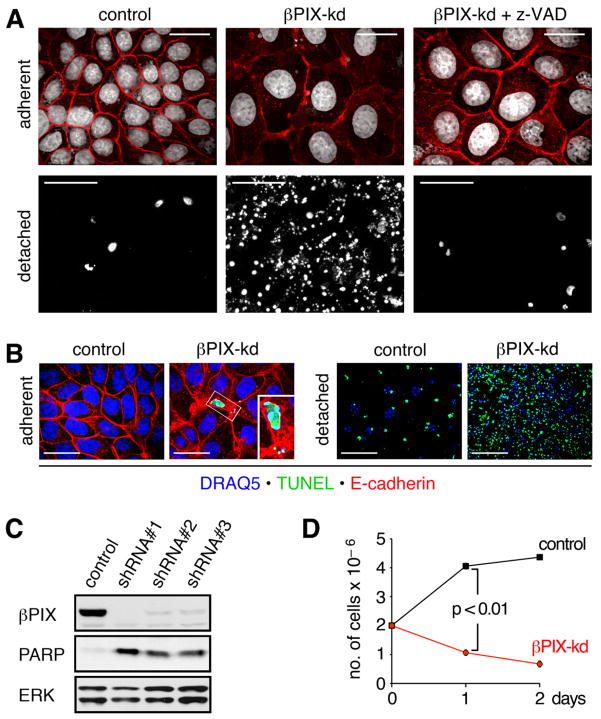
βPIX knockdown at low cell density resulted in fully viable cultures, which exhibited disorganized monolayer morphology and enhanced cell spreading (Figure S1C). βPIX-depleted MDCK cells could readily be propagated, provided the cultures were maintained at sparse density. However, at high density, βPIX-kd cultures succumbed to what visually appeared to be substantial apoptotic death (Figure S1C). To better define the role for βPIX in survival signaling, we devised a strategy for acutely inducing high-density cell-cell contact in adherent cultures. In this protocol, cells from fully viable low-density cultures are harvested and replated at confluent density and form a well-organized monolayer with no cell multilayering. Under these conditions, both control and βPIX depleted cells rapidly adhered to the culture plate and established intercellular adhesions (Figure 1A; adherent). Acute replating of βPIX-kd cells at confluent density, again resulted in apparent apopototic cell death, evidenced by the accumulation of detached cells in the medium (Figure 1A; detached). In contrast, minimal death was observed in control cells. While we detected very few TUNEL positive adherent cells in βPIX-depleted cultures, the vast majority of detached cells stained positively (Figure 1B). This result suggested that high-density contact of βPIX-kd cells elicited apoptosis, which was followed by rapid cell extrusion. To confirm this, we treated βPIX-kd cells with the pan-caspase inhibitor z-VAD-FMK at the time of replating. While z-VAD-FMK treatment did not prevent morphological effects of βPIX-depletion (Figure 1A; adherent), it attenuated cell death, as well as cell detachment (Figure 1A; detached). Examination of the extrusion process revealed the formation of a purse string around apoptotic cells in the process of extrusion. The purse string was enriched in actin and the tight junction constituent ZO-1, akin to processes previously reported to play important roles in tissue homeostasis (movie S1) [8]. To rule out off-target effects, we generated additional shRNAs targeting βPIX, which similarly elicited apoptosis upon replating, as evidenced by cleavage of the DNA-binding protein PARP (Figure 1C). shRNAs eliciting the most effective knockdown resulted in the highest levels of apoptosis (Figure S1D).
Figure 1. βPIX knockdown induces apoptosis of MDCK monolayers at high cell density.
(A) Sparse cultures of dox treated control and βPIX-kd cells were replated at a high density of 2×106 cells per 35 mm dish and cultured in the absence or presence of 50 μM z-VAD-FMK for 16 h. Adherent cells (scale bars 20 μm) were labeled with E-cadherin antibodies (red) and DRAQ5 to detect nuclei (white). Detached cells (scale bars 200 μm) were centrifuged onto coverslides and stained with DRAQ5 (white). (B) Adherent and detached cells were labeled by TUNEL (green). Detached cells were labeled with DRAQ5 to detect nuclei (blue), while adherent cells were also immunostained to detect E-cadherin (red). Scale bars for adherent and detached cells correspond to 10 μm and 100 μm, respectively. (C) Sparse cultures of control or βPIX-kd cell lines were replated at high density and cultured for 16 h. Total cell lysates of pooled adherent and detached cells were immunoblotted for βPIX, PARP, and ERK. The 24 kD PARP cleavage product generated in apoptotic cells is shown for the three most potent shRNAs targeting distinct sequences in βPIX. (D) Sparse cultures of control or βPIX-kd cells were replated at high density. After 1 and 2 days in culture, viable adherent cells, identified by trypan blue exclusion, were counted. The graph shows the number of cells determined in triplicate from a representative experiment (n=3). See also Figure S1 and Movie S1.
To better evaluate the loss of viability observed in βPIX-kd cultures, we employed a trypan blue exclusion assay to calculate cell numbers following high-density replating. As expected, βPIX-depletion resulted in a rapid and highly significant reduction in cell numbers, showing an approximately 50% and 70% reduction in viable adherent cells after 1 and 2 days, respectively (Figure 1D). Moreover, when plated on permeable supports and grown to confluence, βPIX-deficient cells formed confluent monolayers of highly spread cells with electrical resistance above controls, thus indicating that gross assembly of junctional complexes remains intact (Figure S1E). Despite robust BrdU incorporation, βPIX-kd cells achieve a maximum cell density that is 15% of control cells (6.6×105 versus 4.4×106 cells per 35 mm dish), at which point cell death and proliferation are in equilibrium (Figure S1F). Taken together, these results indicate that βPIX-deficient cells have a specific defect that renders them exquisitely sensitive to apoptosis upon cell-cell contact.
