Abstract
Objectives
To evaluate ROP screening rates in a population-based cohort; To identify characteristics of patients that were missed.
Study design
We used the California Perinatal Quality Care Collaborative data from 2005-2007 for a cross sectional study. Using eligibility criteria, screening rates were calculated for each hospital. Multivariable regression was used to assess associations between patient clinical and socio-demographic factors and the odds of missing screening.
Results
Overall rates of missed ROP screening decreased from 18.6% in 2005 to 12.8% in 2007. Higher gestational age (odds ratio [OR] 1.25 for increase of one week, 95% confidence interval [CI] 1.21-1.29), higher birth weight (OR 1.13, 95% CI 1.10-1.15), and singleton birth (OR 1.2, 95% CI 1.07-1.34) were associated with higher probability of missing screening. Level II NICUs and NICUs with lower volume were more likely to miss screenings.
Conclusion
Although ROP screening rates improved over time, larger and older infants are at risk for not receiving screening. Furthermore, large variations in screening rates exist among hospitals in California. Identification of gaps in quality of care creates an opportunity to improve ROP screening rates and prevent impaired vision in this vulnerable population.
Keywords: Retinopathy of Prematurity, Premature, Quality of Care, Neonatal
Retinopathy of Prematurity (ROP) is a leading cause of childhood vision impairment and blindness.(1-5) It is one of the major morbidities faced by infants born prematurely, and infants with poor visual outcomes due to ROP have a lower health-related quality of life than those infants who do not develop severe visual impairment.(6) Importantly, progression of disease to more severe disability is treatable with screening and early treatment.(2-4, 7-9)
Both the CRYO-ROP and ETROP studies showed considerable risk for ROP in premature infants with a significant reduction in unfavorable outcomes as a result of peripheral retinal ablation.(7, 10) For these reasons, screening for ROP has been identified as an important first step in preserving vision in premature infants. Furthermore, national organizations such as the National Quality Forum have identified ROP screening as an area of particular importance in delivering high quality of care to neonates,(11) and the American Academy of Pediatrics (AAP), American Academy of Ophthalmology (AAO), and American Association for Pediatric Ophthalmology and Strabismus (AAPOS) have developed guidelines specifying which neonates should be screened.(8)
What is not known is how well neonatal intensive care units (NICUs) adhere to the guidelines. In one US study that examined ROP screening behavior, there was large variation in how children are identified for ROP screening and how screening and treatment are provided.(12) Although that study involved a survey of 300 neonatologists, no study has evaluated actual ROP screening rates in a population-based sample.
We examined overall ROP screening rates in California and investigated factors associated with missed screening. Using the California Perinatal Quality Care Collaborative (CPQCC) database,(13) we identified infants who qualified for ROP screening by the AAP, AAO and AAPOS criteria and determined what percent of these infants did not receive screening. We then investigated patient and hospital characteristics associated with missed screening.
Methods
This cross-sectional study used CPQCC data from 2005-2007. The CPQCC collects data in a prospective manner for neonates born at member hospitals in California. Membership is offered to any hospital in California that provides neonatal intensive care. During the study period, eligible patients were cared for in 126 member hospitals, representing more than 90% of NICUs. Data are abstracted by NICU personnel including physicians, nurses and other trained data abstractors. Annual training sessions help to promote accuracy and uniformity in data abstraction. Each record has range and logic checks both at the time of data collection and data closeout, with auditing of records with excessive missing data. Data on race and ethnicity were obtained through the use of a linkage with the California Vital Records, which was made possible with support from the March of Dimes and has been described previously.(14)
The AAP AAO and AAPOS recommend ROP screening for all infants with gestational age less than or equal to 30 weeks or birth weight less than or equal to 1500g.(8) Screening is also recommended for infants greater than 30 weeks or 1500 grams at birth who have an unstable clinical course. For our study, we considered as eligible subjects those infants born at 30 weeks or less or 1500 grams or less, as this was a homogeneous population that should have all received screening according to the guidelines, regardless of clinical course. Specific timing of the initial ROP screen is also recommended based on the infant’s age. Infants born between 27 and 30 weeks gestation should be screened at age 4 weeks. Infants born between 22 and 26 weeks gestation should be screened one week later for each additional week of prematurity. For example, infants born at 22 weeks are screened at 9 weeks of age. We excluded those who died or were discharged or transferred to another hospital before the first eye exam was due. We also excluded those infants who had missing data about the eye exam. Due to standard data collection definitions, we were only able to determine if an ROP screen should have been performed some time during the hospitalization, not if it was performed at the earliest suggested time as outlined by the screening guidelines.
