Abstract
Background
Comparisons between animal and human neurotoxicology studies are a foundation of risk assessment, but are hindered by differences in measured behaviors. The Radial Arm Maze (RAM), a rodent visuospatial learning and memory task, has a computerized version for use in children, which may help improve comparisons between animal and human studies.
Objective
To describe the characteristics and correlates of the Virtual Radial Arm Maze (VRAM) in 255 children age 10–15 years from Italy.
Methods
We administered the VRAM using a laptop computer and measured children’s performance using the latency, distance, and working/reference memory errors during eight trials. Using generalized linear mixed models, we described VRAM performance in relation to demographic factors, child activities, and several standard neuropsychologic tests (Italian translations), including the Conners Parent Rating Scales-Short Version (CPRS), California Verbal Learning Test (CVLT), Wechsler Intelligence Scales for Children, finger tapping speed, reaction time, and motor skills.
Results
Children’s VRAM performance tended to improve between trials 1–6 and then plateaued between trials 6–8. Males finished the task 14 seconds faster (95% Confidence Interval [CI]:-20, -9) than females. Children who played 2+ hours of video games per day finished 16 seconds faster (CI:-26, -6) and with 34% (CI:5, 54%) fewer working memory errors than children who reported not playing video games. Higher IQ and better CVLT scores were associated with better VRAM performance. Higher Cognitive/Inattention CPRS scores were associated with more working (11%; CI:1, 22) and reference memory errors (7%; CI:1, 12).
Conclusions
Consistent with animal studies, VRAM performance improved over the course of test trials and males performed better than females. Better VRAM performance was related to higher IQ, fewer inattentive behaviors, and better verbal memory. The VRAM may help improve the integration and comparison between animal and epidemiological studies of environmental neurotoxicants.
Keywords: Child behavior, computerized tests, environmental chemicals, epidemiology, toxicology
Introduction
Gestational and childhood environmental chemical exposure may adversely impact nervous system development and potentially increase the risk of childhood behavioral disorders like attention-deficit hyperactivity disorder (ADHD), autism, and learning disabilities (Anderko et al., 2010, Axelrad et al., 2007, Braun et al., 2006, Braun et al., 2011, Eubig et al., 2010, Miodovnik et al., 2011). Experimental studies confirm that exposure to environmental chemicals can impact specific aspects of brain development and alter behavioral phenotypes in rodents and non-human primates (Chapin et al., 2008, Jones and Miller, 2008, Lidsky and Schneider, 2003, Schantz and Widholm, 2001). Translating the results between experimental neurotoxicology and epidemiology studies is hindered by many factors, including biases arising from non-randomized exposure in humans, higher exposures in animals, pharmacokinetic differences of chemicals across species, differences in developmental trajectories across species, and comparability of animal and human behavioral outcomes.
Typically, animal and human studies conducted on the same neurotoxic chemicals do not measure the same behavioral endpoints, often because directly comparable tests do not exist for humans and animals. The Radial Arm Maze (RAM) – a sensitive animal test of visuospatial learning and memory commonly used in neurotoxicity studies– has been adapted for use in humans using a personal computer (PC) (Anger, 1991, Astur et al., 2004, Haider et al., 2005, Levin et al., 2001, Munoz et al., 1988). The eight arm RAM is a commonly used paradigm in which eight equally spaced arms extend from a central circular platform and four of the eight arms are baited with a food reward. Over the course of successive daily test trials, the rodent is expected to learn which arms are baited (or never baited) and efficiently retrieve the food rewards at the ends of four baited arms using visuospatial cues in the room. The rodent’s performance is measured by the time and distance to complete each trial, as well as the number of times the animal goes down a never baited arm (between trial or reference memory error) or re-entry into an arm it already visited on that trial (within trial or working memory error). Experimental studies have observed RAM performance deficits in rodents exposed to manganese (Mn), lead (Pb), and chlorpyrifos (Haider, Shameem, 2005, Kern et al., 2010, Levin, Addy, 2001).
The Virtual Radial Arm Maze (VRAM) is a computerized version of the RAM that can be completed using a PC and joystick. Few studies have used the VRAM maze in humans and we are not aware of any using it in children or adolescents (Astur et al., 2005, Astur, Tropp, 2004, Goodrich-Hunsaker and Hopkins, 2010). Little is known about correlates of VRAM performance in children. Such information is critical to epidemiological studies using this test since it can help inform confounder selection and may elucidate underlying neuropsychological functions associated with VRAM performance.
Since the VRAM has potential applications in the study of neurotoxicants in children, we sought to describe characteristics and correlates of test performance in 255 adolescents from northern Italy. Specifically, we examined whether the sex of the child, leisure activities, neuropsychological test performance, and parent-reported behavior predicted VRAM performance. We hypothesized that children who were males, played more video games, and had better scores on measures of IQ, reaction time, motor skills, and verbal memory would have better VRAM performance, defined as shorter completion time, shorter distance to completion, and fewer working/reference memory errors. We also hypothesized that parent-reported measures of inattention and hyperactivity would be associated with poorer VRAM performance.
Methods
Study Sample
Data for this study come from an ongoing study of manganese (Mn) exposure and child neurodevelopment in the Bagnolo Mella, Valcamonica, and Garda Lake regions in the province of Brescia Italy. These regions were selected based on the presence of active, historical, or no Mn ferro-alloy smelting. Children ages 10–14 years were identified through the local school district (junior high school) in the study regions and recruited through mailed letters to their parents or public presentations at their school. Interested subjects were contacted by telephone and in-home interviews were conducted where the study objectives, methods, and protocols were explained to the children and parents before written informed consent was obtained. Study protocols were approved by institutional review board at the Ethical Committee of the Public Health Agency of Brescia, University of Brescia, University of California-Santa Cruz, and Harvard School of Public Health.
We excluded children with known hand or finger motor deficits, visual deficits that were not adequately corrected, and neurological, metabolic, hepatic, or endocrine diseases. We also excluded children who currently or had previously received total parenteral nutrition, were currently taking prescription psychoactive drugs, or had psychiatric disturbances. The larger, parent study plans to enroll 450 participants; here we present results from the first 255 eligible children who were enrolled from the three different study regions.
VRAM Testing Procedures
The VRAM is a computerized maze task that assesses visuospatial, reference, and working memory (Astur, Tropp, 2004). The test was administered in a quiet room at the child’s school on a laptop computer and children used a Microsoft Sidewinder joystick (Microsoft Corp, Seattle, WA) to navigate the maze. When the test began, children found themselves in a virtual room and on the center of a circular platform with eight arms extending from the center of the platform (Figure 1). The room contained various visual cues on each of the four walls that can be used for orientation. Children were told to find four hidden rewards as quickly as possible in four of eight arms extending from the central platform. When children correctly located an arm with a reward the word ‘Reward!’ flashed up on the screen and the computer emitted a swooshing sound. For each subject, the same four arms were rewarded across the eight trials. Children were allowed to navigate forward and turn left or right, but were not allowed to move backwards in order to create conditions similar to the animal version of the test. In order to turn around children were required to pan left or right for 180 degrees. A trial ended when the child retrieved all four rewards or 180 seconds elapsed. The screen went black for 3 seconds before the initiation of a new trial. All eight trials were conducted on the same day. The same instructions for completing the test were provided to each child and are shown in Appendix A (Italian to English translation).