βPIX confers protection against anoikis
Anoikis is presently defined as apoptosis elicited by deprivation of cell-matrix contact and acquisition of anoikis resistance is thought to represent an obligate step in tumor progression [9, 10]. Nevertheless, normal epithelial cells grown in suspension rapidly re-establish functional cadherin-based adhesion, and a potential influence of cadherin adhesion on modulation of anoikis has largely been overlooked. Yet, many proteins, which have been assigned a function in suppression of anoikis due to their effects on cell-matrix adhesion, also signal at adherens junctions in polarized epithelia [11–13]. Our previous work established that βPIX translocates from focal adhesions in subconfluent cells to adherens junctions in confluent monolayers [6]. In this context, our current findings demonstrating that apoptosis in βPIX-depleted MDCK cells resulted from density-dependent apoptotic stimuli lead us to investigate a role for βPIX in anoikis. To this end, viable low-density cultures of control and βPIX-kd cells were trypsinized and cultured in suspension at 1×106 cells/ml. At this density, MDCK cells rapidly undergo E-cadherin-dependent aggregation. While control cells cultured under these conditions remained viable for many hours, βPIX-depleted cells exhibited rapid cell death, as evidenced by phase contrast microscopy and trypan blue permeability (Figures S2A and S2B). Moreover, βPIX-kd cells showed enhanced PARP cleavage, indicating that cell death resulted from apoptosis (Figures 2A and 2B). The obligatory requirement for βPIX signaling to suppress anoikis extended to immortalized but non-transformed porcine renal LLC-PK1 cells, as well as the relatively well-differentiated human mammary carcinoma cell lines T47D and MCF7 (Figures 2C and 2D).
Figure 2. βPIX confers protection against anoikis.
(A) Lysates of suspension cultures of dox treated control and βPIX-kd cells were processed for immunoblotting with a PARP antibody. In βPIX-depleted cells, the amount of uncleaved PARP decreased over time as the 24 kD PARP fragment, specific for apoptotic cells, accumulated. As lysates were not adjusted for protein content, the decrease in PARP signal observed in βPIX-kd cells results from the loss in cell numbers occurring over the time course. (B) The PARP signals from the representative experiment shown in (A) were quantified by densitometry and the ratio of cleaved/full-length PARP was determined. (C) LLC-PK1, T47D, and MCF7 were transfected with siRNA targeting βPIX and assayed for anoikis. (D) Anoikis index for the results shown in (C) is presented as mean ± SD of three experiments for each of LLC-PK1, T47D, and MCF7 cells (*p<0.05; **p<0.02; ***p< 0.01). It should be noted that, using a rhodamine-labeled duplex to estimate transfection efficiency, approximately 30% of cells did not take up siRNA. Thus, the results underestimate the effects of βPIX-depletion on anoikis. See also Figure S2.
Cadherins are required for eliciting apoptosis in βPIX-depleted cells
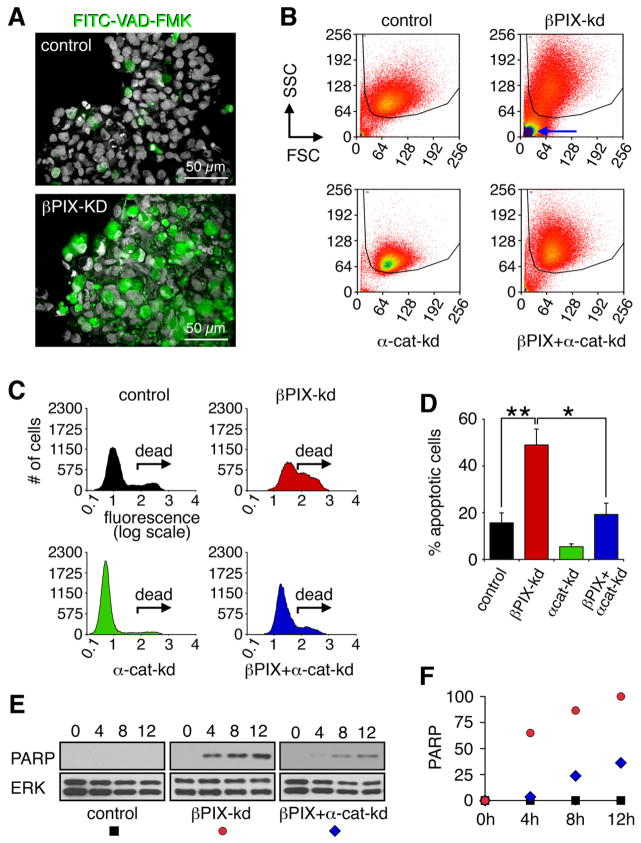
We next determined the role of cadherins in anoikis elicited by βPIX-depletion. MDCK cells express high levels of E-cadherin and cadherin-6 [3]. In addition, lower levels of seven other cadherins are expressed, as determined by mRNA profiling of MDCK cells (table S1). To avoid confounding effects stemming from functional redundancy of cadherin family members, we decided to abrogate cadherin-mediated cell-cell adhesion by knockdown of α-catenin (Figure S1B) [14]. Knockdown of α-catenin disrupted E-cadherin-dependent adhesion, as evidenced by reduced cell aggregation in suspension (Figure S3A). Control, βPIX-kd, α-cat-kd and βPIX+α-cat-kd cells were cultured overnight in suspension and pelleted onto coverslides. Knockdown of α-catenin resulted in a dramatic reduction in anoikis of βPIX-kd cells that was accompanied by internalization of E-cadherin (Figure S3B).
FITC-VAD-FMK binds irreversibly to activate caspases and provides a quantitative method for assaying apoptosis on a cellular level. When cultured in suspension, βPIX-kd cells stained positively for FITC-VAD-FMK (Figure 3A). To quantify anoikis, FITC-VAD-FMK labeled control, βPIX-kd, α-cat-kd and βPIX+α-cat-kd cells were analyzed by flow cytometry (Figure 3B). Forward and side scatter analysis revealed the presence of two distinct populations. Cellular debris including late apoptotic cells, which exhibit low forward and side scatter, are prominent in βPIX-kd samples (Figure 3B; blue arrow). These were necessarily excluded from the subsequent analysis, even though it led to an underestimation of the effects of βPIX-depletion on anoikis. The remaining βPIX-kd cells exhibited intense fluorescence labeling with FITC-VAD-FMK (Figure 3C; “dead”), with ~50% of βPIX-kd cells positively labeled compared to ~15% of control cells (Figure 3D). Consistent with a potential pro-apoptotic function for E-cadherin, we observed a reduction in FITC-VAD-FMK labeling of α-cat-kd cells (Figure 3C). Importantly, when compared to βPIX-kd cells, βPIX+α-cat-kd cells exhibited significantly diminished anoikis (Figure 3D). α-catenin-kd also conferred sustained and robust protection against anoikis at 16 h when apoptosis in βPIX-kd cells is well past peak death (Figure S2B).