We calculated annual rates of missed screening by hospital and various socio-demographic and hospital characteristics. As described above, our rates were based on only those infants who met the criteria for screening at the time of hospitalization based on the screening guidelines. Multivariable logistic regression (PROC LOGISTIC, SAS 9.2, SAS Institute, Cary, NC) was used to assess the associations between various socio-demographic characteristics (race, ethnicity, and prenatal care), clinical factors (sex, gestational age, and birth weight), and hospital variables (NICU level of care and annual NICU volume) and the odds of receiving screening. NICU level of care was based on California Children’s Services (CCS) classification. The CCS classifies NICUs into three levels – Regional, Community, and Intermediate NICUs—based on the services provided at each center with regional NICUs being equivalent to the AAP’s Levels IIIC and IIID designation, community NICUs equivalent to AAP’s Level IIIA and IIIB, and Intermediate NICUs being equivalent to AAP’s Level II designation.(15)
During the study period, there were 20,595 infants born at CPQCC hospitals who qualified for ROP screening by the AAP, AAO and AAPOS criteria of birth weight or gestational age. We excluded: 3,386 infants who died before discharge (mean gestational age 25.0 weeks, birth weight 746 grams), 3,887 infants who were discharged home or transferred to another hospital before the first eye exam was due (mean gestational age 30.0 weeks, birth weight 1221 grams), and 40 infants who had a missing variable for the eye exam (mean gestational age 27.0 weeks, birth weight 1064 grams).
This study was approved by the Institutional Review Boards of the University of California, San Francisco and Stanford University.
Results
The final cohort eligible for the screening consisted of 13,282 infants with mean gestational age 28.1 weeks (standard deviation 2.3 weeks) and 5th and 95th percentiles at 24 and 31 weeks respectively. The eligible cohort had mean birth weight of 1101 grams (standard deviation 303 grams) and 5th and 95th percentiles at 620 and 1545 grams respectively.
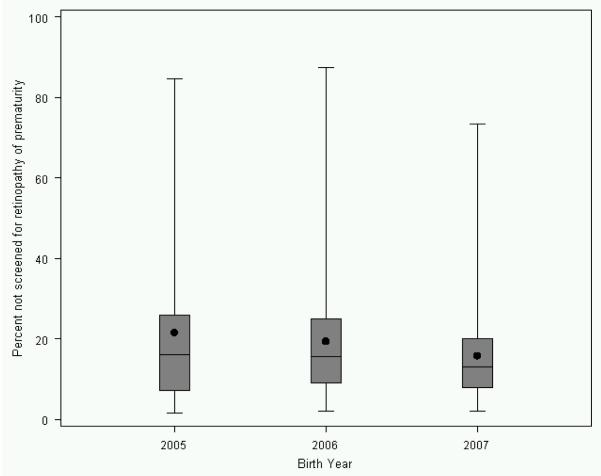
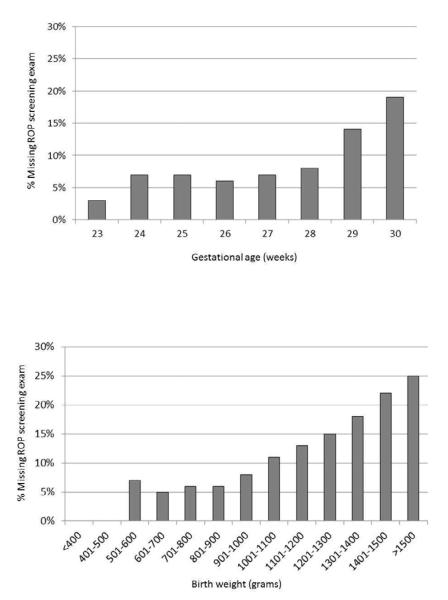
The rates of missed ROP screening decreased over time from 18.6% in 2005 to 12.8% in 2007 (p<0.0001). Individual hospital screening rates varied widely for all years. When examining hospitals with at least 12 eligible patients per year, individual hospital rates for missed ROP screening rates ranged widely (Figure 1). In 2007, the median rate of missed screening was 13.0%, with interquartile range of 7.8% to 20.0%, and total range of 1.9% to 73.3%. The percentage of infants appropriately screened also varied with the maternal race/ethnicity, with infants born to African American mothers having the highest rates of missed screening (Table I). Regional (Level IIIC/D NICU) hospitals and those with the highest patient volume were less likely to have missed screenings (Table I). Higher gestational age was associated with increased rates of missed screening, particularly above 28 weeks (Figure 2). Larger birth weight was also associated with increased rates of missed screening (Figure 2).