Figure 1.

Screenshot from the virtual radial arm maze task
Performance measures included the time to complete the maze (latency), distance traveled, entry into an arm that is never baited (reference memory), and entry into an arm that had already been entered on that trial (working memory). A maximum of four reference memory errors could be committed on a single trial since subsequent entries into non-rewarded arms during that trial were counted as working memory errors. Entry into a non-rewarded arm on the first trial was counted as a reference memory error.
Child Demographics and Test Factors
Demographic factors, including child sex and age were collected with standardized forms over the telephone or during in-person interviews. Child socioeconomic status was categorized as low, medium, and high based on parental occupation and education (Cesana et al., 1995). Examiners asked the children whether they were feeling well during the inter-trial interval to determine if they were experiencing motion sickness. They did not explicitly ask if the child was experiencing motion sickness. Parents reported whether their child experienced nausea or dizziness during routine travel (e.g., motion sickness).
Child Leisure and Recreation Activities
Trained research assistants administered standardized questionnaires to assess children’s leisure activities. We asked children to estimate the number of hours per day they spent playing video games, surfing the internet, or watching television. Children also reported the number of books they read per month, the number of times they played sports per week, and whether they participated in competitive sports.
Child Psychometric Testing
Trained examiners administered several neuropsychological tests to children and parents reported on their child’s behavior. All neuropsychological tests were completed in the child’s school in a quiet room before administering the VRAM. Children completed Italian translations of the Wechsler Intelligence Scales for Children-III (WISC-III) and California Verbal Learning Test-Child Version (CVLT-C) (Delis et al., 2000, Wechsler, 1991). Scores from the WISC-III are age-standardized. Children completed the Picture Completion, Information, Coding, Similarities, Picture Arrangements, Arithmetic, Block Design, Vocabulary, Object Assembly, Comprehension, and Digit Span subtests of the WISC-III. We examined the Full Scale, Performance, and Verbal IQ (FSIQ, PIQ, and VIQ) in our primary analyses.
The CVLT is an age and sex-standardized test of verbal memory where the child is asked to recall a “shopping list” of 15 words over a series of five trials. Children are then asked to recall the list after a short (5 minutes) and long (20 minutes) delay. We analyzed five parameters from the CVLT; the number of words recalled on trial 5, the numbers of words recalled after the short and long delays, the number of perseverations (repeating the same word on a trial), and intrusions (stating a word that is not on the list).
We administered the Swedish Performance Evaluation System (SPES) to assess simple reaction time and finger tapping speed (Iregren et al., 1996). Reaction time was assessed using a laptop PC. The child was asked to press a key as quickly as possible when a white square (n=96 over a six minute testing period) appeared on the monitor. Visual stimuli appeared every 2.5 to 5.0 seconds; the first minute of the test was designated as practice while the last five minutes were recorded. We examined the mean reaction time and variability (i.e., standard deviation [SD]). In the finger tapping speed test children were asked to use their index finger to tap a keyboard key as rapidly as possible for 8 trials of 10 seconds each with a 15 second rest interval. The children alternated hands for each trial so that a total of four trials were completed for both the dominant and non-dominant hands. The mean number of taps per hand was used to assess performance.
Children completed five subtests of motor coordination from the Luria-Nebraska Motor Battery including dominant/non-dominant hand clench, alternative hand clench, and finger-thumb touching with dominant/non-dominant hand. We used the sum of these 5 subtests to yield a partial Luria motor score (Golden et al., 1980, Lucchini et al., 2012).
Child Behavior
Parents, typically mothers, completed the Conners Parent Rating Scales Revised-Short Version (CPRS) (Conners, 1997). The CPRS is an age and sex standardized instrument consisting of 27 items that are used to construct a summary ADHD index and three subscales of behaviors typical of children with ADHD: 1) cognitive problems/inattention, 2) hyperactivity, and 3) oppositional behaviors. In descriptive analyses, CPRS scores were dichotomized to identify children with behaviors suggestive of a clinical ADHD diagnosis. (T score > 60).
Statistical Analysis
We began by describing the demographic characteristics of the children (counts, percents, and means). We then computed univariate statistics (mean, quartiles, standard deviation [SD], and range) of the latency, distance, working memory errors, and reference memory errors on each trial of the VRAM. Next, we calculated univariate statistics for the CBCL, WISC-III, and CVLT scores.
We used generalized linear mixed models to account for the within-person correlation when examining the relationship between various predictors and VRAM latency, distance, working memory errors, and reference memory errors. We modeled the latency, distance, working memory errors, and reference memory errors as a function of the different predictors. We started by modeling VRAM performance measures as a function of trial (continuous variable). Then, we created separate models for each predictor so that each model included the predictor and trial, since trial accounted for a large proportion of the variance. We assumed a normal distribution of residuals and used an identity link when modeling time and distance. Because the working and reference memory errors were recorded as counts, we used a log-link function and Poisson residual distribution; however, we had to use a negative binomial distribution with log-link for analyzing the association between Luria motor scores and reference memory errors. In all the models, each child was given a random intercept for trial and we used an unstructured covariance matrix. Models containing behavioral and neuropsychological predictors were adjusted for trial, child age, sex, and motion sickness during the test.
We estimated the mean change in the four VRAM measures compared to the reference category for categorical variables or with a 1 SD increase in psychometric test score. We used SDs based on the neuropsychological tests’ standardization samples, when available; otherwise we used the SD from our sample of children. We calculated the count ratio (CR) from the working and reference memory error models, which has a null value of 1.0 and values >/<1.0 indicate more/fewer errors, respectively, compared to the reference category. For example, a CR of 0.68 indicates that the index group had 32% fewer errors than the reference group; whereas a CR of 1.32 indicates that the index group had 32% more errors than the reference group.
Secondary Analyses
We conducted three secondary analyses to verify the robustness of our results. First, we analyzed our reference memory error data with Trial 1 excluded from the analysis to determine if including these errors on the first trial biased our results since children were penalized for a reference memory error on trial 1. Second, we examined what specific subscales of the WISC-III were most strongly associated with VRAM performance. Finally, we examined whether the association between child sex and VRAM performance was attenuated after adjustment for video game use.
Results
Child Demographics and Test Factors
A total of 255 children participated in our study and between 248 and 255 had complete VRAM and predictor data available for one or more analysis. Most children were 1st or 2nd born and most parents had completed senior high school (Table 1). On average, children were 13 years of age (SD: 0.9, range 10 to 15 years) at enrollment. Most children had above average scores on the WISC-III FSIQ, VIQ, and PIQ (Table 2). Forty-four children (16%) had an ADHD Index score indicative of a possible ADHD diagnosis.
Table 1.
Demographic characteristics of 255 participating children and their parents
| Characteristic | Mean/N (SD/%) |
|---|---|
| Child Sex | |
| Girl | 125 (49.0) |
| Boy | 130 (51.0) |
| Study Site | |
| Garda Lake | 33 (12.9) |
| Valamonica | 102 (40.0) |
| Bagnolo | 120 (47.1) |
| Birth Order | |
| 1 | 130 (53.3) |
| 2 | 90 (36.9) |
| 3+ | 24 (9.8) |
| Number of Children in Home | |
| 1 | 138 (73.8) |
| 2 | 36 (19.3) |
| 3+ | 13 (7.0) |
| Maternal Education | |
| ≤ Junior High School | 86 (36.3) |
| Senior High School | 113 (47.7) |
| > Senior High School | 38 (16.0) |
| Paternal Education | |
| ≤ Junior High School | 102 (42.5) |
| Senior High School | 112 (46.7) |
| > Senior High School | 26 (10.8) |
| Socioeconomic Status Category | |
| High | 83 (34.9) |
| Medium | 101 (42.4) |
| Low | 54 (22.7) |
| Child Weight (kg) | 52 (13) |
| Child Height (cm) | 158 (9) |
| Child Body Mass Index (kg/m2) | 21 (4) |
| Child Age (years) | 13 (1) |
Table 2.