Figure 3. βPIX confers protection against anoikis elicited by cadherin adhesion.
(A) Dox treated control and βPIX-kd cells were cultured in suspension for 16 h in the presence of 50 μM FITC-VAD-FMK. Cells were centrifuged onto glass slides, fixed, and stained with DRAQ5 to label nuclei (white). Apoptotic cells were identified by FITC-VAD-FMK labeling (green). Scale bars correspond to 50 μm. (B) Cells cultured in suspension for 16 h were incubated with 10 μM FITC-VAD-FMK and analyzed by flow cytometry. Forward and side scatter profiles show gated populations (50,000 cells per sample) analyzed for fluorescence intensity. The blue arrow highlights cellular debris prominent in βPIX-depleted cultures that is not included in the FACS analysis and leads to an underestimation of the amount of cell death. (C) Representative distributions of FITC-VAD-FMK labeled cells identify apoptotic populations (labeled “dead”). (D) Percentages of apoptotic cells from four independent experiments are reported as mean ± SD (*p<0.05; **p<0.02). (E) Dox treated control, βPIX-kd, and βPIX+α-cat-kd cells were replated at high cell density and processed for immunoblotting to detect cleaved PARP. (F) The PARP signal from the representative experiment shown in (E) was quantified by densitometry and presented as a percent of the signal at the 12 h time point for βPIX-kd cells. See also Figure S3 and Table S1.
We next probed the effects of α-cat-kd on apoptosis elicited by βPIX-depletion in adherent cells plated at high cell density. To this end, control, βPIX-kd cells, and βPIX+α-cat-kd cells propagated at low cell density were replated at confluent density, and total cell lysates prepared 0, 4, 8 and 12 h after replating. Compared to βPIX-kd cells, βPIX+α-cat-kd cells exhibited significantly reduced PARP cleavage, suggesting that cadherin-mediated cell adhesion promoted apoptosis in βPIX-kd cells (Figures 3E and F).
Anti-apoptotic functions independent of its role in cadherin-mediated adhesiveness have been invoked for α-catenin [15]. Therefore, as an alternate approach, we expressed dominant negative E-cadherin (DN-E-cad) to perturb cadherin function and determined the effects on anoikis in MDCK cells. Expression of DN-E-cad conferred protection against anoikis (Figures S3C and S3D). DN-E-cad also significantly attenuated anoikis elicited by depletion of βPIX (Figures S3E and F). Collectively, these data established that βPIX plays a fundamental role in suppression of apoptosis promoted by cadherin engagement, in both adherent and suspension cultures. Moreover, these results clearly demonstrate the fundamental role that adherens junction assembly plays in epithelial cell viability.
Scrib sensitizes epithelial cells to apoptosis
Scrib requires E-cadherin for targeting to lateral membranes where Scrib stabilizes adherens junctions [3, 4]. Moreover, βPIX binds directly to one or more PDZ interaction domains found in Scrib [5, 16]. Therefore, we determined whether Scrib was essential for proper localization of βPIX to adherens junctions. While Scrib remained associated with lateral membranes in βPIX-depleted cells (Figure S4A), knockdown of Scrib prevented βPIX from localizing to adherens junctions (Figure S4B). In contrast, βPIX was retained at focal adhesions in Scrib depleted cells (Figure S4C and S4D), thus demonstrating that the requirement for Scrib to recruit βPIX was confined to adherens junctions. To delineate the link between Scrib and βPIX, we first compared the magnitude of apoptosis induced by replating of βPIX-kd and βPIX+Scrib-kd cells at high density. As determined by PARP cleavage, apoptosis of βPIX-kd cells was virtually blocked by the concomitant knockdown of Scrib, suggesting that Scrib sensitizes cells to apoptosis triggered by cadherin-mediated cell-cell adhesion (Figures 4A and 4B). Importantly, knockdown of Scrib expression did not affect βPIX levels and vice versa (Figure S1B).
Figure 4. Scrib sensitizes cells to apoptosis and anoikis elicited by cell-cell contact.
(A) Dox treated control, βPIX-kd and βPIX+Scrib-kd cells were replated at high density and total cell lysates were immunoblotted to detect cleaved PARP. (B) The PARP signal from the representative experiment shown in (A) was quantified by densitometry and presented as a percent of the signal at the 12 h time point for βPIX-kd cells. (C) Distributions of 16 h suspension cultures labeled with FITC-VAD-FMK and analyzed by flow cytometry. (D) Percentages of “dead” FITC-VAD-FMK labeled populations from four independent experiments are reported as mean ± SD (* p<0.05; ** p<0.02). See also Figure S4.
Next, we compared the effects of knocking down Scrib in control and βPIX-depleted cells grown in suspension. In contrast to the significant acceleration of anoikis observed upon depletion of βPIX alone, knockdown of Scrib resulted in attenuated anoikis both in control cells and βPIX-kd cells (Figures 4C and 4D), as well as reduced cadherin-dependent cell aggregation (Figure S3A). Collectively, these results establish that, in the absence of βPIX, Scrib functions as a potent sensitizer of apoptosis promoted by cell-cell contact.
PAK2 signals downstream of βPIX to promote epithelial cell survival
PAKs 1–6 are divided into class I and class II, comprising PAKs 1–3 and 4–6, respectively, of which only class I PAKs bind βPIX [17]. PAKs promote survival via phosphorylation of targets such as BAD [18]. Knockout of PAK2 but not PAK1 is embryonically lethal in mice [17]. Therefore, as MDCK cells express PAK1 and PAK2 [6], we determined the effects of PAK1 or PAK2 depletion on cell survival. Mirroring the effects of βPIX depletion, PAK2 knockdown resulted in cell death at high cell density (Figure 5A), an effect observed with five shRNAs targeting distinct sequences in the PAK2 mRNA (Figures 5B; for quantification see Figure S5A). In contrast knockdown of PAK1 failed to elicit apoptosis at high cell density (Figure S5B). Moreover, in contrast to knockdown of PAK2, which significantly augmented anoikis, knockdown of PAK1 yielded only a minor increase in anoikis (Figures S5C and S5D).
