Abstract
Background
Evidence supports a role for the noradrenergic system in alcohol drinking in animals and humans. Our previous studies demonstrated the efficacy of prazosin, an α1-adrenergic antagonist, in decreasing alcohol drinking in rat models of alcohol dependence. Prazosin has also been shown to decrease alcohol drinking in treatment-seeking alcohol-dependent men. Clinically, the use of prazosin is limited by the requirement for multiple daily administrations, whereas doxazosin, a structurally similar α1-adrenergic antagonist, requires only once-daily dosing. In this study, we tested the hypothesis that doxazosin, like prazosin, would decrease alcohol drinking in rats selectively bred for alcohol preference (P line).
Methods
Adult male P rats were given 2 hour/day scheduled access to a 2-bottle choice (15% v/v alcohol versus water) session 5 days/week (M-F), with food and water available ad libitum 24 hour/day. Rats were injected with doxazosin (0 - 10 mg/kg IP) 40 minutes prior to initiation of the alcohol access session in 3 trials (of 3, 5, and 5 consecutive days) each separated by 5-8 weeks. The third trial included one day without alcohol access (for locomotor testing), and one day of a single hour of alcohol access (for plasma alcohol determination).
Results
Doxazosin significantly reduced alcohol intake in all 3 trials. The 5 mg/kg dose consistently reduced alcohol intake, increased water drinking, did not affect locomotor activity, and resulted a lower plasma alcohol concentration, suggestive that the doxazosin-induced reduction in alcohol drinking was not dependent on a motor impairment or an alteration in alcohol clearance.
Conclusions
Doxazosin decreases voluntary alcohol consumption by male alcohol-preferring (P) rats, supporting a role for the noradrenergic system in alcohol drinking in P rats and suggesting that doxazosin could potentially be an effective once-daily pharmacotherapeutic agent for the treatment of alcohol use disorders.
Keywords: Noradrenergic, Doxazosin, Alcohol, Alcohol-Preferring, P Rats
INTRODUCTION
Alcohol dependence is wide-spread, with a life-time prevalence of 12.5% in the United States, and is associated with significant personal and societal costs (Hasin et al., 2007; Samokhvalov et al., 2010; Thavorncharoensap et al., 2009). Psychosocial therapies are associated with high relapse rates of 40-70% in the first year (Finney et al., 1996). Current US Food and Drug Administration (FDA) approved pharmacologic treatment options – disulfiram, acamprosate, and oral or intramuscular naltrexone – also have considerable limitations to their use and effectiveness (Garbutt, 2009). These medications primarily interact with aldehyde dehydrogenase, the glutamatergic system, and the opioidergic system, respectively (Garbutt, 2009). Additional treatment strategies and targets are needed., and a variety of novel neuropharmacological targets are currently being investigated (recently reviewed by Leggio et al., 2010)
Another neurotransmitter system that appears to play a role in alcohol drinking is the noradrenergic system, although its specific relationships to alcohol reinforcement remain to be resolved. Self-administration of alcohol is suppressed by noradrenergic depletion in rats (Amit et al., 1977; Brown et al., 1977; Davis et al., 1978). Repeated alcohol exposure has been reported to induce sensitization of noradrenergic neurons (Lanteri et al., 2007). Hyperexcitability has been proposed as an underlying feature in the genetic predisposition to alcoholism (Begleiter and Porjesz, 1999), and is associated with adrenergic activation (Stevens et al., 1994). Consistent with this hypothesis, enhanced startle – a response that is mediated by α1-adrenergic mechanisms and reflects a hyperexcitable state (Stevens et al., 1994) – is exhibited by animal models (Chester et al., 2004; Rasmussen et al., 2005) and groups of patients (persons with post-traumatic stress disorder, PTSD: Butler et al., 1990; abstinent alcoholics: Krystal et al., 1997) that are vulnerable to excessive alcohol intake. Another link between the noradrenergic system and alcohol dependence is in stress-induced relapse/reinstatement of drug-seeking; norepinephrine is involved in the response to stress (Cecchi et al., 2002). Yohimbine, an anxiogenic drug that antagonizes the α2 adrenoceptor and induces increases in extracellular norepinephrine (Abercrombie and Keller, 1988), reinstates alcohol seeking in a rat relapse model (Lê et al., 2005). Footshock stress will also reinstate alcohol seeking (Lê et al., 1998), and prazosin, a selective α1-adrenergic receptor antagonist, has been reported to block both yohimbine and footshock-induced reinstatements (Lê et al., 2011). An additional possible connection between noradrenergic regulation and alcohol is the ability of norepinephrine to regulate the dopaminergic neurons of the ventral tegmental area via α1 adrenoceptors (Paladini and Williams, 2004; although see also Arencibia-Albite et al., 2007), an area considered to be important in the reinforcing effects of alcohol (Ikemoto and Wise, 2004).
We have previously shown the efficacy of prazosin in decreasing alcohol dependence-induced operant responding for alcohol during acute withdrawal by Wistar rats (Walker et al., 2008) and in decreasing voluntary alcohol drinking in alcohol-preferring (P) rats (Rasmussen et al., 2009). Prazosin has likewise been shown to decrease relapse alcohol drinking in a pilot study of treatment-seeking alcohol-dependent men (Simpson et al., 2009). It has been established that prazosin penetrates the brain (Menkes et al., 1981; Rogawski and Aghajanian, 1982), and its effect on alcohol intake is hypothesized to be central (Rasmussen et al., 2009). Prazosin, however, has a half-life of 2-3 hours in humans (Jaillon, 1980), requiring dosing multiple times per day. Doxazosin, a structurally related selective α1 adrenoceptor antagonist, has a half-life of 9-22 hours in humans (Babamoto et al., 1992), allowing once daily dosing. Doxazosin has been thought to be predominantly peripherally acting, and to have limited brain penetration (Guo et al., 1991; Prys-Roberts and Farndon, 2002), but there are some indications that doxazosin may cross the blood-brain-barrier more than commonly believed. Not only is its reported dose-dependent side effect of somnolence suggestive of a central action, but it has been reported in a pilot study to effectively reduce PTSD symptoms (De Jong et al., 2010). Guo et al. (1991) examined doxazosin's ability to block the attenuating effect of cirazoline (a centrally active α1-adrenergic receptor agonist) on the hypnotic effect of dexmedetomidine (a centrally active α2 agonist). Cirazoline alone attenuated dexmedetomine's hypnotic effect (relative to the control group), but doxazosin+cirazoline did not, consistent with a central effect of doxazosin. Finally, it has recently been reported that treatment with a low, well tolerated dose of doxazosin (4 mg/day, PO) decreases positive subjective responses to cocaine in humans (Newton et al., 2012), further suggesting a central action. Together, these findings suggest that doxazosin, an α1-adrenergic antagonist with a significantly longer half-life than prazosin, may – like prazosin – decrease alcohol drinking, potentially providing an alternative, more convenient, treatment approach that could increase medication compliance. Since lack of medication compliance is an important reason why medications for alcoholism may show a modest effect (Weiss, 2004; Swift et al., 2011), this alternative treatment approach potentially could increase effectiveness of α1-adrenergic receptor antagonist pharmacotherapy for some alcohol use disorders.
Accordingly, in the current study we investigated the effects of doxazosin on voluntary alcohol consumption in P rats, utilizing methods similar to those we previously used to investigate the effects of prazosin (Rasmussen et al., 2009). The P rat line is a well characterized animal model of excessive voluntary alcohol drinking (Bell et al., 2006). We hypothesized that doxazosin administration would attenuate voluntary alcohol drinking in this model, without compromising locomotor function or capability to drink.
MATERIALS AND METHODS
Subjects
Forty-six alcohol-naïve male rats from generation 71 of selective breeding for alcohol preference were provided by the Alcohol Research Resource Center of the Indiana Alcohol Research Center. The alcohol-preferring (P) rats were 58 ± 1 (mean ± SEM) days of age and weighed 243 ± 7 g at the start of the study. The rats were individually housed in stainless steel hanging cages in an isolated vivarium with controlled temperature (21 ± 1°C) and a 12 hour light/dark cycle (lights off at 10 AM). All rats were acclimated to individual housing and the light/dark cycle for 10 days prior to the start of the study. Standard rodent chow (Laboratory Rodent Diet 5001, PMI Nutrition International, Brentwood, MO) was available ad libitum throughout the study. Water was available ad libitum throughout the study except during the first 4 days of the alcohol drinking induction phase when 10% (v/v) alcohol was the only source of fluid. All experiments were approved by the Veterans Administration Puget Sound Health Care System Institutional Animal Care and Use Committee and conducted in strict compliance with the NIH Guide for the Care and Use of Laboratory Animals.