Figure 1.
Rates of missed retinopathy of prematurity screening by year for hospitals with at least twelve eligible patients
Table 1.
Rates of missed retinopathy of prematurity screening
| N* | % ROP exam missed | P value | |
|---|---|---|---|
| Year | |||
| 2005 | 4145 | 18.6% | |
| 2006 | 4544 | 16.3% | |
| 2007 | 4593 | 12.8% | < 0.0001 |
|
Maternal race /
ethnicity |
|||
| Non-Hispanic White | 3554 | 16.1% | |
| Hispanic White | 6350 | 15.1% | |
| African American | 1592 | 18.1% | |
| Native American | 69 | 13.0% | |
| Asian | 1452 | 15.6% | |
| Other / multi-racial | 250 | 15.6% | 0.093 |
| Sex | |||
| Female | 6534 | 15.9% | |
| Male | 6745 | 15.6% | 0.65 |
| Prenatal care | |||
| Yes | 12550 | 15.6% | |
| No | 643 | 18.9% | 0.03 |
| Multiple gestation | |||
| No | 9615 | 16.2% | |
| Yes | 3665 | 14.7% | 0.031 |
| CCS Level (AAP Level) | |||
| Regional (III C/D) | 4831 | 12.6% | |
| Community (III A/B) | 6873 | 15.8% | |
| Intermediate (II) | 757 | 29.7% | |
| Non-CCS | 821 | 21.4% | < 0.0001 |
|
Hospital volume
(patients/year) |
|||
| < 25 | 1842 | 21.4% | |
| 25 – 49 | 4330 | 18.6% | |
| 50-99 | 4079 | 15.4% | |
| >= 100 | 3031 | 8.8% | < 0.0001 |
ROP – retinopathy of prematurity; CCS – California Children’s Services; AAP – American Academy of Pediatrics
Total numbers reflect data that was available in our dataset. Incomplete records are responsible for different totals in each category.
Figure 2.
Rates of missed retinopathy of prematurity screening by gestational age and birth weight
In multivariable regression, infants born at older gestational ages had 1.25 times the odds of being missed for screening for every week older they were at birth (Table II). For every increase of 100 grams at birth, infants were 1.13 times as likely to be missed (Table II). We saw a protective effect of being born more recently, with infants born in 2005, the earliest year included in the study, having 1.39 times the odds of being missed for screening. Race was also a risk factor for missing screening, as African American babies had 1.4 times the odds of being missed as compared with non-Hispanic Whites. And infants born to mothers without prenatal care were 1.32 times as likely to be missed for ROP exams. At the hospital level, NICU level of care was found to be a risk factor for missing screening, with infants admitted to Intermediate CCS (Level II) NICUs at 1.61 times the odds of being missed as compared with Regional (Level IIIC/D) NICUs.
Table 2.
Multivariable model assessing risk for not receiving appropriate retinopathy of prematurity screening
| 95% confidence interval | |||
|---|---|---|---|
| Odds ratio | Lower | Upper | |
|
Birth weight (increase of 100
grams) |
1.13 | 1.10 | 1.15 |
| Gestational age (increase of 1 week) | 1.25 | 1.21 | 1.29 |
| Year | |||
| 2005 | 1.00 | (ref.) | |
| 2006 | 0.82 | 0.72 | 0.92 |
| 2007 | 0.61 | 0.54 | 0.69 |
| Maternal race / ethnicity | |||
| Non-Hispanic White | 1.00 | (ref.) | |
| Hispanic White | 0.97 | 0.86 | 1.10 |
| African American | 1.40 | 1.19 | 1.66 |
| Native American | 0.63 | 0.30 | 1.32 |
| Asian | 0.96 | 0.81 | 1.15 |
| Other / multi-racial | 1.07 | 0.74 | 1.55 |
| Sex | |||
| Female | 1.05 | 0.95 | 1.16 |
| Male | 1.00 | (ref.) | |
| Prenatal care | |||
| Yes | 1.00 | (ref.) | |
| No | 1.32 | 1.06 | 1.64 |
| Multiple gestation | |||
| No | 1.20 | 1.07 | 1.34 |
| Yes | 1.00 | (ref.) | |
| CCS Level (AAP Level) | |||
| Regional (III C/D) | 1.00 | (ref.) | |
| Community (III A/B) | 0.93 | 0.82 | 1.05 |
| Intermediate (II) | 1.61 | 1.31 | 1.98 |
| Non-CCS | 1.12 | 0.91 | 1.38 |
|
Hospital volume (increase of 10
patients/year) |
0.94 | 0.93 | 0.95 |
CCS – California Children’s Services; AAP – American Academy of Pediatrics
Discussion
Our study of California NICUs revealed that a significant number of eligible patients did not receive ROP screening as suggested by the guidelines developed by the AAP, AAO and AAPOS. These findings are concerning, as screening identifies infants who should be treated, and the importance of treating ROP has been repeatedly demonstrated in improving structural and visual outcomes.(7, 10, 16) We also identified several individual level risk factors that put patients at higher risk of missing screening, including higher gestational age, heavier birth weight, singleton birth, African American maternal race, and lack of prenatal care.