Figure 5. PAK2 confers protection against apoptosis and anoikis elicited by cell-cell contact.
(A) Phase contrast images reveal apoptosis in dox treated PAK2-kd cells, which occurred only at high cell density. (B) Dox treated PAK2-kd cell lines, expressing five distinct shRNAs, succumbed to apoptosis at high density, as evidenced by PARP cleavage. PAK2 was detected with Cell Sig. Tech. #2608. (C) Constitutive expression of human PAK2 (hsPAK2) prevented anoikis in MDCK cells elicited by an shRNA that targets endogenous canine, but not human, PAK2. (D) Anoikis index for the data in (C) presented as mean ± SEM of five independent experiments (***p<0.01). See also Figure S5.
Sensitization to apoptosis in replating and anoikis assays was observed with a total of eight shRNAs and two siRNAs yielding highly efficient knockdown of either βPIX or PAK2. To further control for off-target effects, we generated cells constitutively expressing Myc-tagged human PAK2, which is resistant to knockdown by PAK2 shRNA#5 (Figure 5C). While expression of exogenous PAK2 alone had no detectable effect on viability, it completely rescued anoikis elicited by knockdown of endogenous PAK2 (Figures 5C and 5D). To formally test whether the kinase activity of PAK2 and formation of a βPIX-PAK2 complex is required to confer protection against anoikis, we expressed Myc-tagged, kinase-dead PAK2(K278R) or PAK2(K278R/P185G/R186A), termed PAK2(K278R/ΔPIX), a kinase-dead PAK2 mutant deficient in βPIX-binding, under control of the TetR in MDCK cells. Expression of kinase-dead PAK2(K278R) sensitized MDCK cells to anoikis (Figures 6A and 6B). In contrast, cells expressing PAK2(K278R/ΔPIX) did not differ significantly from control cells in their sensitivity to anoikis (Figures 6A and 6B). These results demonstrate that PAK2 kinase activity and βPIX-PAK2 complex formation are required for anoikis resistance.
Figure 6. PAK2 requires kinase activity and β PIX binding to suppress anoikis.
(A) Expression of dominant inhibitory kinase-dead PAK2 (K278R) but not kinase-dead PAK2 (K278R/ΔPIX), which cannot bind βPIX, promoted anoikis of MDCK cells. (B) Anoikis index for the experiment shown in (A), presented as mean ± SD of three independent experiments (*p<0.05). (C) The LRR region of Scrib, as well as LRR-PAK2*, localizes predominantly to areas of cell-cell contact, as revealed here by immunofluorescence to detect the Myc-tag on these proteins. (D) LRR-PAK2* fusion protein, but not the LRR of Scrib alone, attenuates anoikis elicited by βPIX depletion. (E) Anoikis index for the data in (D) presented as mean ± SD of four independent experiments (***p<0.01). See also Figure S6.
Our data suggested a model whereby the targeting of PAK2 to adherens junctions, via a βPIX-dependent association with Scrib, is required to counteract apoptosis sensitization elicited by Scrib and E-cadherin. To test this hypothesis, we took advantage of the observation by others that the LRR region in Scrib is both sufficient to mediate membrane targeting and tumor suppression [19, 20]. We generated a chimeric protein consisting of the LRR region of Scrib fused to the amino-terminus of constitutively active, Myc-tagged PAK2, which furthermore was rendered defective in binding βPIX to avoid confounding effects from interaction with βPIX. The resulting LRR-PAK2(T402E,P185G,R186A) fusion protein, termed LRR-PAK2*, localized to areas of cell-cell contact similar to LRR-Myc alone (Figure 6C). Notably, expression LRR-PAK2* but not LRR-Myc conferred protection against anoikis in cells depleted of βPIX (Figure 6D and 6E). In contrast, expression of a corresponding LRR-PAK1(T423E,P193G,R194A) fusion protein, termed LRR-PAK1*, did not attenuate anoikis in βPIX-kd cells (Figure S6A and S6B). Intriguingly, at earlier time points LRR-Myc showed a pronounced enhancement of death in βPIX-kd cells, thus suggesting that this domain was sufficient to confer sensitization to apoptosis (Figures S6C and S6D). However, overexpression of LRR-Myc in MDCK cells with normal levels of βPIX had no effect on cell viability (Figure S6E). This was not surprising, because the levels of LRR-Myc expression achieved were insufficient to displace endogenous Scrib from membranes and suggested that the endogenous Scrib-βPIX-PAK2 complex functioned in trans to suppress apoptosis promoted by LRR-Myc. Finally, of note, neither LRR-Myc nor LRR-fusion protein affected cell-cell adhesion, as evidenced by cell-cell aggregation assays (Figure S3A).
Targeting of PAK2 by Scrib to adherens junctions is required for tolerance of osmotic stress
Our results demonstrate that activated PAK2 is required for survival of confluent, but not subconfluent, epithelial monolayers. PAK2-dependent survival signaling requires its association with βPIX, which facilitates the indirect association of PAK2 with Scrib. In turn, Scrib targets the βPIX-PAK2 complex to adherens junctions, where our data suggest activated PAK2 signals survival. To test this model in a normal physiological context, we determined the localization and function of the complex in response to hyperosmolarity. While PAK has been reported to be a component of the MAPK signaling pathway activated hyperosmotic shock [21], the significance of PAK activation in adaptation to osmotic stress is unknown. Moreover, osmotic stress was recently shown to alter the localization of several polarity proteins, including Scrib, and there is growing evidence to suggest that Scrib may be intimately linked to the control of MAPK kinase signaling [22, 23].