Alcohol and Water Intake
Free choice between alcohol and water was introduced as described below. The alcohol solution was prepared by diluting 95% alcohol (ethanol) with deionized water to make a 10% or 15% (v/v) solution. Alcohol and water were presented in two 150 ml glass drinking tubes (BioServ, Frenchtown, NJ) – one containing alcohol and the other containing water – placed on the front of each cage with positions of the tubes alternated daily to control for potential side preferences. Fluid intakes were determined by weighing each drinking tube to the nearest 0.1 g. Alcohol and water tubes were also placed on two empty cages to determine fluid loss due to spillage and/or evaporation; the average losses in these two cages on each day were subtracted from all corresponding (i.e., alcohol or water) intake values for that day. Net alcohol intake was converted to g alcohol/kg body weight.
Alcohol Drinking Induction Phase
After the acclimation period, all rats were initially provided with 24-hour access to 10% (v/v) alcohol as the only source of fluid for 4 days, followed by 24-hour free-choice access to water vs 10% (v/v) alcohol for 11 days. Access to 10% alcohol was then reduced to daily 2-hour scheduled access (starting 1 hour after lights-off) 5 days/week (Monday to Friday) in which the drinking tubes were placed on the front of the cage at the start of the 2-hour access period and removed at the end of the 2-hour period under dim red illumination. Water was otherwise always available from a standard sipper tube water bottle that was removed during the 2-hour trials. After 2 weeks the alcohol concentration was increased to 15% (v/v) for 4 weeks prior to administration of doxazosin in the first experiment, and was maintained at 15% (v/v) for the remainder of the study. Body weights were measured weekly.
Administration of Drugs
Doxazosin mesylate (Sigma-Aldrich, St. Louis, MO) was dissolved in 45 mM lactate buffer (pH 5.2). Doxazosin in lactate buffer or lactate buffer vehicle alone were injected intraperitoneally (IP; 4 ml/kg body weight) under dim red illumination. Injections were staged (1 minute intervals between rats) so the time to subsequent placements and removals of test drinking tubes on each individual cage could be maintained relatively constant.
Assignment to Treatment Groups
In the week prior to onset of doxazosin administration, baseline alcohol intake was calculated for each rat and the rats were ranked in descending order in terms of average daily alcohol consumption. Rats were assigned to drug treatment groups in a manner that assured that the groups did not differ in baseline alcohol intake prior to doxazosin administration. Specifically, the top 4 alcohol drinkers were pseudo-randomly assigned to the vehicle or 1 of 3 doxazosin treatment groups, followed by the next 4 highest alcohol drinkers, etc.
Trial 1 (doxazosin treatment for 3 days - low dose range)
Average daily alcohol intake over 5 baseline days was determined and the rats were ranked and pseudo-randomly assigned to treatment groups following the procedure described above. Doxazosin (1.25, 2.5, or 5 mg/kg) or vehicle alone (i.e., 0 mg doxazosin/kg), was administered 30-40 minutes prior to the daily 2-hour alcohol access period on each of 3 consecutive days. Each treatment group (11-12 rats/group) received a single dose of doxazosin or vehicle on all 3 days, starting on a Tuesday
Trial 2 (doxazosin treatment for 5 days - higher dose range)
After completion of Trial 1, the rats continued to receive 2-hour daily scheduled access to alcohol (15%, v/v) for 5 days/week for 8 weeks prior to initiating Trial 2. Average daily alcohol intake over 5 baseline days was determined and the rats were again ranked and pseudo-randomly assigned to treatment groups following the procedure described above. Doxazosin (2.5, 5, or 10 mg/kg) or vehicle alone was administered 30-40 minutes prior to the daily 2-hour alcohol access period on each of 5 consecutive days. Each treatment group (11-12 rats/group) received a single dose of doxazosin or vehicle on all 5 days. These treatments were initiated on a Monday; since alcohol access was not provided on weekends, this trial followed immediately after the weekly 2-day alcohol deprivation.
On the final (fifth) day of treatments, initial tests of treatment effects on arousal and ingestive behavior were conducted. In the first test, each rat was observed (under dim red light illumination) at the time of placement of the alcohol and water tubes on the front of the cage at the start of the 2-hour alcohol vs water access period (i.e., 30-40 minutes after administration of doxazosin) to determine if the rat would investigate (sniff, lick) and/or drink from the tubes within 1 minute (i.e., that the rat was not sleeping or sedated at the start of the trial and was motivated and competent to investigate the tubes). The second test was conducted at completion of the 2-hour alcohol vs water access period (i.e., 150-160 minutes after administration of doxazosin) when the alcohol and water drinking tubes were removed from the front of the cage; a chow pellet (identical to the chow pellets always available in the food hopper already in the cage) was placed on the floor of the cage through the hole that had accommodated the alcohol tube and it was determined (by observation under dim red illumination) whether the rat would recover and chew on the pellet within 15 seconds (i.e., that the rat was not sleeping or sedated at the end of the trial and was motivated and competent to recover and to begin eating the pellet).
Trial 3 (second doxazosin treatment for 5 days - with tests of effects on ability to drink, locomotor activity and plasma alcohol concentration)
After completion of Trial 2, the rats continued to receive 2-hour daily scheduled access to alcohol (15%, v/v) 5 days/week for 5 weeks prior to initiating Trial 3. Average daily alcohol intake over 2 baseline days was determined and the rats were again ranked and pseudo-randomly assigned to treatment groups following the procedure described above. Doxazosin (2.5, 5, or 10 mg/kg) or vehicle alone was administered 30-40 minutes prior to the daily 2-hour alcohol access period on each of 5 consecutive days. Each treatment group (11-12 rats/group) received a single dose of doxazosin or vehicle on all 5 days. These treatments were initiated on a Monday; since alcohol access was not provided on weekends, this trial followed immediately after the weekly 2-day alcohol deprivation.
On Day 3, a test of treatment effect on ability to drink was conducted at the midpoint of the 2-hour alcohol access period by presenting 1 ml of sweet solution (2% sucrose + 0.2% saccharin) in a small cup (the 0.5 inch conical base of a polypropylene 50 ml centrifuge tube; Fisher Scientific; Pittsburgh, PA) under dim red illumination. The rats had previously each received 1 ml of this sweet solution on 3 occasions during the 5 week interval between Trials 2 and 3, to discover its palatability and minimize neophobia. It was determined whether each rat had consumed the entire 1 ml of sweet solution within 1 minute.
On Day 4, the rats received doxazosin or vehicle treatment consistent with the other trial days, but did not receive access to alcohol. In a test of response to doxazosin treatment independent of concurrent pharmacologic effects of alcohol, 1 ml of the sweet solution was presented 40 minutes after the doxazosin vs vehicle injections (i.e., at the start of the 2-hour period when effects on alcohol vs water drinking were usually tested, but with no alcohol access during this trial) and again at 100 minutes after the injections (i.e., at the midpoint of the usual 2-hour access period, but with no alcohol). As on Day 3, it was determined if each rat had consumed the entire 1 ml of sweet solution within 1 minute after each presentation. Between these two presentations of sweet solution, each rat was transferred to a clean plastic shoebox cage (in the same room, under dim red illumination) for 5 minutes and locomotor activity was quantified as number of infra-red beam breaks with an Opto-Varimex Mini animal activity monitoring system (Columbus Instruments, Columbus, OH). These tests of locomotor activity were conducted in the same order as the preceding pseudo-randomized doxazosin or treatment administrations. The testing was conducted in sets of 4, with each rat in a separate visually-isolated activity monitoring system.
On Day 5, the alcohol access session was only 1 hour, with tail blood collection at the end of this hour for determination of plasma alcohol concentration with a NAD-ADH enzymatic Ethanol Assay (Genzyme Diagnostics; Charlottetown, PE, Canada). Tail blood was collected after only 1 hour because it was noticed during the previous trials that most or all alcohol intake usually occurred within the first 15-30 minutes of the alcohol access sessions.
Data Analyses
Alcohol and water intakes during doxazosin treatment trials were analyzed by two-way analysis of variance (ANOVA) with repeated measures (dose × day with repeated measures on day) followed – when justified by determination of significant differences or interactions in the ANOVA – by pairwise multiple comparisons using Student Newman-Keuls tests unless otherwise noted. Pre-treatment baseline and post-treatment alcohol and water intakes, locomotor activity and plasma alcohol levels were each individually analyzed by one-way ANOVA followed – when justified by a significant difference in the ANOVA – by pairwise multiple comparisons using Student Newman-Keuls test. Associations between plasma alcohol levels and preceding alcohol intake were evaluated by Pearson Product Moment Correlation analyses. All analyses were conducted using Sigmaplot Version 11 software (Systat Software, Inc., Chicago,IL) with significance accepted at p < .05. Data are presented as mean ± SEM.