Univariate description of child psychometric tests
| Neuropsychological Scale | N | Mean (SD) | Range |
|---|---|---|---|
| Full Scale IQ | 254 | 108 (12) | (68, 147) |
| Performance IQ | 254 | 111 (13) | (55, 143) |
| Verbal IQ | 254 | 104 (13) | (71, 146) |
| Conner’s ADHD Index | 248 | 51 (12) | (39, 100) |
| Conner’s Hyperactivity | 248 | 49 (10) | (36, 89) |
| Conner’s Cognitive/Inattention | 248 | 48 (11) | (41, 100) |
| Conner’s Oppositional | 248 | 48 (7) | (40, 90) |
| California Verbal Learning-Trial 5 | 255 | 12 (2) | (7, 15) |
| California Verbal Learning-Short Recall | 255 | 11 (2) | (5, 15) |
| California Verbal Learning-Long Recall | 255 | 12 (2) | (5, 15) |
| California Verbal Learning-Perseverations | 255 | 7 (6) | (0, 44) |
| California Verbal Learning-Intrusions | 255 | 3 (4) | (0, 20) |
| Reaction Time (ms) | 253 | 307 (47) | (230, 598) |
| Reaction Time Variability (ms) | 253 | 69 (25) | (23, 184) |
| Finger Tapping Dominant (taps) | 252 | 60 (6) | (40, 77) |
| Finger Tapping Non-Dominant (taps) | 252 | 52 (6) | (36, 69) |
| Luria-Nebraska Motor Sum | 255 | 61 (12) | (35, 101) |
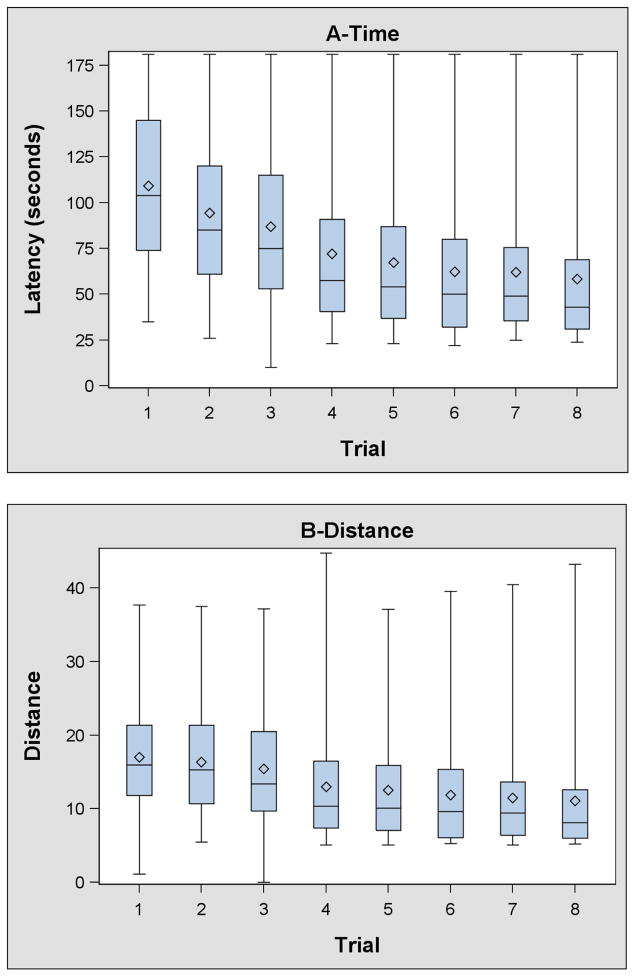
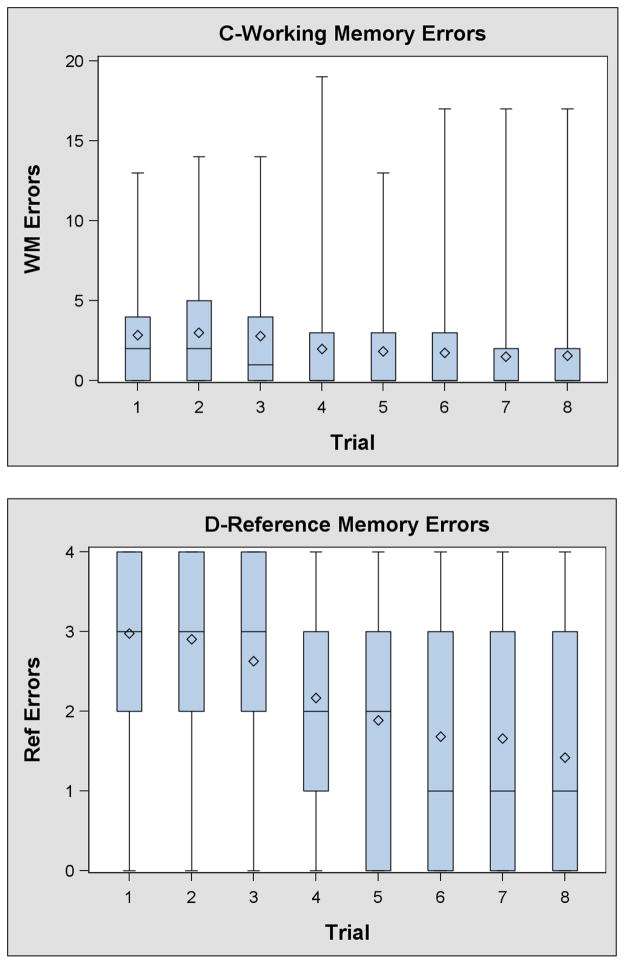
The number of children who did not find all four rewards in ≤ 180 seconds decreased from 33 (13%) on trial 1 to ≤ 12 (<5%) on trials 6–8. On average children completed the VRAM in 110 seconds (SD: 43 seconds) on the first trial and in 58 seconds (SD: 41 seconds) on the eighth trial (Supplemental Tables 1–4). Approximately 90% of children completed trial 6, 7, or 8 faster and in less distance than trial 1. A smaller percent completed trial 6, 7, or 8 with fewer working (67%) or reference (82%) memory errors; however, at least 90% completed one of the last 3 trials with an equivalent number of errors to their first trial.
Children’s time and distance to complete the VRAM, as well as the number of working and reference memory errors decreased over the first six trials and plateaued between trials 6 and 8 (Table 3, Supplemental Tables 1–4, and Figure 2). The relationship between trial and latency (β: −2 seconds/trial; 95% CI [CI]: −5, 1) distance (β: −0.4 maze units/trial; CI; −0.9, 0.1), working memory errors (CR: 0.95; CI: 0.89, 1.02), and reference memory errors (CR: 0.92; CI: 0.86, 0.99) was attenuated towards the null when we restricted our analysis to trials 6–8.