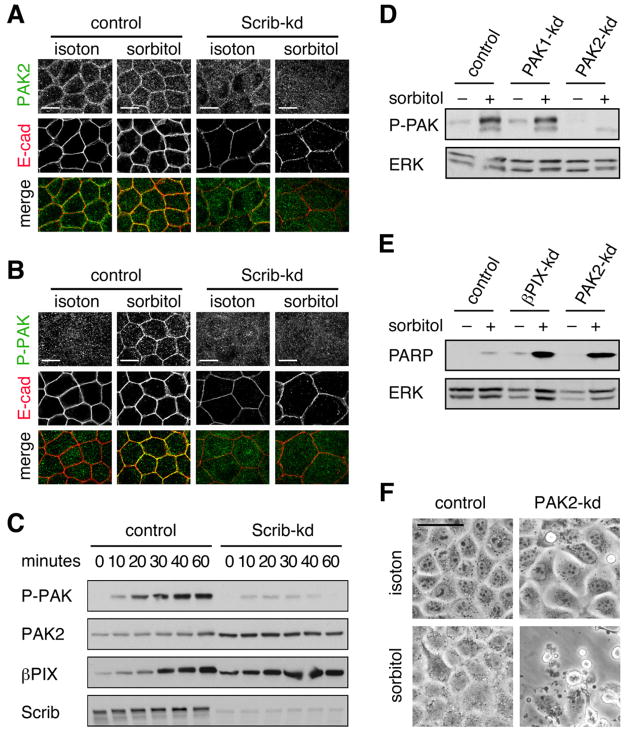
As previously reported in cultured endothelial cells [24], confluent MDCK cells exposed to osmotic shock show enhanced membranous staining of E-cadherin, indicative of dynamic reorganization of adherens junctions (Figures 7A). PAK2 specific antibodies confirmed its localization to adherens junctions (Figure 7A). Importantly, localization of PAK2 to adherens junctions was enhanced by osmotic shock. To determine whether PAK2 associated with adherens junctions was active we stained cells with an antibody capable of detecting phosphorylation of PAK1 and PAK2 at a site thought to reflect complete kinase activation. Indeed, osmotic shock resulted in an acute increase in the levels of phosphorylated PAK associated with adherens junctions (Figure 7B). Like βPIX, the localization of activated PAK to adherens junctions was dependent on Scrib (Figures 7A and 7B). As an independent readout of PAK activation, we performed western blotting analysis of cell lysates using an antibody that detects autophosphorylated PAK1 and PAK2. Osmotic shock induced rapid activation of a single protein that ran at a molecular weight consistent with PAK2 (Figure 7C). Consistent with PAK2 functioning at adherens junction in polarized epithelia, activation of PAK2 by osmotic shock was dependent on Scribble (Figure 7C). Interestingly, the levels of endogenous βPIX increased rapidly following osmotic shock, again suggesting an intimate connection between pathways activated under hyperosmotic conditions and the βPIX-PAK2 complex. Specific knockdown of PAK1 and PAK2 established the selective activation of PAK2 by osmotic shock in MDCK cells (Figure 7D).
Figure 7. Scrib-β PIX-PAK2 complex participates in osmotic stress response.
(A) Dox treated control and Scrib-kd cells cultured in isotonic or hypertonic (0.6 M sorbitol) medium for 1 h were methanol/acetone fixed and stained with antibodies against PAK2 (green; Santa Cruz, E-9) and E-cad (red). (B) Treated cells were stained with antibodies against E-cadherin (red) and phosphorylated PAK (red), detecting pThr423 and pThr402 in PAK1 and PAK2, respectively. (C) Lysates of control and Scrib-kd cells cultured in isotonic or hypertonic (0.6 M sorbitol) medium for the indicated time points were immunoblotted with antibodies detecting PAK autophosphorylated at pSer199/204 and pSer192/197 in PAK1 and PAK2, respectively, as well as PAK2 (Cell Sig. Tech. #2608), βPIX and Scrib. (D) PAK1-kd and PAK2-kd cells were cultured in isotonic or hypertonic medium for 1 h and blotted for autophosphorylated PAK. (E) Control, βPIX-kd and PAK2-kd cells were cultured in isotonic or sublethal hypertonic (0.3 M sorbitol) medium for 3 h and immunoblotted for cleaved PARP and ERK. (F) Control and PAK2-kd cells cultured in isotonic or hypertonic (0.3 M sorbitol) medium for 3 h. See also Figure S7.
Finally, we examined the effect of βPIX or PAK2 knockdown on the ability of cells to tolerate hypertonic stress. These experiments were conducted at a cell density when cells had just reached confluency and below the threshold of significant apoptosis in βPIX-kd or PAK2-kd cultures. Osmotic shock induced substantially increased apoptosis in βPIX-kd and PAK2-kd cells, indicating reduced tolerance to a normal physiological environment of kidney epithelial cells (Figures 7E and 7F). Taken together, these results suggest an obligatory role for the Scrib-βPIX-PAK2 complex in modulating signaling and cellular response to physiogical stress.
DISCUSSION
Here we establish that differentiated epithelial cells rely on a survival signaling network associated with adherens junctions that is distinct from that used at cell-matrix adhesions (Figure S7). Specifically, we demonstrate that epithelial cells are critically dependent on Scrib-mediated localization of a βPIX-PAK2 complex to adherens junctions to counterbalance the apoptosis promoting effects of E-cadherin engagement. As a result, disruption of βPIX-PAK2 signaling results in a near complete loss of epithelial viability at confluent density. Consistent with an established role in phosphorylating cellular targets involved in apoptosis [18], a functional kinase domain is required for PAK2-dependent survival signaling. In subconfluent cultures, where the βPIX-PAK2 complex localizes to focal adhesions, the complex is dispensable for cell survival. Thus, as epithelial cells form cell-cell junctions and polarize, they become critically dependent on a pro-survival signal provided by the βPIX-PAK2 complex at adherens junctions.
To date, the only direct evidence for the function of PAKs at adherens junctions is in the regulation of cadherin adhesiveness and actin-dependent cell contractility [25, 26]. While invoked by prior findings, our results demonstrate for the first time that Scrib via its association with βPIX promotes targeting of PAK2 to adherens junctions. In cultured human keratinocytes PAK1 has been reported to augment cadherin adhesiveness in response to activated Rac [26]. PAK1 and PAK2 have also been reported to enhance and suppress, respectively, loss of cell-cell contacts in response to Hepatocyte Growth Factor [27]. Our results suggest that neither PAK1 nor PAK2 play a major role in steady state junctional integrity in MDCK cells. However, as MDCK cells express both PAK1 and PAK2, we cannot exclude the possibility that they may function redundantly in regulation of E-cadherin adhesiveness and/or junctional remodeling.