Of the total 828 alcohol consumption determinations and 828 water consumption determinations attempted in trials 1-3, a few (16/828 alcohol; 15/828 water) were unsuccessful due to technical problems (e.g., spills or breakage of glass drinking tubes during removal from the cage and weighing; data entry error; injection error; unexplained acute illness/lethargy of one vehicle control-treated rat requiring euthanasia on the last day of trial 3). A few additional determinations (11/828 alcohol; 7/828 water) were corrupted due to major leakage from the drinking bottles producing puddles of either water or alcohol below the hanging cage. This occurred when the seal for a drinking bottle cap was cracked or when the cap was not screwed on tightly enough. More commonly, it was noted that a rat would repeatedly disturb a drinking bottle during the trial, banging it from side to side and splashing out fluid, or lean into the spout, providing a “wick” for the fluid to flow out freely. Finally, some aberrant values (45/828 alcohol; 47/828 water) exceeding 2 standard deviations from the mean of the remaining values in each [treatment × day] group were designated to be outliers. These aberrant values consistently exceeding the other values in the group were presumably also due to undocumented leakage or spillage. No more than one determination of the 11-12 determinations in any [treatment × day] group was identified as an outlier and removed. Any determinations that were missing or removed were handled as missing values in the statistical analyses.
RESULTS
Trial 1: 3-Day Treatment, Doxazosin (1.25 – 5 mg/kg) Effects on Alcohol and Water Intake
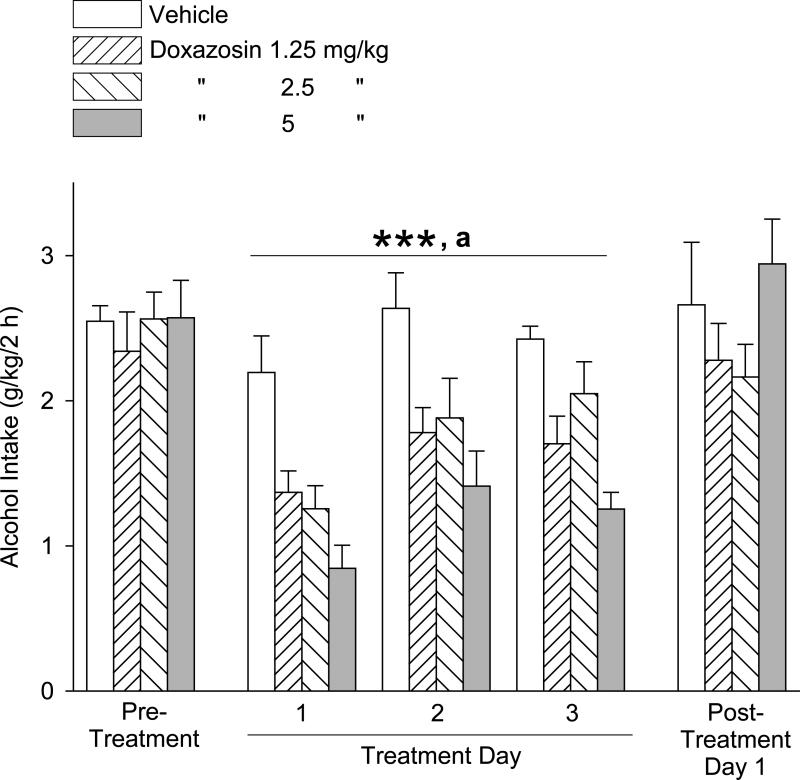
The effects of doxazosin treatment on alcohol intake during daily 2-hour alcohol access periods on 3 consecutive days are presented in Fig. 1. One-way ANOVAs revealed no significant differences in alcohol intake between the treatment groups (dose) in either the pretreatment period [F(3,40) = 0.26, p = .86] or the post-treatment days [F(3,39) = 1.47, p = .24]. Two-way repeated measures ANOVA (dose × day, with repeated measures on day) over the 3 drug treatment days revealed significant main effects of dose [F(3,106) = 21.53, p < .001] and day [F(2,106) = 9.66, p < .001], but no dose × day interaction [F(6,106) = 1.145, p = .34]. Student-Newman-Keuls post-hoc pairwise comparisons revealed that alcohol intake (independent of day) was suppressed by all 3 doses of doxazosin (1.25, 2.5, and 5 mg/kg) relative to alcohol intake in vehicle-treated (0 mg doxazosin) control rats (p < .001 for each). The 5 mg/kg doxazosin dose resulted in significantly less alcohol intake relative to the 2.5 and 1.25 mg/kg doses (p < .01 for each). Pairwise comparisons of day revealed that alcohol intake (independent of dose) was less on Day 1 relative to Days 2 and 3 (p ≤ .001 for each).
Fig. 1.
Trial 1, 3-day treatment: Effects of doxazosin (1.25 – 5 mg/kg, IP) on alcohol intake (mean ±S.E.M). Doxazosin dose-dependently decreased alcohol intake, independent of day. Each bar represents data from 11-12 rats. ***p < .001 versus vehicle control treatment for 1.25, 2.5 and 5 mg/kg; a p < .01, 5 mg/kg versus 1.25 and 2.5 mg/kg. For significant differences between days, see text. Pre-treatment values reflect the averages from 5 daily sessions.
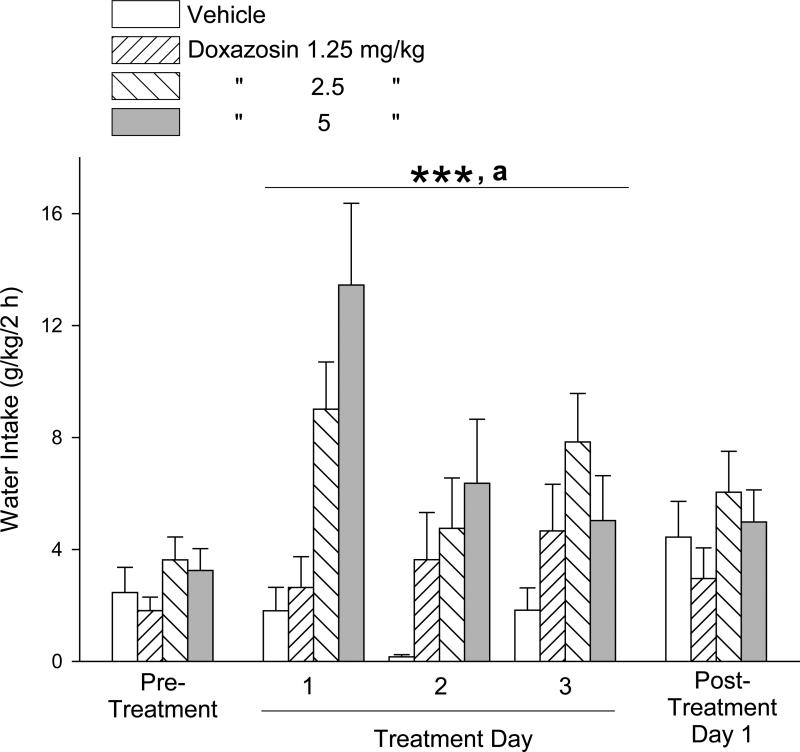
The effects of doxazosin treatment on water intake during these daily 2-hour periods are presented in Fig. 2. Due to the lack of homogeneity of variance of the water intake measures on the pre-treatment day, a Kruskal-Wallis one-way ANOVA on ranks was calculated, revealing no significant difference in water intake between the treatment groups (H = 2.66, 3 df, p = .45). One-way ANOVA revealed no significant difference in water intake between treatment groups on the post-treatment day [F(3,40) = 1.00, p = .40]. Two-way repeated measures ANOVA over the 3 drug treatment days, as above, revealed a significant main effect of dose [F(3,108) = 9.52, p < .001], but not of day [F(2,108) = 2.39, p = .10] and no dose × day interaction [F(6,108) = 1.50, p = .19]. Student-Newman-Keuls post-hoc pairwise comparisons revealed that the 2.5 and 5 mg/kg doses of doxazosin resulted in greater water intake (independent of day) relative to vehicle administration (p < .001 for each) and relative to 1.25 mg/kg doxazosin (p < .05 for each).
Fig. 2.
Trial 1, 3-day treatment: Effects of doxazosin (1.25 – 5 mg/kg, IP) on water intake (mean ±S.E.M). Doxazosin dose-dependently increased water intake, independent of day. Each bar represents data from 11-12 rats. ***p < .001, 2.5 and 5 mg/kg versus vehicle control treatment; a p < .05, 2.5 and 5 mg/kg versus 1.25 mg/kg. Pre-treatment values reflect the averages from 5 daily sessions.