Table 3.
Change in VRAM performance measures according to child demographic and testing factors*
| N | Latency, seconds (95% CI) | Distance, maze units (95% CI) | Working Memory Count Ratio† (95% CI) | Reference Memory Count Ratio† (95% CI) | |
|---|---|---|---|---|---|
| Trial (per trial) | 255 | −7 (−8, −6) | −0.9 (−1.0, −0.8) | 0.90 (0.89, 0.91) | 0.89 (0.88, 0.91) |
| Age (per year) | 255 | −6 (−9, −3) | −1.2 (−1.7, −0.6) | 0.76 (0.68, 0.85) | 0.91 (0.86, 0.97) |
| Sex | |||||
| Girls | 126 | Ref | Ref | Ref | Ref |
| Boys | 129 | −14 (−20, −9) | −1.2 (−2.2, −0.3) | 0.81 (0.66, 0.99) | 0.88 (0.79, 0.98) |
| Socioeconomic Status Category | |||||
| High | 83 | Ref | Ref | Ref | Ref |
| Medium | 101 | −2 (−9, 5) | 0.0 (−1.1, 1.1) | 1.05 (0.82, 1.35) | 0.97 (0.85, 1.10) |
| Low | 54 | 3 (−5, 11) | 1.2 (−0.1, 2.5) | 1.25 (0.93, 1.67) | 1.06 (0.91, 1.23) |
| Reported Sensitivity to Motion Sickness | |||||
| No | 209 | Ref | Ref | Ref | Ref |
| Yes | 46 | 1 (−7, 8) | 0.0 (−1.3, 1.3) | 1.06 (0.81, 1.39) | 1.02 (0.88, 1.17) |
| Dizziness During Testing | |||||
| No | 235 | Ref | Ref | Ref | Ref |
| Yes | 20 | 20 (8, 32) | 2.1 (0.1, 4.1) | 1.57 (1.08, 2.30) | 1.17 (0.95, 1.44) |
| Visual Deficits | |||||
| None | 181 | Ref | Ref | Ref | Ref |
| Astigmatism | 21 | 0 (−10, 11) | −0.3 (−2.1, 1.5) | 1.01 (0.69, 1.47) | 0.95 (0.78, 1.16) |
| Myopia | 32 | −1 (−10, 8) | 0.1 (−1.4, 1.5) | 0.94 (0.68, 1.28) | 0.99 (0.84, 1.17) |
| Both | 12 | −1 (−14, 13) | 0.2 (−2.1, 2.5) | 1.04 (0.64, 1.70) | 0.92 (0.71, 1.19) |
| Other | 9 | −13 (−28, 3) | −2.6 (−5.2, 0.0) | 0.43 (0.24, 0.79) | 0.73 (0.54, 1.00) |
| Any | 74 | −1 (−4, 1) | −0.2 (−0.7, 0.2) | 0.92 (0.84, 1.01) | 0.96 (0.91, 1.01) |
-All models are adjusted for trial.
-Count ratios represent the fold-difference in the number of working or reference memory errors relative to the reference group. Ratios >1 indicate more errors, while ratios <1 indicate less errors.
-Bolded estimates are statistically significant (p<0.05). Null values for latency and distance are 0 and 1.0 for working memory and reference memory errors.
Figure 2.
Box and whisker plots of VRAM time, distance, working memory errors, and reference memory errors.*
*- Whiskers represent the minimum and maximum. The bottom and top of the box represents the 25th and 75th percentiles, respectively. The lines in the box represent the median. The diamond represents the mean.
Twenty children (12%) experienced some nausea or dizziness during the testing. None of these children completed the entire VRAM. In addition, these children had longer latency and distance to completion, as well as more working and reference memory errors than children who did not experience motion sickness during the testing (Table 3). Children who reported sensitivity to motion sickness (not during the testing) were less likely to complete all 8 trials than children who were not (85 vs. 94%), but did not perform substantially worse than children without motion sickness sensitivity (Table 3). Children with any diagnosed, but corrected visual deficits did not perform more poorly than children without visual deficits.
On average, boys completed the VRAM 14 seconds faster than girls (95% confidence interval [CI]: -20, -9). Boys also completed the VRAM in less distance and with fewer errors than girls (Table 3). Older children tended to perform better on the VRAM than younger children.
Child Leisure and Recreation Activities
After adjusting for trial, age, sex, and motion sickness during the test, children’s daily video game use predicted better performance on all four VRAM performance measures in a monotonic dose-response fashion (Table 4). Children who played ≥ 2 hours of video games/day finished the maze 16 seconds faster (CI: −25, −6) and in 2.4 fewer maze units (CI: −4.1, −0.7) than children who rarely played video games. Children who spent ≥2 hours playing video games also had 34% (CI: 5, 54) and 21% (CI: 4, 35) fewer working and reference memory errors, respectively. Compared to children in the reference category, children who spent the 2+ hours per day on the internet or watching television completed the maze somewhat faster and in less distance, although the 95% CIs for this contrast included the null value.
Table 4.
Change in VRAM performance measures according to child activity factors*
| N | Latency, seconds (95% CI) | Distance, maze units (95% CI) | Working Memory Count Ratio† (95% CI) | Reference Memory Count Ratio† (95% CI) | |
|---|---|---|---|---|---|
| Books Read per Month | |||||
| Doesn’t read/Rarely | 53 | Ref | Ref | Ref | Ref |
| 1 | 143 | −1 (−14, 11) | 0.1 (−2.0, 2.3) | 0.91 (0.57, 1.45) | 0.92 (0.72, 1.19) |
| 2 | 17 | 1 (−7, 9) | 0.6 (−0.8, 1.9) | 1.07 (0.80, 1.42) | 1.12 (0.96, 1.30) |
| 3+ | 41 | −1 (−11, 8.4) | 0.2 (−1.5, 1.8) | 0.94 (0.66, 1.33) | 1.14 (0.94, 1.37) |
| Duration of Daily Video Game Use | |||||
| Doesn’t play | 28 | Ref | Ref | Ref | Ref |
| Rarely plays | 69 | −8 (−18, 1) | −1.5 (−3.1, 0.2) | 0.76 (0.53, 1.08) | 0.91 (0.76, 1.10) |
| 1 hour/day | 98 | −11 (−20, −2) | −1.7 (−3.3, −0.1) | 0.77 (0.54, 1.08) | 0.84 (0.70, 1.00) |
| 2+ hours/day | 57 | −16 (−25, −6) | −2.4 (−4.1, −0.7) | 0.66 (0.46, 0.95) | 0.79 (0.65, 0.96) |
| Duration of Daily Internet Use | |||||
| Doesn’t surf | 38 | Ref | Ref | Ref | Ref |
| <1 hour/day | 148 | −9 (−16, −1) | −0.8 (−2.1, 0.6) | 0.79 (0.60, 1.06) | 0.89 (0.76, 1.03) |
| 1–2 hours/day | 46 | −7 (−16, 3) | −0.1 (−1.7, 1.5) | 0.89 (0.63, 1.26) | 0.88 (0.73, 1.07) |
| 2+ hours/day | 22 | −12 (−23, 0) | −0.8 (−2.9, 1.2) | 0.93 (0.60, 1.45) | 0.82 (0.64, 1.04) |
| Duration of Daily TV Viewing | |||||
| Rarely | 29 | Ref | Ref | Ref | Ref |
| <1 hour/day | 84 | −4 (−14, 5) | −0.2 (−1.8, 1.5) | 0.95 (0.67, 1.35) | 1.00 (0.83, 1.20) |
| 1–2 hours/day | 87 | −4 (−13, 5) | −0.5 (−2.1, 1.2) | 0.93 (0.66, 1.32) | 0.94 (0.78, 1.14) |
| 2+ hours/day | 54 | −8 (−18, 2) | −0.8 (−2.6, 1.0) | 0.86 (0.59, 1.25) | 0.95 (0.78, 1.16) |
| Frequency of Playing Sports | |||||
| ≤ 2 times/week | 130 | Ref | Ref | Ref | Ref |
| ≥ 3 times/week | 124 | −2 (−8, 3) | −0.2 (−1.2, 0.7) | 0.96 (0.78, 1.17) | 0.98 (0.88, 1.09) |
| Competitive Sports | |||||
| No | 100 | Ref | Ref | Ref | Ref |
| Yes | 112 | −3 (−9, 3) | −0.5 (−1.5, 0.6) | 0.88 (0.70, 1.10) | 0.96 (0.85, 1.09) |
| Previously | 42 | 0 (−8, 8) | 0.1 (−1.3, 1.4) | 0.95 (0.70, 1.27) | 0.94 (0.80, 1.10) |
-All models are adjusted for trial (continuous), child age (continuous in years), child sex (males and females), and motion sickness during the test (yes and no).