βPIX and PAKs have been most extensively characterized for their role in focal adhesion dynamics, where they play a coordinated role in regulating turnover of these integrin attachment sites and promoting directional motility [28]. PAK1 and PAK2 appear to have non-redundant functions in cell invasion and motility [29, 30]. There is moreover evidence to suggest that when localized to focal adhesions the βPIX-PAK complex promotes mitogenic signaling and that redistribution of the complex from focal adhesions to adherens junctions contributes to the cessation of epithelial proliferation and establishment of contact inhibition [31, 32]. Scribb also shuttles between adherens junctions and protrusive membrane structures where it promotes βPIX-PAK complex-dependent cell motility [2]. It will be interesting to determine if Scrib will play a role in redistribution of the βPIX-PAK2 complex from focal adhesions to adherens junctions, which occurs as cells undergo contact inhibition [31, 32]. In brief, taken together with our present work, these studies suggest that PAKs have distinct roles when localized to focal adhesions and adherens junctions. When localized to focal adhesions, PAKs participate in signaling pathways that promote proliferation and motility, while at adherens junctions PAKs regulate adhesiveness and survival. As such, the translocation of the βPIX-PAK complex from to lateral membranes upon formation of stable adherens junctions likely plays a fundamental role in the transition from a motile/mitogenic state to a non-motile/quiescent state.
E-cadherin is a potent inhibitor of multiple signaling pathways and plays a fundamental role in suppression of motility and proliferation upon establishment of cell-cell contact [33]. The ability of Scrib to promote E-cadherin adhesiveness may underlie some of its tumor suppressive potential in mammalian cells [3]. However, increasing evidence suggests that Scrib plays E-cadherin-independent roles in the regulation of signaling pathways, such as inhibition of ERK and AKT, as well as activation of Hippo signaling [23, 34, 35]. These Scrib-dependent effects would be predicted to sensitize cells to apoptosis; a prediction supported by our present results, which furthermore establish that Scrib-mediated apoptosis is counterbalanced by its recruitment of active PAK2. Taken together, these findings demonstrate that Scrib transduces both pro- and anti-apoptotic stimuli.
Metastasis requires that cells tolerate the loss of matrix adhesion, i.e. that they are protected against anoikis [10]. However, in spite of seminal work by Frish and Francis suggesting a role for cell-cell adhesion in modulating anoikis [36], the role for epithelial architecture in anoikis remains unappreciated and poorly characterized. Nevertheless, there is growing evidence indicating that loss of E-cadherin function suffices to abrogate anoikis [37–39]. Our results support a key role of adherens junctions in modulating anoikis. In simple polarized epithelia, which likely rely on the summation of survival signals emanating from both focal adhesions and adherens junctions, apoptotic stimuli emanating from E-cadherin will sensitize cells to anoikis (Figure S7). In contrast, suprabasal cells in stratified epithelia depend exclusively on survival signals from adherens junctions. PAK2 is highly expressed in suprabasal keratinocytes, which may suggest that upregulation of PAK2-dependent survival signals emanating from adherens junctions are required to offset loss of integrin-mediated survival signaling [40]. In summary, our results indicate that E-cadherin does not function simply as a pro-survival or pro-apoptotic factor, but rather as a regulatory node to coordinate death and survival signaling. The balance between pro- and anti-apoptotic signaling emanating from adherens junctions plays a key role in epithelial cell viability and is likely essential in regulating diverse processes, including epithelial morphogenesis, wound healing, physiological stress, and metastasis.
EXPERIMENTAL PROCEDURES
Antibodies, plasmids, and reagents
A description of antibodies, plasmids and other reagents is included in Supplemental Information.
Cell culture and retroviral transduction
TR-T10 MDCK cells expressing TetR were kindly provided by Sanjay Pimplikar and propagated in DMEM with 10% FCS. Retroviruses for transduction of shRNA constructs were produced in 293 Phoenix cells and used to infect TR-T10 cells. Infected cells were selected in 2.5 μg/ml of puromycin or 200 μg/ml hygromycin. Knockdown was induced in subconfluent cultures by addition of 4 μg/ml of doxycycline (dox) for a minimum of 72 h prior to experimentation.
Tet-off MDCK cells permitting conditional expression of HA-tagged dominant negative E-cadherin (DN-E-cad) were cultured as described elsewhere [41]. This DN-E-cad has had 390 amino acids of the extracellular domain of E-cadherin deleted and an HA-tag inserted in the remaining extracellular moiety [42]. To induce expression of DN-E-cad, dox was removed three days before experimentation. Transfection with siRNA duplexes was performed with cells grown in the absence of dox, as it reduced transfection efficiency. After transfection, dox was added back to suppress expression of DN-E-cad where required.
LLC-PK1, T47D, and MCF7 cells were grown in DMEM containing 10% FCS. The medium for MCF7 cells was further supplemented with 100 ng/ml insulin.
Confocal fluorescence microscopy
Cells processed for GIT2, PAK2 and phospho-PAK staining were fixed for 2 min at room temperature with methanol/acetone (50:50 v/v). All other samples were processed as previously described [6].
Replating assay
Dox treated cell lines grown at subconfluent density for 72 h were harvested by trypsinization and 2×106 single cells in were plated in a volume of 2 ml into 35 mm wells. At designated time points, detached cells in the medium were pelleted onto glass coverslips by cytospin centrifugation. Adherent and detached cells were fixed with 2% paraformaldehyde and processed for immunofluorescence. For preparation of total cell lysates, adherent cells were harvested by cell scraping and pelleted along with detached cells. Cells were lysed in Laemmli buffer containing 6 M urea, boiled, and subjected to immunoblotting. To block apoptosis, z-VAD-FMK was used at 50 μM. The 24 kD PARP fragment, quantified by densitometry using ImageJ, was used as a read-out of apoptosis.