Trial 2: 5-Day Treatment, Doxazosin (2.5 – 10 mg/kg) Effects on Alcohol and Water Intake, and Motor Function
The effects of doxazosin treatment on alcohol intake in a second trial with daily 2-hour alcohol access periods on 5 consecutive days are presented in Fig. 3. One-way ANOVA revealed no significant difference in alcohol intake between the treatment groups in the pre-treatment period [F(3,42) = 0.06, p = .98]. Two-way repeated measures ANOVA (dose × day, with repeated measures on day) over the 5 drug treatment days revealed significant main effects of dose [F(3,151) = 19.8, p < .001], day [F(4,151) = 24.68, p < .001], and dose × day interaction [F(12,151) = 3.26, p < .001]. Student-Newman-Keuls post-hoc pairwise comparisons revealed that on Day 1 all 3 doses of doxazosin (2.5, 5, and 10 mg/kg) significantly decreased alcohol intake relative to vehicle-treated control rats, by 37% (p < .05), 82% (p < .001) and 96% (p < .001), respectively. Dose dependence was also evident, as alcohol intake in response to all 3 doxazosin doses differed significantly from each other (5 and 10 mg/kg relative to 2.5 mg/kg, p ≤ .001 for each; 5 mg/kg relative to 10 mg/kg, p < .05). On Days 2-5, the response to the lowest doxazosin dose, 2.5 mg/kg, was not significantly different from the response to vehicle administration. The response to 5 mg/kg doxazosin relative to the response to vehicle was diminished (p < .001, Day 2 versus Day 1) but still significant on Day 2 (48%, p < .05), then was no longer significant on Days 3-5. The alcohol intake on Days 3, 4, and 5, however, did not significantly differ from that on Day 2 for the 5 mg/kg doxazosin group. The 10 mg/kg doxazosin dose maintained significant suppression of alcohol intake on all 5 treatment days relative to vehicle administration (by 96%, 76%, 75%, 55%, and 57%, respectively, p < .001 for each), though this suppression was attenuated with repeated administrations during the 5 day trial – i.e., Days 4 and 5 produced significantly less suppression than Day 3 (p < .01 for each), Day 2 (p < .05 for each), and Day 1 (p < 0.001 for each). One-way ANOVA revealed a significant difference between the treatment groups on the post-treatment day [F(3,35) = 5.97, p < .01], and Student-Newman-Keuls post-hoc pairwise comparisons revealed that the alcohol intake of the 10 mg/kg doxazosin group was greater than the vehicle-treated (p < .01) and 2.5 mg/kg doxazosin (p < .01) groups.
Fig. 3.
Trial 2, 5-day treatment: Effects of doxazosin (2.5 – 10 mg/kg, IP) on alcohol intake (mean ±S.E.M). Each bar represents data from 11 - 12 rats. “A” and “B” indicate behavioral tests discussed in the Results section. *p < .05, **p < .001, ***p < .001 versus vehicle control treatment within individual days. For significant differences between doxazosin doses and between days, see text. Pre-treatment values reflect the averages from 5 daily sessions.
The effects of doxazosin treatment on water intake during these daily 2-hour periods are presented in Fig. 4. One-way ANOVAs revealed no significant difference in water intake between the treatment groups in either the pre-treatment period [F(3,42) = 0.20, p = .90] or post-treatment day [F(3,38) = 0.20, p = .90]. Two-way repeated measures ANOVA over the 5 drug treatment days, as above, revealed significant main effects of dose [F(3,151) = 2.78, p = .05], day [F(4,151) = 16.06, p < .001], and dose × day interaction [F(12,151) = 2.24, p < .05]. Student-Newman-Keuls post-hoc pairwise comparisons revealed that water intake was significantly increased by the 5 mg/kg doxazosin dose relative to the response to vehicle (by 253%, p < .05), and to the 10 mg/kg doxazosin group (p < .01) on Day 1. Water intake was increased by the 2.5 mg/kg doxazosin dose relative to the response to vehicle (by 203%, p < .05) and to the 10 mg/kg doxazosin group (p < .01) on Day 1. The 10 mg/kg doxazosin group did not differ significantly from the vehicle-treated group on any of the treatment days. The 2.5 mg/kg doxazosin dose resulted in greater water intake on Day 1 relative to Day 2 (p < .05) and Day 4 (p < .05). The 5 mg/kg doxazosin dose resulted in significantly greater water intake on all days relative to Day 4 (Day 1, 3, and 5, p < .001 for each; Day 2, p < .01). This doxazosin dose also resulted in greater water intake on Day 5 relative to Day 2 (p < .05). The 10 mg/kg doxazosin dose resulted in significantly greater water intake on Days 3 (p < .01 for each) and 5 (p < .001 for each) relative to Days 1, 2, and 4. For a simpler analysis of overall doxazosin effects on water intake, we also calculated the mean water intake of individual rats across all 5 days of the trial. One-way ANOVA of these 5-day average data revealed a significant difference [F(3,42) = 3.64, p < .05] among treatment groups (vehicle: 2.6 ± 0.6 g/kg; 2.5 mg/kg: 5.3 ± 1.6 g/kg; 5 mg/kg: 7.0 ± 1.0 g/kg; 10 mg/kg: 3.2 ± 1.0 g/kg). Student-Newman-Keuls post-hoc pairwise comparisons revealed that the water intake of the 5 mg/kg group was greater than the vehicle-treated and 10 mg/kg groups (p < .05 for each)
Fig. 4.
Trial 2, 5-day treatment: Effects of doxazosin (2.5 – 10 mg/kg, IP) on water intake (mean ±S.E.M). Each bar represents data from 11 - 12 rats. *p < .05 versus vehicle control treatment, a p < .01 versus 10 mg/kg, within individual days. For significant differences between days, see text. Pre-treatment values reflect the averages from 5 daily sessions.