-Count ratios represent the fold-difference in the number of working or reference memory errors relative to the reference group. Ratios >1 indicate more errors, while ratios <1 indicate less errors.
-Bolded estimates are statistically significant (p<0.05). Null values for latency and distance are 0 and 1.0 for working memory and reference memory errors.
Child Psychometric Tests
In general, faster and more efficient VRAM performance was associated with higher FSIQ, PIQ, and VIQ scores (Table 5). These associations were among the strongest observed among all the different psychometric tests. Scores indicative of better verbal memory on the CVLT were associated with improvements in VRAM performance, although the 95% CI included the null value. Each SD increase in CVLT perseveration scores was associated with longer VRAM latency and distance, as well as more working and reference memory errors.
Table 5.
Adjusted change in VRAM performance measures with one standard deviation increase in psychometric test score*
| Neuropsychological Test | N | SD | Latency, seconds (95% CI) | Distance, maze units (95% CI) | Working Memory Count Ratio (95% CI) † | Reference Memory Count Ratio† (95% CI) |
|---|---|---|---|---|---|---|
| Full Scale IQ | 254 | 15 | −9 (−12, −6) | −1.6 (−2.2, −1.1) | 0.71 (0.63, 0.79) | 0.85 (0.79, 0.90) |
| Performance IQ | 254 | 15 | −8 (−11, −6) | −1.4 (−1.9, −0.9) | 0.73 (0.66, 0.82) | 0.86 (0.81, 0.91) |
| Verbal IQ | 254 | 15 | −7 (−10, −4) | −1.5 (−2.0, −0.9) | 0.74 (0.66, 0.84) | 0.87 (0.82, 0.93) |
| Conners ADHD Index | 247 | 10 | 1 (−1, 4) | 0.4 (0.0, 0.7) | 1.08 (1.00, 1.18) | 1.04 (1.00, 1.09) |
| Conners Oppositional | 247 | 10 | 0 (−2, 3) | 0.0 (−0.4, 0.5) | 1.00 (0.91, 1.11) | 1.01 (0.96, 1.07) |
| Conners Cognitive/Inattention | 247 | 10 | 2 (0, 5) | 0.5 (0.0, 0.9) | 1.11 (1.01, 1.22) | 1.07 (1.01, 1.12) |
| Conners Hyperactivity | 247 | 10 | 0 (−3, 4) | 0.4 (−0.3, 1.0) | 1.11 (0.97, 1.27) | 1.06 (0.99, 1.14) |
| CVLT Trial 5 | 254 | 2 | −2 (−5, 0) | −0.4 (−0.8, 0.1) | 0.92 (0.83, 1.01) | 0.95 (0.90, 1.01) |
| CVLT Short Recall | 254 | 2 | −2 (−5, 0) | −0.3 (−0.8, 0.1) | 0.95 (0.86, 1.04) | 0.99 (0.94, 1.04) |
| CVLT Long Recall | 254 | 2 | −2 (−4, 1) | −0.4 (−0.8, 0.0) | 0.93 (0.84, 1.02) | 0.97 (0.93, 1.02) |
| CVLT Perseverations | 254 | 6 | 2 (0, 5) | 0.5 (0.1, 0.9) | 1.10 (1.00, 1.20) | 1.05 (1.00, 1.11) |
| CVLT Intrusions | 254 | 4 | 2 (−1, 5) | 0.3 (−0.2, 0.7) | 1.05 (0.95, 1.17) | 1.02 (0.96, 1.08) |
| Reaction Time | 252 | 47 | 3 (0, 5) | 0.2 (−0.3, 0.6) | 1.02 (0.92, 1.13) | 0.99 (0.94, 1.05) |
| Reaction Time Standard Deviation | 252 | 25 | 3 (0, 6) | 0.2 (−0.3, 0.7) | 1.03 (0.93, 1.15) | 1.03 (0.97, 1.08) |
| Finger Tapping Speed Dominant | 251 | 6 | −2 (−5, 1) | −0.3 (−0.7, 0.2) | 0.98 (0.89, 1.09) | 0.97 (0.92, 1.03) |
| Finger Tapping Speed Non-Dominant | 251 | 6 | −2 (−5, 1) | −0.2 (−0.7, 0.3) | 0.99 (0.89, 1.10) | 1.00 (0.94, 1.06) |
| Luria-Nebraska Motor Sum | 254 | 12 | −2 (−5, 1) | −0.4 (−0.8, 0.1) | 0.93 (0.85, 1.02) | 0.92 (0.83, 1.02) |
-All models are adjusted for trial (continuous), child age (continuous in years), child sex (males and females), and motion sickness during the test (yes and no).
-Count ratios represent the fold-difference in the number of working or reference memory errors relative to the reference group. Ratios >1 indicate more errors, while ratios <1 indicate less errors.
-Bolded estimates are statistically significant (p<0.05). Null values for latency and distance are 0 and 1.0 for working memory and reference memory errors.
SD-Standard Deviation
In general, scores on measures of reaction time and motor skills were in the anticipated direction. Higher RT variability was associated with longer VRAM completion times. Higher Luria-Nebraska motor scores were associated with less working and reference memory errors, although the 95% CIs included the null value. (Table 5)
Child Behavior
There was a suggestive trend of more working (CR: 1.08; CI: 1.00, 1.18) and reference (CR: 1.04; CI: 1.00, 1.09) memory errors among children with higher CPRS ADHD Index scores (Table 5). We observed a stronger association between Hyperactive or Cognitive/Inattentive scores and VRAM performance with some 95% CIs excluding the null value. In contrast, the association between oppositional problems and VRAM performance was null.