Anoikis assay
Dox treated knockdown cells grown at subconfluent density for 72 h were harvested by trypsinization, and 2×106 single cells in a volume of 2 ml were plated into 24 well ultra low attachment plates (Corning). For immunofluorescence microscopy, cells were first triturated by 20 passages through a 2 ml tissue culture pipette. 1×105 in 200 μl cells were pelleted onto coverslides by cytospin centrifugation and fixed with 2% paraformaldehyde. For fluorescence microscopy, cells were incubated for 16 h with 50 μM FITC-VAD-FMK and fixed with 2% paraformaldehyde but not detergent permeabilized. For flow cytometry, cells were labeled for 16 h with 10 μM FITC-VAD-FMK. Labeled cultures were pelleted, suspended in 1 ml trypsin, and incubated for 5 min at 37°C. The trypsin was neutralized by addition 1 ml FCS and the cells triturated by 20–30 passages through a 2 ml tissue culture pipette. Cells were then pelleted and resuspended in PBS at a concentration of 4×106 cells/ml and analyzed by flow cytometry.
For PARP assays, the 24 kD PARP fragment was used to detect apoptosis and quantified by densitometry using ImageJ as detailed in Figure S2C.
TUNEL analysis
Cells were fixed with 2% paraformaldehyde for 30 min, rinsed twice with PBS and once with distilled water. Cells were permeabilized with ice-cold 70% (v/v) ethanol for 30 min on ice and subsequently labeled according to the manufacturer’s instructions (In situ Cell Death Detection Kit; Roche).
Statistical analyses
Unpaired two-tailed Student t tests were performed as previously detailed [43].
Supplementary Material
Acknowledgments
We are grateful to Giorgio G. Galli, William G. Kaelin, Ed Manser, Sanjay Pimplikar, and Mirjam M.P. Zegers for providing reagents and/or expert advice, and to Jessica S. Wagner and Elizabeth B.G. Boush for technical assistance. This work utilized Harvard Digestive Diseases Center core facilities and was supported by NIH R01 CA142647, the Danish Cancer Society, the Novo Nordic Foundation, the Roy and Lynne Frank Foundation, and funds from Children’s Hospital Boston (all to SHH).
Footnotes
Supplemental Information attached as a separate file includes Supplemental Figures S1–7, movie S1 and table S1, Supplemental Experimental Procedures and Supplemental References.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin-Belmonte F, Perez-Moreno M. Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer. 2012;12:23–38. doi: 10.1038/nrc3169. [DOI] [PubMed] [Google Scholar]
- 2.Humbert PO, Grzeschik NA, Brumby AM, Galea R, Elsum I, Richardson HE. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene. 2008;27:6888–6907. doi: 10.1038/onc.2008.341. [DOI] [PubMed] [Google Scholar]
- 3.Qin Y, Capaldo C, Gumbiner BM, Macara IG. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J Cell Biol. 2005;171:1061–1071. doi: 10.1083/jcb.200506094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navarro C, Nola S, Audebert S, Santoni MJ, Arsanto JP, Ginestier C, Marchetto S, Jacquemier J, Isnardon D, Le Bivic A, et al. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene. 2005;24:4330–4339. doi: 10.1038/sj.onc.1208632. [DOI] [PubMed] [Google Scholar]
- 5.Audebert S, Navarro C, Nourry C, Chasserot-Golaz S, Lecine P, Bellaiche Y, Dupont JL, Premont RT, Sempere C, Strub JM, et al. Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr Biol. 2004;14:987–995. doi: 10.1016/j.cub.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 6.Zegers MM, Forget MA, Chernoff J, Mostov KE, Ter Beest MB, Hansen SH. Pak1 and PIX regulate contact inhibition during epithelial wound healing. Embo J. 2003;22:4155–4165. doi: 10.1093/emboj/cdg398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank SR, Adelstein MR, Hansen SH. GIT2 represses Crk- and Rac1-regulated cell spreading and Cdc42-mediated focal adhesion turnover. Embo J. 2006;25:1848–1859. doi: 10.1038/sj.emboj.7601092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol. 2001;11:1847–1857. doi: 10.1016/s0960-9822(01)00587-5. [DOI] [PubMed] [Google Scholar]
- 9.Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005;24:425–439. doi: 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- 10.Horbinski C, Mojesky C, Kyprianou N. Live free or die: tales of homeless (cells) in cancer. Am J Pathol. 2010;177:1044–1052. doi: 10.2353/ajpath.2010.091270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pece S, Chiariello M, Murga C, Gutkind JS. Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell-cell junctions. Evidence for the association of phosphatidylinositol 3-kinase with the E-cadherin adhesion complex. Journal of Biological Chemistry. 1999;274:19347–19351. doi: 10.1074/jbc.274.27.19347. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa M, Fukata M, Yamaga M, Itoh N, Kaibuchi K. Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J Cell Sci. 2001;114:1829–1838. doi: 10.1242/jcs.114.10.1829. [DOI] [PubMed] [Google Scholar]
- 13.Noren NK, Niessen CM, Gumbiner BM, Burridge K. Cadherin engagement regulates Rho family GTPases. J Biol Chem. 2001;276:33305–33308. doi: 10.1074/jbc.C100306200. [DOI] [PubMed] [Google Scholar]
- 14.Capaldo CT, Macara IG. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 2007;18:189–200. doi: 10.1091/mbc.E06-05-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamin JM, Nelson WJ. Bench to bedside and back again: molecular mechanisms of alpha-catenin function and roles in tumorigenesis. Semin Cancer Biol. 2008;18:53–64. doi: 10.1016/j.semcancer.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Yeh S, Appleton BA, Held HA, Kausalya PJ, Phua DC, Wong WL, Lasky LA, Wiesmann C, Hunziker W, et al. Convergent and divergent ligand specificity among PDZ domains of the LAP and zonula occludens (ZO) families. J Biol Chem. 2006;281:22299–22311. doi: 10.1074/jbc.M602902200. [DOI] [PubMed] [Google Scholar]
- 17.Arias-Romero LE, Chernoff J. A tale of two Paks. Biol Cell. 2008;100:97–108. doi: 10.1042/BC20070109. [DOI] [PubMed] [Google Scholar]