All rats, with the exception of one that had received a dose of 5 mg/kg doxazosin, investigated the alcohol and water tubes within 1 minute of placement on the cage at the start of the session on Day 5. The one rat that did not initially investigate the tubes was sleeping at the time of their placement; when he awoke, he ate and then returned to the back of the cage, ignoring the bottles. Immediately following the 2-hour alcohol access period, all rats recovered and chewed on a novel chow pellet within 15 seconds after its introduction into the cage.
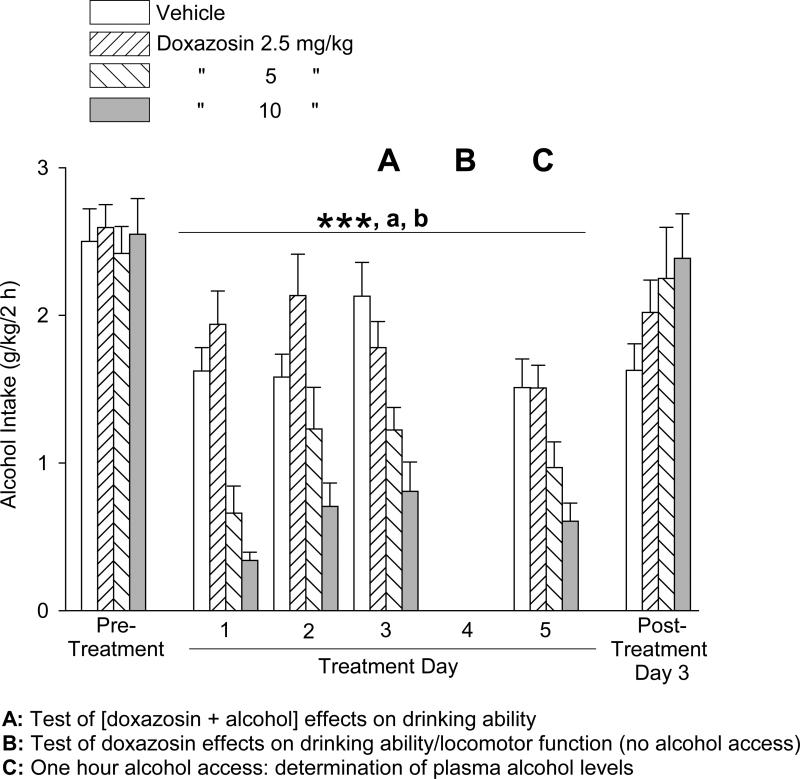
Trial 3: 5-Day Treatment, Doxazosin (2.5 – 10 mg/kg) Effects on Alcohol and Water Intake, Drinking Ability, Motor Function, and Plasma Alcohol Levels
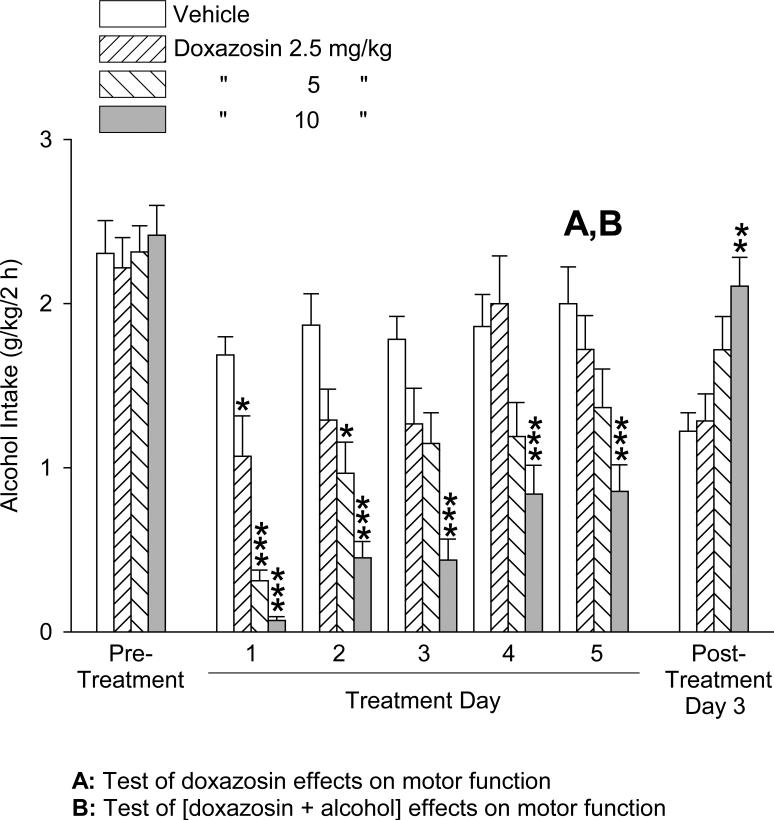
In the third trial, doxazosin versus vehicle was again administered over a period of 5 consecutive days prior to the daily 2-hour alcohol access sessions, except on Day 4 no alcohol was provided. On Day 4 behavioral tests were conducted when the alcohol access session would otherwise have occurred. On Day 5, the alcohol access session was limited to 1 hour, with tail blood sampling at the end of this hour. Although the alcohol and water consumption trial was limited to 1 hour on day 5, the alcohol and water intakes by vehicle-treated rats on this day were not significantly different from intakes on the other treatment days, consistent with the observation that most or all drinking occurred within the first 15-30 minutes of the trials. Consequently, the repeated measures analyses of alcohol and water intake extended across all treatment days. The effects of doxazosin treatment on alcohol intake are presented in Fig. 5. One-way ANOVAs revealed no significant differences in alcohol intake between the treatment groups in either the pre-treatment period [F(3,41) = 0.13, p = .94] or the post-treatment day [F(3,36) = 1.41, p = .26]. Two-way repeated measures ANOVA (dose × day, with repeated measures on day) over the 4 treatment days with alcohol intake revealed a significant main effect of dose [F(3,111) = 18.50, p < .001] and day [F(3,111) = 7.66, p < .001], but no significant dose × day interaction [F(9,111) = 1.73, p = .09]. Student-Newman-Keuls post-hoc pairwise comparisons revealed that alcohol intake was dose-dependently suppressed (independent of day) by administration of doxazosin: both 5 and 10 mg/kg doses resulted in significantly less alcohol intake than the vehicle administration (p ≤ .001 for each) and the 2.5 mg/kg dose (p ≤ .001 for each), and the 10 mg/kg dose of doxazosin resulted in significantly less alcohol intake than the 5 mg/kg dose (p < .05). There was significantly less alcohol intake (independent of dose) on Day 1 and Day 5 than Day 2 (p < .05 and p < .01, respectively) and Day 3 (p < .001 for each).
Fig. 5.
Trial 3, 5-day treatment: Effects of doxazosin (2.5 – 10 mg/kg, IP) on alcohol intake (mean ±S.E.M). On Treatment Day 4 no alcohol was provided. On Treatment Day 5, the alcohol session was limited to 1 hour. Doxazosin dose-dependently decreased alcohol intake, independent of day. Each bar represents data from 11-12 rats. “A”: Time of test of doxazosin + alcohol effects on drinking ability (see discussion in Results section); “B”: Time of tests of doxazosin effects on drinking ability (see discussion in Results section) and locomotor function (see Fig. 7); “C”: Time of determination of plasma alcohol levels (see Fig. 8). ***p ≤ .001, 5 and 10 mg/kg versus vehicle control treatment; a p ≤ .001, 5 and 10 mg/kg versus 2.5 mg/kg; b p < .05, 10 mg/kg versus 5 mg/kg. For significant differences between days, see text. Pre-treatment values reflect the averages from 2 daily sessions.
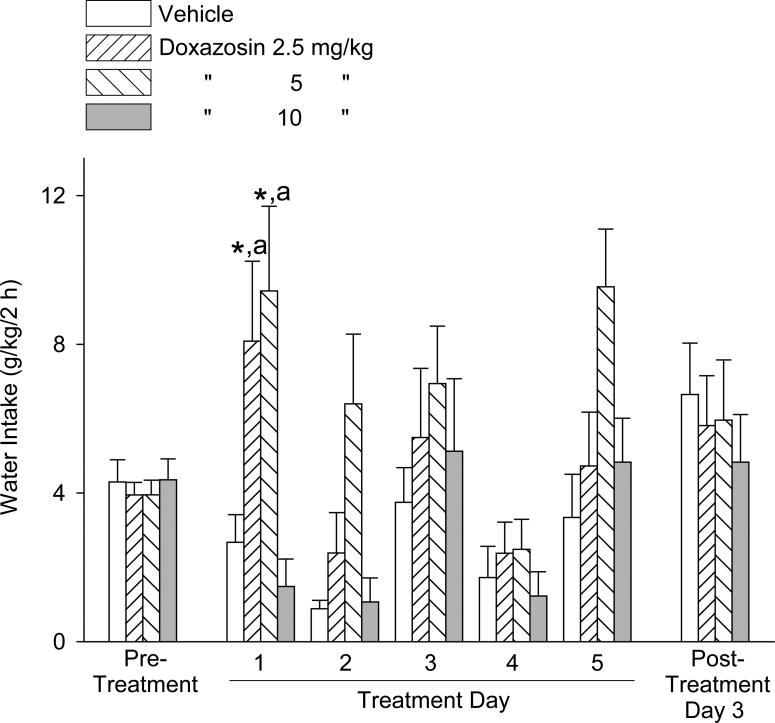
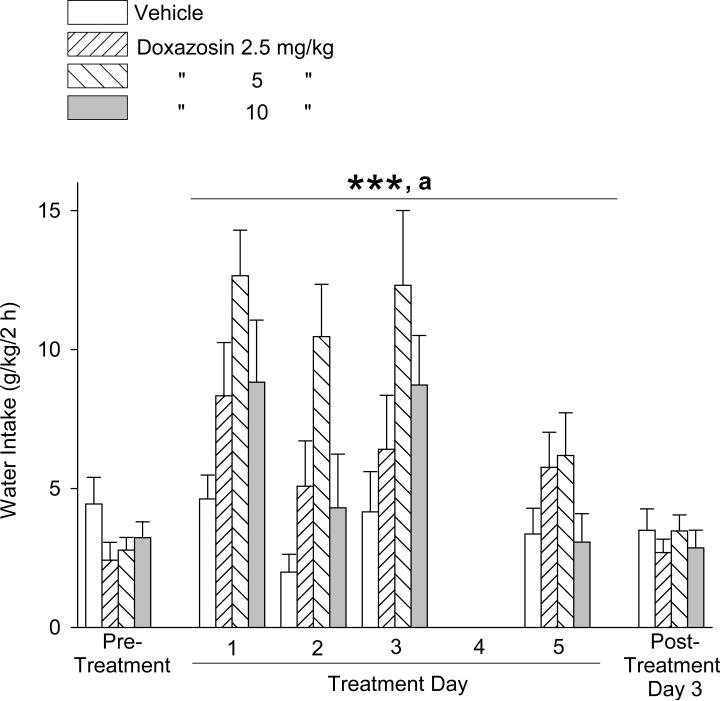
The effects of doxazosin treatment on water intake during the alcohol access sessions are presented in Fig. 6. One-way ANOVAs revealed no significant difference in water intake between the treatment groups in either the pre-treatment period [F(3,41) = 1.21, p = .32] or post-treatment day [F(3,40) = 0.40, p = .75]. Two-way repeated measures ANOVA calculations, as above, revealed a significant main effect for dose [F(3,111) = 6.25, p ≤ .001] and day [F(3,111) = 9.06, p < .001], but no significant dose × day interaction [F(9,111) = 1.27, p = .26]. Student-Newman-Keuls post-hoc pairwise comparisons revealed that water intake (independent of day) was increased by the 5 mg/kg doxazosin dose relative to the response to vehicle administration (p < .001), 2.5 mg/kg doxazosin (p < .05), and 10 mg/kg doxazosin (p < .05). Water intake was greater (independent of dose) on Day 1 and Day 3 relative to Day 2 (p < .001 and p < .01, respectively) and Day 5 (p < .001 and p < .01, respectively).
Fig. 6.
Trial 3, 5-day treatment: Effects of doxazosin (2.5 – 10 mg/kg, IP) on water intake (mean ±S.E.M). On Treatment Day 4 no alcohol was provided. On Treatment Day 5, the alcohol access session was limited to 1 hour. Doxazosin dose-dependently increased water intake, independent of day. Each bar represents data from 11 – 12 rats. ***p < .001, 5 mg/kg versus vehicle control treatment; a p < .05, 5 mg/kg versus both 2.5 and 10 mg/kg. For significant differences between days, see text. Pre-treatment values reflect the averages from 2 daily sessions.
