Abstract
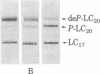
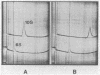
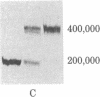
Smooth muscle myosin filaments formed in 0.15 M KCl are depolymerized by MgATP to a 10S component, rather than to the 6S component typical of myosin monomer in high salt concentrations. This 10S species is also monomeric as determined by sedimentation equilibrium and calculated from the diffusion and sedimentation coefficients. The conformation of 10S myosin is, however, very different from that of 6S myosin, which has a flexible but extended rod. The Stokes radius and the viscosity of 10S myosin are less than those of 6S myosin, consistent with a structure in which the rod is bent. Electron microscopy of rotary-shadowed preparations confirmed that the light meromyosin region of the rod is bent back on subfragment 2, that region of the rod adjacent to the two globular heads. MgATP and dephosphorylation of the 20,000 molecular weight light chain increase the amount of 10S myosin present in 0.15 M KCl; addition of salt converts 10S myosin back to the typical 6S conformation. We conclude that smooth muscle myosin preferentially forms a bent or folded conformation instead of the extended shape usually associated with skeletal muscle myosin, provided that the salt concentration is kept sufficiently low.
Full text
PDF
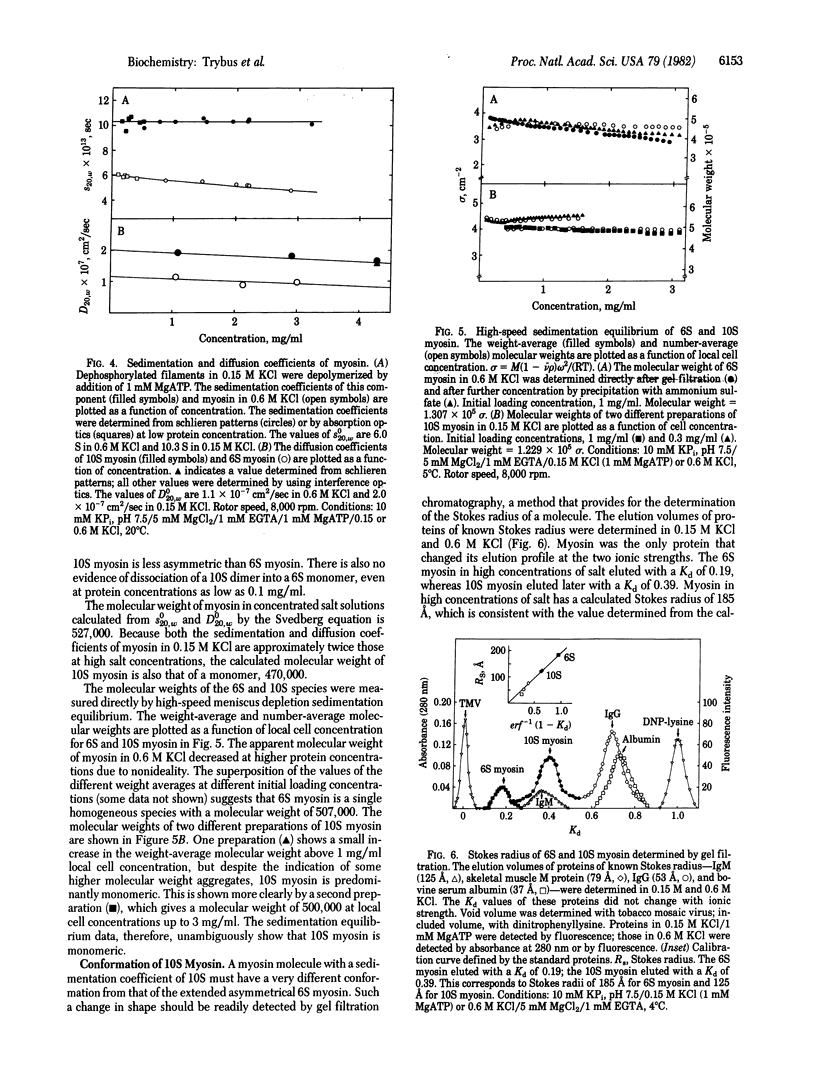
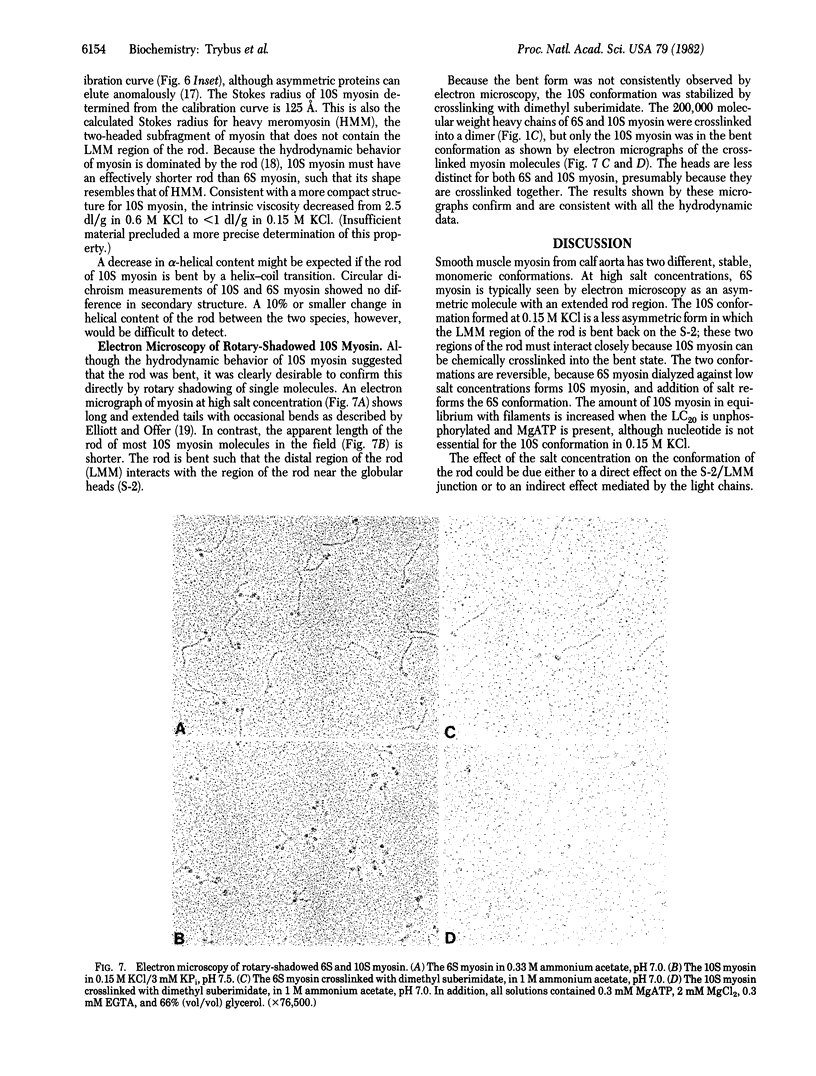

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansevin A. T., Roark D. E., Yphantis D. A. Improved ultracentrifuge cells for high-speed sedimentation equilibrium studies with interference optics. Anal Biochem. 1970 Mar;34:237–261. doi: 10.1016/0003-2697(70)90103-x. [DOI] [PubMed] [Google Scholar]
- Burke M., Himmelfarb S., Harrington W. F. Studies on the "hinge" region of myosin. Biochemistry. 1973 Feb;12(4):701–710. doi: 10.1021/bi00728a020. [DOI] [PubMed] [Google Scholar]
- Craig R., Megerman J. Assembly of smooth muscle myosin into side-polar filaments. J Cell Biol. 1977 Dec;75(3):990–996. doi: 10.1083/jcb.75.3.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRosier D. J., Munk P., Cox D. J. Automatic measurement of interference photographs from the ultracentrifuge. Anal Biochem. 1972 Nov;50(1):139–153. doi: 10.1016/0003-2697(72)90493-9. [DOI] [PubMed] [Google Scholar]
- Elliott A., Offer G. Shape and flexibility of the myosin molecule. J Mol Biol. 1978 Aug 25;123(4):505–519. doi: 10.1016/0022-2836(78)90204-8. [DOI] [PubMed] [Google Scholar]