Because of the limited information in our dataset, our study cannot address why older and larger infants were less likely to be screened. Although most screens happen at 3 to 4 weeks of life and infants of older gestational age may be discharged to home before their screen is due, we accounted for this possibility by excluding infants who had already been discharged before the first exam was due. Furthermore, this study only included infants who qualified for screening based on birth weight or gestational age. Older and larger infants who may have qualified for screening based on an unstable clinical course were not included in our study, and our results may in fact be underestimating the severity of the discrepancy in screening rates between infants at each end of the spectrum of gestational age and birth weight.
It is possible that older and larger infants are perceived to be healthier than their smaller, younger counter parts and are therefore not considered a priority for screening, although they still qualify as being at risk by the screening criteria. In a study of antenatal steroid administration practices for premature birth, there was a similar finding of decreased application of a well-accepted practice for larger birth weight and higher gestational age, perhaps reflecting a similar complacency in this “moderately” preterm population.(14) There may be some complacency in treating this population that actually has relatively high respiratory morbidity and would benefit from increased antenatal steroid use.(17-19) Another potential reason for decreased screening rates for this group may be limited resources at the NICU in which they received their care, reserving the screen for higher risk infants. The AAO recently found that fewer pediatric ophthalmologists and retinal specialists are willing to perform ROP exams due to liability concerns, poor reimbursement, and the complexity of scheduling care.(12) This may force NICUs to select the highest risk patients for screening. We do not know if this is appropriate, as the existing guidelines do not have a risk stratified approach for screening in the population of infants born before 30 weeks gestation or with birth weight less than 1500 grams.
We also found a differential in screening rates by maternal race. In particular, infants born to African American women were less likely to be screened than those born to Caucasian, Asian or Native American women. This may reflect a perception that African American infants are at lower risk because some studies have shown that they are slightly less likely to have severe disease.(20) The CRYO-ROP study showed that black infants had a 65% lower risk of reaching threshold disease as compared with white infants.(20, 21) However, another study identified Asians and blacks as being at higher risk for developing threshold ROP compared with white infants.(22) With the current evidence, race should not be considered an eligibility factor for screening.
One striking finding in our study was the large variation in screening rates among hospitals. Even though overall screening rates reached 87% for the whole group in 2007, there was wide variation in rates with some hospitals missing screening for the majority of eligible patients (Figure 1). These patients may have received screening after discharge home, but would have received this screening after the recommended date. There were also several hospitals with 100% screening rates. This wide range in screening rates suggests that there is a very significant opportunity for quality improvement initiatives in this area. Lessons learned from the higher performing hospitals may be applied to the lower performing hospitals in an effort to improve their screening rates. We plan to investigate this in future studies.
Hospitals with higher patient volume had higher screening rates than hospitals with lower patient volume, although the impact of volume was attenuated in multivariable analysis. Higher patient volume has been shown to be associated with improved performance on quality indicators such as operative mortality for various surgeries and neonatal mortality for preterm infants.(23-27) We postulate that hospitals that care for larger numbers of infants are more likely to have a system in place to identify neonates who qualify for screening and potentially more readily available access to an appropriately trained pediatric ophthalmologist.
This study has several limitations. As we only had data about inpatient care, we were unable to evaluate infants who were discharged before their first ROP exam was due. Our study may have underestimated missed screening rates, as infants discharged home could very well miss an outpatient appointment with an ophthalmologist. We were also unable to evaluate certain patient and hospital level variables that were not recorded in the dataset, such as availability of an ophthalmologist to perform ROP exams. Furthermore, as the eye exam data did not include the date of exam, we were only able to evaluate whether an ROP exam was performed, not whether the exam was performed at the exact correct time according to guidelines.
Acknowledgments
Financial Disclosure Supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (K23HD068400 ), NIH LRP (1 L40 EY021928-01), and NIH/NCRR/OD UCSF-CTSI (KL2 RR024130). Data management was funded in part by a community grant from the March of Dimes California Chapter. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding bodies.