Secondary Analyses
Excluding trial 1 from our reference memory analyses generally resulted in the observed associations becoming stronger, but the overall pattern of results remained the same. Examination of the 11 WISC-III subscales revealed that the Arithmetic, Block Design, Digit Span, Information, and Similarities subscales were most strongly associated with faster and more efficient performance. After adjustment for trial, child sex, age, and motion sickness during the testing session, Block Design was associated with the largest decrease in time (β: −9; 95% CI: −12, −6), distance (β: −1.6; 95% CI: −2.1, −1.1), working memory errors (CR: 0.69; 95% CI: 0.62, 0.78), and reference memory errors (CR: 0.85; 95% CI: 0.80, 0.90). In a single model containing trial, child age, sex, and video game use, the associations between child sex and VRAM performance were similar.
Discussion
Several investigators have advocated for and used domain-specific neurobehavioral tests in epidemiological studies (Grandjean et al., 1997, Rice, 2005, Stewart et al., 2003). Computerized analogues of animal neurobehavioral tests go one step further by allowing domain specific testing and more direct comparison of epidemiological and animal results. While using the VRAM in epidemiological studies may facilitate integration and translational comparisons across species, investigators should carefully consider whether the VRAM (or any neurobehavioral test) will be sensitive to the toxicant of interest. For example the VRAM may be appropriate for the study of toxicants like Mn, where the animal research shows that Mn exposure impacts RAM performance in rodents (Kern, Stanwood, 2010). However, the absence of animal data should not discourage investigators from using the VRAM in their test batteries.
Early animal studies revealed performance and learning on the RAM was a function of hippocampal activity, although neurobehavioral processes related to executive function (i.e., frontal lobe activity) are also likely involved in performance on the task (Becker et al., 1980, Olton et al., 1977). In humans VRAM performance is impaired in amnesic patients with hippocampal damage (Goodrich-Hunsaker and Hopkins, 2010). In addition, the hippocampal and frontal cortex regions of the brain are activated when humans are completing the VRAM (Astur, St Germain, 2005). Several environmental chemicals including Pb, Mn, pesticides, and phthalates have been implicated as risk factors for ADHD, specifically impairments in executive function (Bouchard et al., 2010, Engel et al., 2010, Ericson et al., 2007, Eskenazi et al., 2007, Roy et al., 2009). Thus, the VRAM may be a sensitive measure of impairments in executive function and provide a comparable measure to animal studies of these toxicants.
Other researchers have also adapted domain-specific animal behavioral tests to humans. Examples include the Cambridge Neuropsychological Test Automated Batteries (CANTAB), Differential Reinforcement of Low Rates (DRL), and Temporal Response Differentiation (TRD) task (Canfield et al., 2004, Chelonis et al., 2004, Stewart et al., 2006) Consistent with our study, several previous studies have found that higher IQ scores are associated better CANTAB scores (Strauss et al., 2006). Using the CANTAB, Canfield et al reported that children with higher childhood blood Pb concentrations had poorer working memory and planning (Canfield, Gendle, 2004). Stewart and colleagues found that DRL performance was associated with PCB, Hg, and Pb exposure in children. Similar to the positive association we observed between IQ and VRAM performance, Chelonis et al. found that higher IQ scores were associated with less variability on the TRD task, which assesses timing abilities.
Other domain-specific computerized analogues of animal tests are available, including the Virtual Morris Water Maze (VMWM) (Astur, Tropp, 2004, Newhouse et al., 2007). Using a PC and joystick, children navigate around a virtual pool to find a hidden platform. Like the VRAM, the VMWM assesses visuospatial memory and can be used to measure visuospatial reference memory across trials. A previous study found that gestational Pb exposure increases the time to complete the Morris Water Maze (MWM) among rats (Jett et al., 1997) and another study observed altered search strategies and increased MWM latency among chlorpyrifos exposed rats (Moser et al., 2005).
Children in our study improved on all four VRAM performance measures (latency, distance, and working and reference memory errors) over the course of eight trials, indicating that they learned the location of the rewards and became more efficient at finding them. We are not aware of prior studies in which the VRAM was administered to children or adolescents, but this pattern of improved performance is consistent with the pattern observed in adults who completed the VRAM and children who completed another virtualized animal test (Virtual Morris Water Maze [VMWM]) (Astur, Tropp, 2004, Newhouse, Newhouse, 2007, Spieker et al., 2011). A prior study administered a human-scale version of the RAM to school-aged children. Consistent with our findings, they reported that younger children did not perform as well as older children; but this study did not report differences in child performance by child sex (Overman et al., 1996). This discrepancy may be due to Overman using a free and forced choice testing paradigm while we used a fixed-reward paradigm.
Boys in our study completed the VRAM more quickly and efficiently than females. This is consistent with a large body of literature reporting better visuospatial abilities among males compared to females (Cohen-Bendahan et al., 2005). This relationship may be mediated by sex-specific expectations and opportunities that influence activity choices in childhood and adolescences. Thus, the magnitude of any direct effect may be attenuated after adjustment for these factors. Furthermore, differences between the sexes may not represent differences in spatial learning capacity, but rather different search strategies employed by males and females. (Saucier et al., 2002).
Rodent studies demonstrate that gonadal hormone exposures are responsible for the development of improved visuospatial abilities in males (Gibbs and Johnson, 2008, Lund and Lephart, 2001, Lund et al., 2001). While less is known with regards to humans, the results from studies of children with congenital adrenal hyperplasia – a genetic disorder resulting in excessively high levels of gestational androgens – suggest that gonadal hormone exposure masculinizes VMWM performance in girls with CAH (Mueller et al., 2008). Given the known role of gonadal hormones in the development of visuospatial abilities and the potential for endocrine disrupting compounds to perturb both the gestational hormone milieu and other pathways associated with executive function, future epidemiological studies could use the VRAM to study endocrine disrupting compounds (Eubig, Aguiar, 2010, Li et al., 2008, Mueller, Temple, 2008, Ryan and Vandenbergh, 2006).
Consistent with our a priori hypothesis, more video game use predicted better VRAM performance in a monotonic dose-response fashion, and the relationships between video game use and VRAM performance measures were independent of child sex and duration of daily TV viewing. This could be due to a practice effect among children who play more video games. On the other hand, children with better visuospatial abilities may play more video games because they are better at them than other children. Future studies using the VRAM may need to adjust for video game use as a confounder since the presence of a video game console or computer might be associated with socioeconomic factors that predict both environmental chemical exposure and VRAM performance.
While FSIQ was associated with better VRAM performance, some domains of IQ were more strongly associated with performance than others. Among WISC-III subtests, Block Design showed the strongest association with VRAM performance measures. Both arithmetic and digit span, which comprise the freedom from distractibility factor of the WISC-III, were also positively associated with VRAM performance. Freedom from distractibility scores are usually lower in children with ADHD. (Mayes and Calhoun, 2006) This suggests that executive function plays an important role in VRAM performance. Surprisingly, better information, vocabulary, and comprehension subtest scores, which all are part of the verbal comprehension factor of the WISC-III, also predicted better VRAM performance.
Children with higher ADHD Index, hyperactivity, and inattentive CPRS scores had worse performance on the VRAM. This is not surprising since children need to use executive function processes such as working and reference memory to remember which arms they have visited on a given trial and which arms are rewarded across trials. The association between between both hyperactive and inattentive behaviors suggests that the VRAM is sensitive to multiple dimensions of ADHD-like behaviors, while the lack of association between oppositional behaviors and VRAM performance suggests that the VRAM is specific to certain features of ADHD-like behaviors.