- García de la Torre J., Bloomfield V. A. Conformation of myosin in dilute solution as estimated from hydrodynamic properties. Biochemistry. 1980 Oct 28;19(22):5118–5123. doi: 10.1021/bi00563a028. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Harrington W. F. A mechanochemical mechanism for muscle contraction. Proc Natl Acad Sci U S A. 1971 Mar;68(3):685–689. doi: 10.1073/pnas.68.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highsmith S., Kretzschmar K. M., O'Konski C. T., Morales M. F. Flexibility of myosin rod, light meromyosin, and myosin subfragment-2 in solution. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4986–4990. doi: 10.1073/pnas.74.11.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highsmith S., Wang C. C., Zero K., Pecora R., Jardetzky O. Bending motions and internal motions in myosin rod. Biochemistry. 1982 Mar 16;21(6):1192–1197. doi: 10.1021/bi00535a013. [DOI] [PubMed] [Google Scholar]
- Hinssen H., D'Haese J., Small J. V., Sobieszek A. Mode of filament assembly of myosins from muscle and nonmuscle cells. J Ultrastruct Res. 1978 Sep;64(3):282–302. doi: 10.1016/s0022-5320(78)90037-0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Kelly R. E., Rice R. V. Ultrastructural studies on the contractile mechanism of smooth muscle. J Cell Biol. 1969 Sep;42(3):683–694. doi: 10.1083/jcb.42.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- Lu R. C. Identification of a region susceptible to proteolysis in myosin subfragment-2. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2010–2013. doi: 10.1073/pnas.77.4.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megerman J., Lowey S. Polymerization of myosin from smooth muscle of the calf aorta. Biochemistry. 1981 Apr 14;20(8):2099–2110. doi: 10.1021/bi00511a006. [DOI] [PubMed] [Google Scholar]
- Mrakovcić-Zenic A., Oriol-Audit C., Reisler E. On the alkali light chains of vertebrate skeletal myosin. Nucleotide binding and salt-induced conformational changes. Eur J Biochem. 1981 Apr;115(3):565–570. doi: 10.1111/j.1432-1033.1981.tb06240.x. [DOI] [PubMed] [Google Scholar]
- Mrakovcić A., Oda S., Reisler E. Salt-induced conformational changes in skeletal myosin light chains, troponin-C, and parvalbumin. Biochemistry. 1979 Dec 25;18(26):5960–5965. doi: 10.1021/bi00593a031. [DOI] [PubMed] [Google Scholar]
- Nozaki Y., Schechter N. M., Reynolds J. A., Tanford C. Use of gel chromatography for the determination of the Stokes radii of proteins in the presence and absence of detergents. A reexamination. Biochemistry. 1976 Aug 24;15(17):3884–3890. doi: 10.1021/bi00662a036. [DOI] [PubMed] [Google Scholar]