On Day 3, one hour into the 2-hour alcohol access session, all rats consumed 1 ml of a sucrose-saccharin solution in less than 1 minute. On Day 4, in the absence of any alcohol availability, all rats consumed the sucrose-saccharin solution in less than 1 minute after presentation at both 40 and 100 minutes after doxazosin administration.
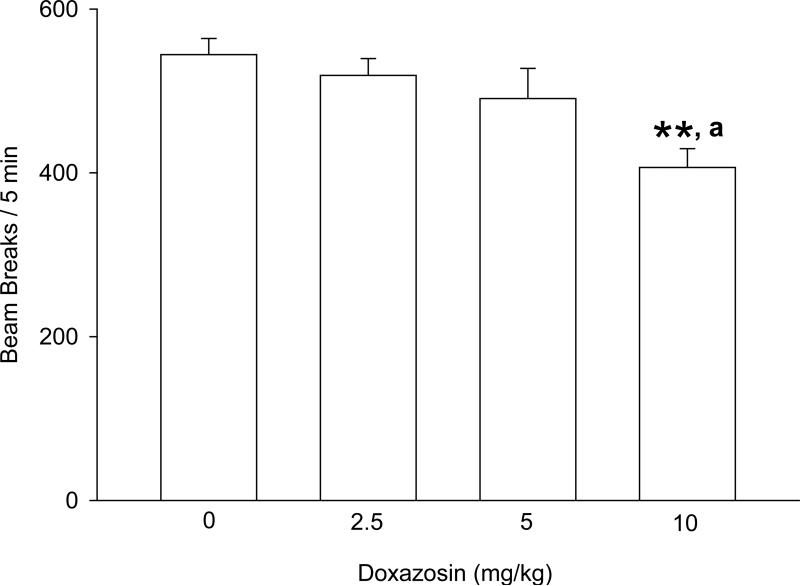
The effect of doxazosin on motor function, as measured by locomotor activity in a novel environment, is presented in Fig. 7. One-way ANOVA revealed a significant difference between treatment groups [F(3,40) = 5.33, p < .01]. Student-Newman-Keuls post-hoc pairwise comparisons revealed that the 10 mg/kg dose of doxazosin resulted in 25% fewer beam breaks relative to vehicle administration (p < .01). The 10 mg/kg doxazosin dose also resulted in significantly fewer beam breaks than the 2.5 and 5 mg/kg doxazosin doses (p < .05 for each).
Fig. 7.
Effects of doxazosin on locomotor activity in a novel environment (“B” in Fig. 5). **p < .01 versus vehicle control treatment; a p < .05 versus 2.5 and 5 mg/kg.
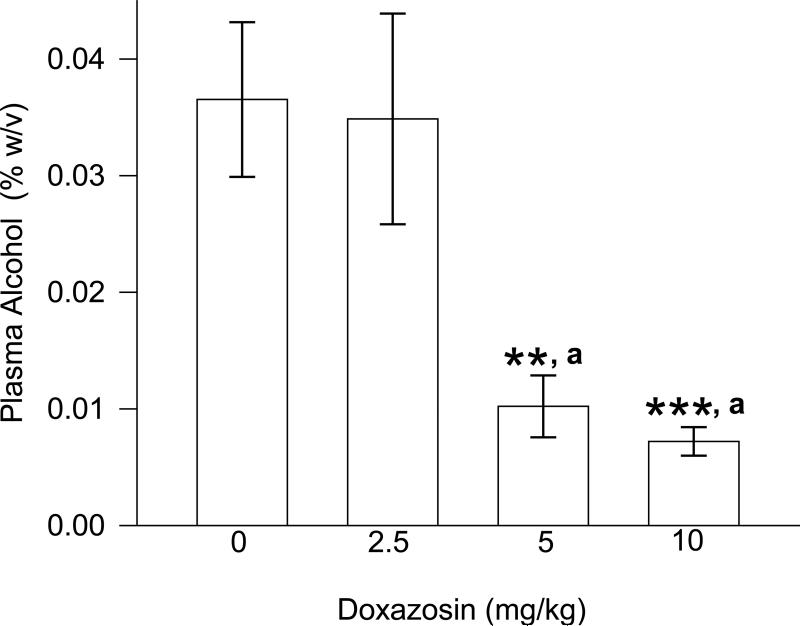
The effect of doxazosin on plasma alcohol concentration after 1-hour of alcohol access on Day 5 is presented in Fig. 8. One-way ANOVA revealed a significant difference between treatment groups [F(3,41) = 8.63, p < .001]. Student-Newman-Keuls post-hoc pairwise comparisons revealed that the 5 and 10 mg/kg doses of doxazosin resulted in significantly lower alcohol concentrations relative to vehicle administration (p < 0.01 and p < .001, respectively) and 2.5 mg/kg doxazosin (p ≤ .01 for each). Pearson Product Moment Correlation analyses revealed that plasma alcohol concentrations were positively correlated with preceding 1 hour alcohol intake across all treatment groups [r = .67; F(1,40) = 32.86, p < .001], and within the vehicle-treated group [r = .629; F(1,9] = 5.88, p < .05] and the 5 mg/kg doxazosin group [r = .78; F(1,8) = 12.10, p < .01]. There was a modest trend for plasma alcohol concentrations to likewise be positively correlated with 1 hour alcohol intake within the 2.5 mg/kg doxazosin group [r = .53; F(1,8) = 3.12, p = .11]. Plasma alcohol levels were not significantly correlated with 1 hour alcohol intake within the 10 mg/kg doxazosin group [r = – .17; F(1,9) = .28, p = .61].
Fig. 8.
Effects of doxazosin on plasma alcohol concentration (C in Fig. 5). ***p < .001 versus vehicle control treatment, **p < .01 versus vehicle control treatment, a p ≤ .01 versus 2.5 mg/kg.
DISCUSSION
The selective α1-adrenergic receptor antagonist, doxazosin, dose-dependently decreased voluntary alcohol drinking by male alcohol-preferring (P) rats. Three trials were performed in order to establish intra-study replication of our findings, to investigate a wide dose range, and to evaluate potential confounds that could contribute to decreased alcohol intake (i.e. drinking ability and motor function). Across the trials, doxazosin was effective in reducing alcohol drinking, consistent with our previous findings that prazosin decreases voluntary alcohol drinking in P rats (Rasmussen et al., 2009) and also decreases alcohol dependence-induced increases in operant responding for alcohol by Wistar rats during acute withdrawal (Walker et al., 2008). The results are also consistent with the pilot demonstration by Simpson et al. (2009) that prazosin decreases alcohol drinking in treatment-seeking alcohol-dependent men.
Water intake was variable, but was not suppressed by doxazosin and was significantly increased by a mid-range dose (5 mg/kg) that suppressed alcohol intake. Although the doxazosin-induced increase in water intake may be a compensatory response to the decreased intake of ethanol in water, it is also plausible that it reflects hypotension-induced thirst (Evered, 1990), or that it is related to the reported inhibitory effect of norepinephrine on water intake in water-deprived rats (Grossman, 1962), which is central α1 adrenoceptor mediated (Oryan et al., 2003). The doxazosin-induced increase in water intake is consistent with our previous demonstration that prazosin administration in doses that decreased alcohol intake also increased water intake in 2-hour trials with male P rats (Rasmussen et al., 2009), and confirms that the suppression of alcohol intake by doxazosin is not due to motor impairment or an inhibition of general ingestive behavior. However, the fact that the 10 mg/kg dose of doxazosin did not increase water intake suggests that an excessive dose of doxazosin may exert some non-specific motor inhibitory effects (which may also have confounded the 10 mg/kg doxazosin-induced suppression of alcohol intake).
The 5 mg/kg dose that consistently decreased alcohol intake and increased water intake is higher than the similarly effective prazosin doses in our prior studies (ranging to 2 mg/kg) (Rasmussen et al., 2009). This may reflect in part the lower potency of doxazosin relative to prazosin (Cubeddu, 1988; Hamilton et al.,1985) and/or its potentially poorer brain penetration. Another possibility is that the timing of our evaluations may not have corresponded to the time of peak efficacy of the drug. We selected to begin the alcohol access sessions 30-40 minutes after the IP injection based in part on the efficacy we had previously shown 15 minutes after an IP administration of prazosin, together with the slower metabolic clearance of doxazosin relative to prazosin. In clinical studies, however, it has been shown that the peak hypotensive effect of doxazosin is distinctly out of phase with its peak serum levels, occurring 4-6 h later; it has been suggested that this delay may be due to the time it takes for doxazosin to distribute to the site of the α1 receptor (the ‘effect compartment’) (Vincent et al., 1983; Meredith et al., 1985). Our results do not address when the peak alcohol intake suppressing effect of doxazosin occurs in rats, but it is plausible that lower doses would have been more effective at a later time point, particularly if the relevant site of action is central.