There was some evidence that CVLT scores, reaction time variability, and finger tapping speed were associated with VRAM performance. These point estimates were not as large as those for WISC-III scores, but they were similar to the magnitude of association between some CPRS subscales and VRAM measures. These other neuropsychological measures may measure various aspects non-visuospatial memory, executive function, and motor skills.
While the VRAM has potential to aid in the study of developmental neurotoxicants, some limitations should be considered. The age of the child may influence VRAM performance. Because we only tested children between 10 and 15 years of age and found evidence that older children may perform better, future studies should determine if the VRAM can be administered to children <10 years of age. Second, 12% of children could not complete the entire VRAM due to motion sickness. Investigators should collect data on children’s experience of motion sickness during the test so that the data for these children can be examined separately. Also, the VRAM should be administered at the end of a test battery to avoid any effects that motion sickness may have on subsequent testing. While we did not find evidence that children with diagnosed visual deficits performed worse on the VRAM, we took special care to make sure children wore corrective lenses during the testing. We recommend that future studies do the same. Third, while the VRAM was designed as an analogue of the RAM, there are still factors that differ in the testing condition and motivation of the subject. For example, rodents are typically food-restricted before testing to ensure they are sufficiently motivated to retrieve baits. We did not evaluate the degree of a child’s motivation to complete the VRAM, though future studies may consider incentives to elicit the best performance. It is also worth noting that rodent studies typically test one trial per day over a period of 12–15 days, so the demands on between-trial (reference) memory is much greater in the RAM than the VRAM. Despite these limitations the VRAM is more comparable to tests administered to animals than other tests typically administered in epidemiological studies of neurotoxicants. Finally, while the VRAM and other domain-specific tests allow better comparability with animal tests, they lack the interpretability that traditional IQ and behavior measures provide. This makes the interpretation of these results more challenging when presenting results to policy makers and regulators (Rice, 2005).
Conclusion
A variety of factors predicted VRAM performance in this cohort. Consistent with rodent studies, we found that boys generally performed better on the VRAM than girls. Among the measured child leisure activities, video game use was the strongest predictor of VRAM performance. Finally, many cognitive and behavioral processes, including some related to ADHD-like behaviors and memory, were associated with VRAM performance. Future research should validate these findings and examine whether environmental chemical exposures impact VRAM performance.
Supplementary Material
Highlights.
Performance on the VRAM improved over the course of 8 trials and boys performed better than girls.
Children who spent more time playing video games performed better independent of child sex, age, and motion sickness during the test.
Higher IQ and fewer inattentive behaviors were associated with better performance.
Acknowledgments
This work was supported by NIEHS (grants P42 ES016454, R01 ES013744, R01 ES019222, R01ES018990, and T32 ES007069), the European Union (Sixth Framework Programme for RTD, contract no. FOOD-CT-2006-016253); and the Leaves of Grass Fund.
Abbreviations
- ADHD
Attention-Deficit/Hyperactivity Disorder
- CANTAB
Cambridge Neuropsychological Test Automated Batteries
- CBCL
Child Behavior Checklist
- CI
95% Confidence Interval
- CPRS
Conners Parent Rating Scales-Short Version
- CR
Count Ratio
- CVLT
California Verbal Learning Test
- DRL
Differential Reinforcement of Low Rates
- FSIQ
Full Scale Intelligence Quotient
- Hg
Mercury
- ICC
Intraclass Correlation Coefficient
- Mn
Manganese
- Pb
Lead
- PCB
Polychlorinated Biphenyl
- PIQ
Performance Intelligence Quotient
- RAM
Radial Arm Maze
- SD
Standard Deviation
- SPES
Swedish Performance Evaluation System
- TRD
Temporal Response Differentiation
- VIQ
Verbal Intelligence Quotient
- VMWM
Virtual Morris Water Maze
- VRAM
Virtual Radial Arm Maze
- WISC-III
Wechsler Intelligence Scales for Children
Appendix A: Instructions given to participants for the VRAM
You will find yourself in a room that has 8 runways extending from its middle. At the end of each runway is an area that may or may not have a reward.
Four (4) of the runways have rewards, and 4 do not have rewards. Your goal is to find all 4 rewards without re-entering a runway where you already retrieved a reward. Avoid the runways that do not have a reward.
You will use a joystick to explore the room. The computer will inform you when you have found all 4 rewards.
Once you have found all the rewards, you will start again, and you are to find the rewards again. The rewards will always be in the same runways. Use the landmarks in the room to remember which runways have the rewards.
If anything is unclear about these instructions, please feel free to ask questions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderko L, Braun JM, Auinger P. Contribution of Tobacco Smoke Exposure to Learning Disabilities. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2010;39:111–7. doi: 10.1111/j.1552-6909.2009.01093.x. [DOI] [PubMed] [Google Scholar]
- Anger WK. Animal test systems to study behavioral dysfunctions of neurodegenerative disorders. Neurotoxicology. 1991;12:403–13. [PubMed] [Google Scholar]
- Astur RS, St Germain SA, Baker EK, Calhoun V, Pearlson GD, Constable RT. fMRI hippocampal activity during a virtual radial arm maze. Appl Psychophysiol Biofeedback. 2005;30:307–17. doi: 10.1007/s10484-005-6385-z. [DOI] [PubMed] [Google Scholar]
- Astur RS, Tropp J, Sava S, Constable RT, Markus EJ. Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation. Behav Brain Res. 2004;151:103–15. doi: 10.1016/j.bbr.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Axelrad DA, Bellinger DC, Ryan LM, Woodruff TJ. Dose-response relationship of prenatal mercury exposure and IQ: an integrative analysis of epidemiologic data. Environmental health perspectives. 2007;115:609–15. doi: 10.1289/ehp.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Walker JA, Olton DS. Neuroanatomical bases of spatial memory. Brain Res. 1980;200:307–20. doi: 10.1016/0006-8993(80)90922-1. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Bellinger DC, Wright RO, Weisskopf MG. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics. 2010;125:e1270–7. doi: 10.1542/peds.2009-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Froehlich TF, Kahn RS, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environmental health perspectives. 2006;114 (12):1904–9. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, et al. Impact of Early-Life Bisphenol A Exposure on Behavior and Executive Function in Children. Pediatrics. 2011;128:873–82. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield RL, Gendle MH, Cory-Slechta DA. Impaired neuropsychological functioning in lead-exposed children. Dev Neuropsychol. 2004;26:513–40. doi: 10.1207/s15326942dn2601_8. [DOI] [PubMed] [Google Scholar]
- Cesana GC, Ferrario M, De Vito G, Sega R, Grieco A. Evaluation of the socioeconomic status in epidemiological surveys: hypotheses of research in the Brianza area MONICA project. Med Lav. 1995;86:16–26. [PubMed] [Google Scholar]
- Chapin RE, Adams J, Boekelheide K, Gray LE, Jr, Hayward SW, Lees PS, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol. 2008;83:157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- Chelonis JJ, Flake RA, Baldwin RL, Blake DJ, Paule MG. Developmental aspects of timing behavior in children. Neurotoxicology and teratology. 2004;26:461–76. doi: 10.1016/j.ntt.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Cohen-Bendahan CC, van de Beek C, Berenbaum SA. Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings. Neurosci Biobehav Rev. 2005;29:353–84. doi: 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners Rating Scale-Revised. Toronto, ON: Multi-Health Systems; 1997. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2. San Antonio: The Psychological Corporation; 2000. [Google Scholar]
- Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, et al. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environmental health perspectives. 2010;118:565–71. doi: 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson JE, Crinella FM, Clarke-Stewart KA, Allhusen VD, Chan T, Robertson RT. Prenatal manganese levels linked to childhood behavioral disinhibition. Neurotoxicology and teratology. 2007;29:181–7. doi: 10.1016/j.ntt.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environmental health perspectives. 2007;115:792–8. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubig PA, Aguiar A, Schantz SL. Lead and PCBs as risk factors for attention deficit/hyperactivity disorder. Environmental health perspectives. 2010;118:1654–67. doi: 10.1289/ehp.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology. 2008;149:3176–83. doi: 10.1210/en.2007-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ, Sweet J, Hammeke T, Purisch A, Graber B, Osmon D. Factor analysis of the Luria-Nebraska Neuropsychological Battery. I. Motor, rhythm, and tactile scales. Int J Neurosci. 1980;11:91–9. doi: 10.3109/00207458009150331. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hopkins RO. Spatial memory deficits in a virtual radial arm maze in amnesic participants with hippocampal damage. Behav Neurosci. 2010;124:405–13. doi: 10.1037/a0019193. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicology and teratology. 1997;19:417–28. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Haider S, Shameem S, Ahmed SP, Perveen T, Haleem DJ. Repeated administration of lead decreases brain 5-HT metabolism and produces memory deficits in rats. Cellular & molecular biology letters. 2005;10:669–76. [PubMed] [Google Scholar]
- Iregren A, Gamberale F, Kjellberg A. SPES: a psychological test system to diagnose environmental hazards. Swedish Performance Evaluation System. Neurotoxicology and teratology. 1996;18:485–91. doi: 10.1016/0892-0362(96)00033-5. [DOI] [PubMed] [Google Scholar]
- Jett DA, Kuhlmann AC, Farmer SJ, Guilarte TR. Age-dependent effects of developmental lead exposure on performance in the Morris water maze. Pharmacol Biochem Behav. 1997;57:271–9. doi: 10.1016/s0091-3057(96)00350-4. [DOI] [PubMed] [Google Scholar]
- Jones DC, Miller GW. The effects of environmental neurotoxicants on the dopaminergic system: A possible role in drug addiction. Biochem Pharmacol. 2008;76:569–81. doi: 10.1016/j.bcp.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Kern CH, Stanwood GD, Smith DR. Preweaning manganese exposure causes hyperactivity, disinhibition, and spatial learning and memory deficits associated with altered dopamine receptor and transporter levels. Synapse (New York, NY. 2010;64:363–78. doi: 10.1002/syn.20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Addy N, Nakajima A, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Brain Res Dev Brain Res. 2001;130:83–9. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- Li AA, Baum MJ, McIntosh LJ, Day M, Liu F, Gray LE., Jr Building a scientific framework for studying hormonal effects on behavior and on the development of the sexually dimorphic nervous system. Neurotoxicology. 2008;29:504–19. doi: 10.1016/j.neuro.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Lidsky TI, Schneider JS. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain. 2003;126:5–19. doi: 10.1093/brain/awg014. [DOI] [PubMed] [Google Scholar]
- Lucchini RG, Guazzetti S, Zoni S, Donna F, Peter S, Zacco A, et al. Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicology. 2012 doi: 10.1016/j.neuro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Lephart ED. Manipulation of prenatal hormones and dietary phytoestrogens during adulthood alter the sexually dimorphic expression of visual spatial memory. BMC Neurosci. 2001;2:21. doi: 10.1186/1471-2202-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, West TW, Tian LY, Bu LH, Simmons DL, Setchell KD, et al. Visual spatial memory is enhanced in female rats (but inhibited in males) by dietary soy phytoestrogens. BMC Neurosci. 2001;2:20. doi: 10.1186/1471-2202-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, et al. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011;32:261–7. doi: 10.1016/j.neuro.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser VC, Phillips PM, McDaniel KL, Marshall RS, Hunter DL, Padilla S. Neurobehavioral effects of chronic dietary and repeated high-level spike exposure to chlorpyrifos in rats. Toxicol Sci. 2005;86:375–86. doi: 10.1093/toxsci/kfi199. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Temple V, Oh E, VanRyzin C, Williams A, Cornwell B, et al. Early androgen exposure modulates spatial cognition in congenital adrenal hyperplasia (CAH) Psychoneuroendocrinology. 2008;33:973–80. doi: 10.1016/j.psyneuen.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz C, Garbe K, Lilienthal H, Winneke G. Significance of hippocampal dysfunction in low level lead exposure of rats. Neurotoxicology and teratology. 1988;10:245–53. doi: 10.1016/0892-0362(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Newhouse P, Newhouse C, Astur RS. Sex differences in visual-spatial learning using a virtual water maze in pre-pubertal children. Behav Brain Res. 2007;183:1–7. doi: 10.1016/j.bbr.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Olton D, Collison C, Werz M. Spatial memory and radial arm maze performance of rats. Learn Motiv. 1977;8:289–314. [Google Scholar]
- Overman WH, Pate BJ, Moore K, Peuster A. Ontogeny of place learning in children as measured in the radial arm maze, Morris search task, and open field task. Behav Neurosci. 1996;110:1205–28. doi: 10.1037//0735-7044.110.6.1205. [DOI] [PubMed] [Google Scholar]
- Rice DC. Assessing the effects of environmental toxicant exposure in developmental epidemiological studies: issues for risk assessment. Neurotoxicology. 2005;26:483–9. doi: 10.1016/j.neuro.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Roy A, Bellinger D, Hu H, Schwartz J, Ettinger AS, Wright RO, et al. Lead exposure and behavior among young children in Chennai, India. Environmental health perspectives. 2009;117:1607–11. doi: 10.1289/ehp.0900625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan BC, Vandenbergh JG. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Hormones and behavior. 2006;50:85–93. doi: 10.1016/j.yhbeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Saucier DM, Green SM, Leason J, MacFadden A, Bell S, Elias LJ. Are sex differences in navigation caused by sexually dimorphic strategies or by differences in the ability to use the strategies? Behav Neurosci. 2002;116:403–10. doi: 10.1037//0735-7044.116.3.403. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ. Cognitive effects of endocrine-disrupting chemicals in animals. Environmental health perspectives. 2001;109:1197–206. doi: 10.1289/ehp.011091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieker EA, Astur RS, West JT, Griego JA, Rowland LM. Spatial memory deficits in a virtual reality eight-arm radial maze in schizophrenia. Schizophr Res. 2011 doi: 10.1016/j.schres.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P, Fitzgerald S, Reihman J, Gump B, Lonky E, Darvill T, et al. Prenatal PCB exposure, the corpus callosum, and response inhibition. Environmental health perspectives. 2003;111:1670–7. doi: 10.1289/ehp.6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PW, Sargent DM, Reihman J, Gump BB, Lonky E, Darvill T, et al. Response inhibition during Differential Reinforcement of Low Rates (DRL) schedules may be sensitive to low-level polychlorinated biphenyl, methylmercury, and lead exposure in children. Environmental health perspectives. 2006;114:1923–9. doi: 10.1289/ehp.9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests. 3. New York, NY: Oxford; 2006. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3. San Antonio: The Psychological Corporation; 1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.