- Onishi H., Suzuki H., Nakamura K., Takahashi K., Watanabe S. Adenosine triphosphatase activity and "thick filament" formation of chicken gizzard myosin in low salt media. J Biochem. 1978 Mar;83(3):835–847. doi: 10.1093/oxfordjournals.jbchem.a131980. [DOI] [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970 Aug;119(1):31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roark D. E., Yphantis D. A. Studies of self-associating systems by equilibrium ultracentrifugation. Ann N Y Acad Sci. 1969 Nov 7;164(1):245–278. doi: 10.1111/j.1749-6632.1969.tb14043.x. [DOI] [PubMed] [Google Scholar]
- Scholey J. M., Taylor K. A., Kendrick-Jones J. Regulation of non-muscle myosin assembly by calmodulin-dependent light chain kinase. Nature. 1980 Sep 18;287(5779):233–235. doi: 10.1038/287233a0. [DOI] [PubMed] [Google Scholar]
- Small J. V., Squire J. M. Structural basis of contraction in vertebrate smooth muscle. J Mol Biol. 1972 Jun 14;67(1):117–149. doi: 10.1016/0022-2836(72)90390-7. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Butler T. M., Bond M., Somlyo A. P. Myosin filaments have non-phosphorylated light chains in relaxed smooth muscle. Nature. 1981 Dec 10;294(5841):567–569. doi: 10.1038/294567a0. [DOI] [PubMed] [Google Scholar]
- Sutoh K., Sutoh K., Karr T., Harrington W. F. Isolation and physico-chemical properties of a high molecular weight subfragment-2 of myosin. J Mol Biol. 1978 Nov 25;126(1):1–22. doi: 10.1016/0022-2836(78)90276-0. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kamata T., Onishi H., Watanabe S. Adenosine triphosphate-induced reversible change in the conformation of chicken gizzard myosin and heavy meromyosin. J Biochem. 1982 May;91(5):1699–1705. doi: 10.1093/oxfordjournals.jbchem.a133861. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Onishi H., Takahashi K., Watanabe S. Structure and function of chicken gizzard myosin. J Biochem. 1978 Dec;84(6):1529–1542. doi: 10.1093/oxfordjournals.jbchem.a132278. [DOI] [PubMed] [Google Scholar]
- Tyler J. M., Branton D. Rotary shadowing of extended molecules dried from glycerol. J Ultrastruct Res. 1980 May;71(2):95–102. doi: 10.1016/s0022-5320(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Ueno H., Harrington W. F. Conformational transition in the myosin hinge upon activation of muscle. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6101–6105. doi: 10.1073/pnas.78.10.6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert P., Craig R. Three-dimensional reconstruction of thin filaments decorated with a Ca2+-regulated myosin. J Mol Biol. 1982 May 15;157(2):299–319. doi: 10.1016/0022-2836(82)90236-4. [DOI] [PubMed] [Google Scholar]