- 18.Schurmann A, Mooney AF, Sanders LC, Sells MA, Wang HG, Reed JC, Bokoch GM. p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis. Mol Cell Biol. 2000;20:453–461. doi: 10.1128/mcb.20.2.453-461.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albertson R, Chabu C, Sheehan A, Doe CQ. Scribble protein domain mapping reveals a multistep localization mechanism and domains necessary for establishing cortical polarity. J Cell Sci. 2004;117:6061–6070. doi: 10.1242/jcs.01525. [DOI] [PubMed] [Google Scholar]
- 20.Zeitler J, Hsu CP, Dionne H, Bilder D. Domains controlling cell polarity and proliferation in the Drosophila tumor suppressor Scribble. J Cell Biol. 2004;167:1137–1146. doi: 10.1083/jcb.200407158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan PM, Lim L, Manser E. PAK is regulated by PI3K, PIX, CDC42, and PP2Calpha and mediates focal adhesion turnover in the hyperosmotic stress-induced p38 pathway. J Biol Chem. 2008;283:24949–24961. doi: 10.1074/jbc.M801728200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massimi P, Narayan N, Thomas M, Gammoh N, Strand S, Strand D, Banks L. Regulation of the hDlg/hScrib/Hugl-1 tumour suppressor complex. Exp Cell Res. 2008 doi: 10.1016/j.yexcr.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Nagasaka K, Massimi P, Pim D, Subbaiah VK, Kranjec C, Nakagawa S, Yano T, Taketani Y, Banks L. The mechanism and implications of hScrib regulation of ERK. Small Gtpases. 2010;1:108–112. doi: 10.4161/sgtp.1.2.13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quadri SK, Bhattacharjee M, Parthasarathi K, Tanita T, Bhattacharya J. Endothelial barrier strengthening by activation of focal adhesion kinase. J Biol Chem. 2003;278:13342–13349. doi: 10.1074/jbc.M209922200. [DOI] [PubMed] [Google Scholar]
- 25.Tay HG, Ng YW, Manser E. A vertebrate-specific Chp-PAK-PIX pathway maintains E-cadherin at adherens junctions during zebrafish epiboly. PLoS One. 2010;5:e10125. doi: 10.1371/journal.pone.0010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nola S, Daigaku R, Smolarczyk K, Carstens M, Martin-Martin B, Longmore G, Bailly M, Braga VM. Ajuba is required for Rac activation and maintenance of E-cadherin adhesion. J Cell Biol. 2011;195:855–871. doi: 10.1083/jcb.201107162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bright MD, Garner AP, Ridley AJ. PAK1 and PAK2 have different roles in HGF-induced morphological responses. Cell Signal. 2009;21:1738–1747. doi: 10.1016/j.cellsig.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Zegers M. Roles of P21-activated kinases and associated proteins in epithelial wound healing. Int Rev Cell Mol Biol. 2008;267:253–298. doi: 10.1016/S1937-6448(08)00606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nola S, Sebbagh M, Marchetto S, Osmani N, Nourry C, Audebert S, Navarro C, Rachel R, Montcouquiol M, Sans N, et al. Scrib regulates PAK activity during the cell migration process. Hum Mol Genet. 2008 doi: 10.1093/hmg/ddn248. [DOI] [PubMed] [Google Scholar]
- 30.Coniglio SJ, Zavarella S, Symons MH. Pak1 and Pak2 mediate tumor cell invasion through distinct signaling mechanisms. Mol Cell Biol. 2008;28:4162–4172. doi: 10.1128/MCB.01532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu F, Jia L, Thompson-Baine AM, Puglise JM, Ter Beest MB, Zegers MM. Cadherins and Pak1 control contact inhibition of proliferation by Pak1-betaPIX-GIT complex-dependent regulation of cell-matrix signaling. Mol Cell Biol. 2010;30:1971–1983. doi: 10.1128/MCB.01247-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livshits G, Kobielak A, Fuchs E. Governing epidermal homeostasis by coupling cell-cell adhesion to integrin and growth factor signaling, proliferation, and apoptosis. Proc Natl Acad Sci U S A. 2012;109:4886–4891. doi: 10.1073/pnas.1202120109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920–6929. doi: 10.1038/onc.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Yang H, Liu J, Schmidt MD, Gao T. Scribble-mediated membrane targeting of PHLPP1 is required for its negative regulation of Akt. EMBO Rep. 2011;12:818–824. doi: 10.1038/embor.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 36.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. Journal of Cell Biology. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645–3654. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 38.Schackmann RC, van Amersfoort M, Haarhuis JH, Vlug EJ, Halim VA, Roodhart JM, Vermaat JS, Voest EE, van der Groep P, van Diest PJ, et al. Cytosolic p120-catenin regulates growth of metastatic lobular carcinoma through Rock1-mediated anoikis resistance. J Clin Invest. 2011;121:3176–3188. doi: 10.1172/JCI41695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar S, Park SH, Cieply B, Schupp J, Killiam E, Zhang F, Rimm DL, Frisch SM. A pathway for the control of anoikis sensitivity by E-cadherin and epithelial-to-mesenchymal transition. Mol Cell Biol. 2011;31:4036–4051. doi: 10.1128/MCB.01342-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Pedersen E, Basse A, Lefever T, Peyrollier K, Kapoor S, Mei Q, Karlsson R, Chrostek-Grashoff A, Brakebusch C. Rac1 is crucial for Ras-dependent skin tumor formation by controlling Pak1-Mek-Erk hyperactivation and hyperproliferation in vivo. Oncogene. 2010;29:3362–3373. doi: 10.1038/onc.2010.95. [DOI] [PubMed] [Google Scholar]
- 41.Jia L, Liu F, Hansen SH, Ter Beest MB, Zegers MM. Distinct roles of cadherin-6 and E-cadherin in tubulogenesis and lumen formation. Mol Biol Cell. 2011;22:2031–2041. doi: 10.1091/mbc.E11-01-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Troxell ML, Chen YT, Cobb N, Nelson WJ, Marrs JA. Cadherin function in junctional complex rearrangement and posttranslational control of cadherin expression. Am J Physiol. 1999;276:C404–418. doi: 10.1152/ajpcell.1999.276.2.C404. [DOI] [PubMed] [Google Scholar]
- 43.Hansen SH, Sandvig K, van Deurs B. Internalization efficiency of the transferrin receptor. Experimental Cell Research. 1992;199:19–28. doi: 10.1016/0014-4827(92)90457-j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.