It should be noted that there appeared to be diminishing efficacy of doxazosin over the days of the second trial. This is in contrast to the pattern that was observed with prazosin in our prior study utilizing P rats (Rasmussen et al., 2009); in that study, the lower doses of prazosin became increasingly effective with repeated administration, while the highest doses lost efficacy. The reason for this discrepancy, and the mechanism behind the observed changes in efficacy of both prazosin and doxazosin, is not clear. Further studies would be required to characterize these response patterns and the contributing mechanisms. While our third trial was intended to provide some intra-study replication (as it was also conducted over 5 days), it does not allow for a direct comparison with the second trial (in which the above pattern was observed) due to the lack of any alcohol access on Day 4 and the abbreviated alcohol access session on Day 5.
There was marked suppression of alcohol intake by the highest (10 mg/kg) doxazosin dose on Day 1 of the second trial. This apparent first-day effect potentially could be related to a side effect of doxazosin. When used clinically, dose-dependent postural hypotension can occur with the first administration, although this occurs less with doxazosin than with prazosin (Khoury and Kaplan, 1991). Accordingly, clinical treatment with doxazosin, as with prazosin, is typically initiated with low doses followed by gradual up-titration to avoid this first-dose effect.
An apparent increase in alcohol intake was observed in the 10 mg/kg doxazosin group after termination of treatment in the second trial. This could be suggestive of a potential rebound effect, although it was not demonstrated in the other trials of the current study, nor was it observed with prazosin in our previous study (Rasmussen et al., 2009). It should be noted that this increased intake, relative to the response to vehicle, was observed on a Monday, 3 days after the termination of treatment. Further studies would be required to characterize the pattern of intake in the days immediately following the termination of treatment.
We evaluated motor function and drinking ability in the second and third trials. Our subjective impression was that the rats’ observed overall activity during the 2-hour intake trials was not affected by doxazosin administration. This was supported by responses in the second trial, in which the rats, regardless of treatment group, readily investigated the alcohol and water tubes within 1 minute of insertion into the cage. Also, all of the rats promptly (in less than 15 seconds) recovered and chewed upon a novel chow pellet inserted into the cage at the end of the alcohol access session. In the third trial, we more specifically examined drinking ability. All rats rapidly (in less than 1 minute) consumed 1 ml of a palatable sucrose-saccharin solution at time points following doxazosin administration that corresponded to the start and midpoint of the 2-hour period normally associated with alcohol access, but with no alcohol available. During the 1-hour period corresponding to the first half of the usual 2-hour alcohol access session, locomotor activity was modestly but significantly decreased by the highest dose of doxazosin – a dose that also decreased water intake (relative to intake in response to the 5 mg/kg doxazosin dose) in the alcohol access trials, as discussed above. Nonetheless, even the group of rats that received the highest (10 mg/kg) dose of doxazosin were still active and did not appear lethargic. It should be noted that this locomotor test occurred when the rats were accustomed to having an alcohol access session and had been exposed to at least one cue (i.e., the IP injection) that the session would be forthcoming. Consequently, although the highest doxazosin dose appeared to produce some limited suppression of locomotor activity, a plausible alternate explanation could be that the other groups were exhibiting greater anticipatory anxiety and/or alcohol seeking leading to greater locomotor activity.
Plasma alcohol was measured 1 hour after initiation of the alcohol access session on Day 5 of the third trial. Alcohol levels in the rats receiving 5 and 10 mg/kg doses of doxazosin were significantly lower than those receiving 2.5 mg/kg and vehicle administrations, consistent with the decreased alcohol intake in rats receiving 5 and 10 mg/kg doxazosin. This clearly suggests that the decrease in alcohol intake in response to doxazosin was not mediated by a decrease in alcohol clearance (i.e., if doxazosin had decreased alcohol clearance, the lower alcohol intake in the doxazosin treated rats would not have resulted in correspondingly lower blood alcohol levels). When analyzed within individual treatment groups, plasma alcohol concentrations were positively correlated with alcohol intake in the vehicle treated group and in the 5 mg/kg prazosin group, with a modest trend for positive correlation in the 2.5 mg/kg group. In contrast, the consistently low and commonly undetectable plasma alcohol levels within the 10 mg/kg doxazosin group were not correlated with alcohol intake, probably because the alcohol intake commonly did not exceed alcohol metabolism and clearance. It should be noted that the plasma alcohol levels exhibited 1 hour after the start of alcohol access by even the vehicle treated rats would not be expected to be associated with intoxication, even if all of the consumption had occurred within the first few minutes of access (as was observed in some cases).
The results of the present study suggest that doxazosin could be an effective pharmacotherapeutic intervention in some cases of alcohol dependence. The clinical efficacy of the closely-related α1-adrenergic antagonist prazosin was previously demonstrated in a pilot study on treatment seeking alcohol-dependent men, in which prazosin was administered three times daily, and subjects received text page reminders to take the medication (Simpson et al., 2009). However, compliance with a three times daily administration schedule would likely be more challenging outside of a structured study. Doxazosin has a considerably longer half-life than prazosin (9-22 hours versus 2-3 hours in humans) (Babamoto et al., 1992; Jaillon, 1980), allowing once daily dosing, and is reported to be generally well tolerated in both its immediate-release and extended-release formulations (Kirby et al., 2001). As such, it represents a potentially clinically advantageous alternative to prazosin. However, considering the results of the current study, it is possible that higher doses (relative to prazosin) would be required to achieve efficacy in suppressing alcohol drinking. This potential need for higher clinical doses could limit its utility due to potentially increased peripheral effects, such as hypotension. However, it should be noted that the dose of doxazosin that appeared to be effective in a pilot study addressing treatment of PTSD was 8 mg/day (De Jong et al., 2010) and the dose recently reported to effectively decrease positive subjective effects of cocaine in humans was 4 mg/day (Newton et al., 2012), both of which are less than the total daily dose (16 mg) of prazosin shown to be effective in decreasing relapse alcohol drinking by alcohol-dependent men without significant adverse side effects (Simpson et al., 2009) and also less than the dose of doxazosin approved by the FDA (16 mg/day). Steady-state studies in subjects given doxazosin doses of 2-16 mg once daily show linear kinetics and dose proportionality (Elliot et al., 1987).
In summary, the α1 receptor antagonist doxazosin was found to decrease voluntary alcohol consumption by male alcohol-preferring (P) rats. These results further support a role for the noradrenergic system in alcohol drinking in P rats, and suggest that doxazosin could potentially be an effective once-daily pharmacotherapeutic agent for the treatment of some alcohol use disorders.
Acknowledgments
SUPPORT
This material is based upon work supported in part by resources from the VA Puget Sound Health Care System, Seattle, Washington and by NIH Grant P20 AA017839. P rats were provided by the Indiana Alcohol Research Resource Center, supported by NIH Grant R24 AA015512.
REFERENCES
- Abercrombie E, Keller R. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- Amit Z, Brown ZW, Levitan DE, Ogren SO. Noradrenergic mediation of the positive reinforcing properties of ethanol: I. Suppression of ethanol consumption in laboratory rats following dopamine-beta-hydroxylase inhibition. Arch Int Pharmacodyn Ther. 1977;230:65–75. [PubMed] [Google Scholar]
- Arencibia-Albite F, Paladini C, Williams JT, Jiménez-Rivera CA. Noradrenergic modulation of the hyperpolarization-activated cation current (Ih) in dopamine neurons of the ventral tegmental area. Neuroscience. 2007;149:303–314. doi: 10.1016/j.neuroscience.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babamoto KS, Hirokawa WT. Doxazosin: a new alpha 1-adrenergic antagonist. Clin Pharm. 1992;11:415–427. [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcohol Clin Exp Res. 1999;23:1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Brown ZW, Amit Z, Levitan DE, Ogren SO, Sutherland EA. Noradrenergic mediation of the positive reinforcing properties of ethanol: II. Extinction of ethanol-drinking behavior in laboratory rats by inhibition of dopamine-beta-hydroxylase. Implications for treatment procedures in human alcoholics. Arch Int Pharmacodyn Ther. 1977;230:76–82. [PubMed] [Google Scholar]
- Butler RW, Braff DL, Rausch JL, Jenkins MA, Sprock J, Geyer MA. Physiological evidence of exaggerated startle response in a subgroup of Vietnam veterans with combat-related PTSD. Am J Psychiatry. 1990;147:1308–1312. doi: 10.1176/ajp.147.10.1308. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak D. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Froehlich JC. Acoustic startle reactivity during acute alcohol withdrawal in rats that differ in genetic predisposition toward alcohol drinking: effect of stimulus characteristics. Alcohol Clin Exp Res. 2004;28:677–687. doi: 10.1097/01.alc.0000125345.19665.09. [DOI] [PubMed] [Google Scholar]
- Cubeddu LX. New alpha1-adrenergic receptor antagonists for the treatment of hypertension: role of vascular alpha receptors in the control of peripheral resistance. Am Heart J. 1988;116:133–162. doi: 10.1016/0002-8703(88)90261-x. [DOI] [PubMed] [Google Scholar]
- Davis WM, Smith SG, Werner TE. Noradrenergic role in the self-administration of ethanol. Pharmacol Biochem Behav. 1978;9:369–374. doi: 10.1016/0091-3057(78)90298-8. [DOI] [PubMed] [Google Scholar]
- De Jong J, Wauben P, Huijbrechts I, Oolders H, Haffmans J. Doxazosin treatment for posttraumatic stress disorder. J Clin Psychopharmacol. 2010;30:84–85. doi: 10.1097/JCP.0b013e3181c827ae. [DOI] [PubMed] [Google Scholar]
- Elliot HL, Meridith PA, Reid JL. Pharmacokinetic overview of doxazosin. Am J Cardiol. 1987;59:78G–81G. doi: 10.1016/0002-9149(87)90162-7. [DOI] [PubMed] [Google Scholar]
- Evered MD. Relationship between thirst and diazoxide-induced hypotension in rats. Am J Physiol Regul Integr Comp Physiol. 1990;259:R362–R370. doi: 10.1152/ajpregu.1990.259.2.R362. [DOI] [PubMed] [Google Scholar]
- Finney JW, Hahn AC, Moos RH. The effectiveness of inpatient and outpatient treatment for alcohol abuse: the need to focus on mediators and moderators of setting effects. Addiction. 1996;91:1773–1796. [PubMed] [Google Scholar]
- Garbutt JC. The state of pharmacotherapy for the treatment of alcohol dependence. J Subst Abuse Treat. 2009;36:S15–S23. [PubMed] [Google Scholar]
- Grossman S. Direct adrenergic and cholinergic stimulation of hypothalamic mechanisms. Am J Physiol. 1962;202:872–882. doi: 10.1152/ajplegacy.1962.202.5.872. [DOI] [PubMed] [Google Scholar]
- Guo TZ, Tinkienberg J, Oliker R, Maze M. Central α1-Adrenoceptor Stimulation Functionally Antagonizes the Hypnotic Response to Dexmedetomidine, an α2-Adrenoceptor Agonist. Anesthesiology. 1991;75:252–256. doi: 10.1097/00000542-199108000-00013. [DOI] [PubMed] [Google Scholar]
- Hamilton C, Reid J, Vincent J. Pharmacokinetic and pharmacodynamic studies with two alpha-adrenoceptor antagonists, doxazosin and prazosin in the rabbit. Br J Pharmacol. 1985;86:79–87. doi: 10.1111/j.1476-5381.1985.tb09437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Wise RA. Mapping of chemical trigger zones for reward. Neuropharmacology. 2004;47:190–201. doi: 10.1016/j.neuropharm.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Jaillon P. Clinical pharmacokinetics of prazosin. Clin Pharmacokinet. 1980;5:365–376. doi: 10.2165/00003088-198005040-00004. [DOI] [PubMed] [Google Scholar]
- Khoury AF, Kaplan NM. α-Blocker therapy of hypertension. JAMA. 1991;266:394–398. [PubMed] [Google Scholar]
- Kirby R, Andersen M, Gratzke P, Dahlstrand C, Høye K. A combined analysis of double-blind trials of the efficacy and tolerability of doxazosin-gastrointestinal therapeutic system, doxazosin standard and placebo in patients with benign prostatic hyperplasia. BJU Int. 2001;87:192–200. doi: 10.1046/j.1464-410x.2001.02032.x. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Webb E, Grillon C, Cooney N, Casal L, Morgan C, III, Southwick SM, Davis M, Charney DS. Evidence of acoustic startle hyperreflexia in recently detoxified early onset male alcoholics: modulation by yohimbine and m-chlorophenylpiperazine (mCPP). Psychopharmacology (Berl) 1997;131:207–215. doi: 10.1007/s002130050285. [DOI] [PubMed] [Google Scholar]
- Lanteri C, Salomon L, Torrens Y, Glowinski J, Tassin JP. Drugs of abuse specifically sensitize noradrenergic and serotonergic neurons via a non-dopaminergic mechanism. Neuropsychopharmacology. 2007;33:1724–1734. doi: 10.1038/sj.npp.1301548. [DOI] [PubMed] [Google Scholar]
- Lê A, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology (Berl) 2011;218:89–99. doi: 10.1007/s00213-011-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê A, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Lê A, Quan B, Juzytch W, Fletcher P, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl) 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Leggio L, Cardone S, Ferrulli A, Kenna GA, Dianna M, Swift RM, Addolorato G. Turning the clock ahead: potential preclinical and clinical neuropharmacological targets for alcohol dependence. Curr Pharm Des. 2010;16:2159–2186. doi: 10.2174/138161210791516369. [DOI] [PubMed] [Google Scholar]
- Menkes DB, Baraban JM, Aghajanian GK. Prazosin selectively antagonizes neuronal responses mediated by α 1-adrenoceptors in brain. Naunyn Schmiedebergs Arch Pharmacol. 1981;317:273–275. doi: 10.1007/BF00503830. [DOI] [PubMed] [Google Scholar]
- Meredith PA, Elliott HL, Kelman AW, Reid JL. Application of pharmacokinetic-pharmacodynamic modeling for the comparison of quinazoline α-adrenoceptor agonists in normotensive volunteers. J Cardiovasc Pharmacol. 1985;7:532–537. doi: 10.1097/00005344-198505000-00019. [DOI] [PubMed] [Google Scholar]
- Newton TF, De La Garza R, 2nd, Brown G, Kosten TR, Mahoney JJ, 3rd, Haile CN. Noradrenergic α(1) receptor antagonist treatment attenuates positive subjective effects of cocaine in humans: a randomized trial. PLoS One. 2012;7:e30854. doi: 10.1371/journal.pone.0030854. Epub 2012 Feb 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oryan S, Eidi M, Eidi A, Kohanrooz B. Effects of α1-adrenoceptors and muscarinic cholinoceptors on water intake in rats. Eur J Pharmacol. 2003;477:123–127. doi: 10.1016/j.ejphar.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Williams JT. Noradrenergic inhibition of midbrain dopamine neurons. J Neurosci. 2004;24:4568–4575. doi: 10.1523/JNEUROSCI.5735-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prys-Roberts C, Farndon JR. Efficacy and safety of doxazosin for perioperative management of patients with pheochromocytoma. World J Surg. 2002;26:1037–1042. doi: 10.1007/s00268-002-6667-z. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, Froehlich JC. The α1-Adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2009;33:264–272. doi: 10.1111/j.1530-0277.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen D, Burke B, Crites N. Chronic daily ethanol and withdrawal: melatonin treatment reverses persistently increased acoustic startle response during abstinence. Alcohol Clin Exp Res. 2005;29:16A. [Google Scholar]
- Rogawski MA, Aghajanian GK. Activation of lateral geniculate neurons by locus coeruleus or dorsal noradrenergic bundle stimulation: Selective blockade by the alpha1-adrenoceptor antagonist prazosin. Brain Res. 1982;250:31–39. doi: 10.1016/0006-8993(82)90950-7. [DOI] [PubMed] [Google Scholar]
- Samokhvalov AV, Popova S, Room R, Ramonas M, Rehm J. Disability associated with alcohol abuse and dependence. Alcohol Clin Exp Res. 2010;34:1871–1878. doi: 10.1111/j.1530-0277.2010.01275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, Gross CA, Hart KL, Raskind M. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res. 2009;33:255–263. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Stevens D, McCarley R, Greene R. The mechanism of noradrenergic alpha 1 excitatory modulation of pontine reticular formation neurons. J Neurosci. 1994;14:6481–6487. doi: 10.1523/JNEUROSCI.14-11-06481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift R, Oslin DW, Alexander M, Forman R. Adherence monitoring in naltrexone pharmacotherapy trials: a systematic review. J Stud Alcohol Drugs. 2011;72:1012–1018. doi: 10.15288/jsad.2011.72.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thavorncharoensap M, Teerawattananon Y, Yothasamut J, Lertpitakpong C. The economic impact of alcohol consumption: a systematic review. Substance Abuse Treatment Prev Policy. 2009;4:20. doi: 10.1186/1747-597X-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J, Elliott H, Meredith P, Reid J. Doxazosin, an alpha 1-adrenoceptor antagonist: pharmacokinetics and concentration-effect relationships in man. Br J Clin Pharmacol. 1983;15:719–725. doi: 10.1111/j.1365-2125.1983.tb01556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, Koob GF. α1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD. Adherence to pharmacotherapy in patients with alcohol and opioid dependence. Addiction. 2004;99:1382–1392. doi: 10.1111/j.1360-0443.2004.00884.x. [DOI] [PubMed] [Google Scholar]