Abbreviations
- ROP
Retinopathy of Prematurity
- CPQCC
California Perinatal Quality Care Collaborative
- CCS
California Children’s Services
- AAP
American Academy of Pediatrics
- AAO
American Academy of Ophthalmology
- AAPOS
American Association for Pediatric Ophthalmology and Strabismus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Contributor Information
Lisa Charo Bain, University of California, San Francisco.
R. Adams Dudley, University of California, San Francisco.
Jeffrey B. Gould, Stanford University.
Henry C. Lee, University of California, San Francisco.
References
- 1.Binenbaum G, Ying GS, Quinn GE, Dreiseitl S, Karp K, Roberts RS, et al. A clinical prediction model to stratify retinopathy of prematurity risk using postnatal weight gain. Pediatrics. 2011;127(3):e607–14. doi: 10.1542/peds.2010-2240. Epub 2011/02/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slidsborg C, Forman JL, Rasmussen S, Jensen H, Nissen KR, Jensen PK, et al. A new risk-based screening criterion for treatment-demanding retinopathy of prematurity in Denmark. Pediatrics. 2011;127(3):e598–606. doi: 10.1542/peds.2010-1974. Epub 2011/02/16. [DOI] [PubMed] [Google Scholar]
- 3.Good WV, Hardy RJ, Dobson V, Palmer EA, Phelps DL, Quintos M, et al. The incidence and course of retinopathy of prematurity: findings from the early treatment for retinopathy of prematurity study. Pediatrics. 2005;116(1):15–23. doi: 10.1542/peds.2004-1413. Epub 2005/07/05. [DOI] [PubMed] [Google Scholar]
- 4.Rudanko SL, Fellman V, Laatikainen L. Visual impairment in children born prematurely from 1972 through 1989. Ophthalmology. 2003;110(8):1639–45. doi: 10.1016/S0161-6420(03)00498-6. Epub 2003/08/15. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert C, Foster A. Childhood blindness in the context of VISION 2020--the right to sight. Bulletin of the World Health Organization. 2001;79(3):227–32. Epub 2001/04/05. [PMC free article] [PubMed] [Google Scholar]
- 6.The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Quinn GE, Dobson V, Saigal S, Phelps DL, Hardy RJ, Tung B, et al. Health-related quality of life at age 10 years in very low-birth-weight children with and without threshold retinopathy of prematurity. Archives of ophthalmology. 2004;122(11):1659–66. doi: 10.1001/archopht.122.11.1659. Epub 2004/11/10. [DOI] [PubMed] [Google Scholar]
- 7.The Cryotherapy for Retinopathy of Prematurity Cooperative Group Multicenter trial of cryotherapy for retinopathy of prematurity: preliminary results. Pediatrics. 1988;81(5):697–706. Epub 1988/05/01. [PubMed] [Google Scholar]
- 8.Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006;117(2):572–6. doi: 10.1542/peds.2005-2749. Epub 2006/02/03. [DOI] [PubMed] [Google Scholar]
- 9.O’Keefe M, Kirwan C. Screening for retinopathy of prematurity. Early human development. 2008;84(2):89–94. doi: 10.1016/j.earlhumdev.2007.11.006. Epub 2008/01/31. [DOI] [PubMed] [Google Scholar]
- 10.Jones JG, MacKinnon B, Good WV, Hardy RJ, Dobson V, Palmer EA, et al. The early treatment for ROP (ETROP) randomized trial: study results and nursing care adaptations. Insight. 2005;30(2):7–13. Epub 2005/09/02. [PubMed] [Google Scholar]
- 11.National Quality Forum National Voluntary Consensus Standard for Perinatal Care. Peformance Measure Specifications. 2008 Oct 20; http://www.qualityforum.org.
- 12.Kemper AR, Wallace DK. Neonatologists’ practices and experiences in arranging retinopathy of prematurity screening services. Pediatrics. 2007;120(3):527–31. doi: 10.1542/peds.2007-0378. Epub 2007/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.California Perinatal Quality Care Collaborative [Accessed 11-11-2011]; http://cpqcc.org/research.
- 14.Lee HC, Lyndon A, Blumenfeld YJ, Dudley RA, Gould JB. Antenatal steroid administration for premature neonates in California. Obstetrics and gynecology. 2011;117(3):603–9. doi: 10.1097/aog.0b013e31820c3c9b. Epub 2011/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stark AR. Levels of neonatal care. Pediatrics. 2004;114(5):1341–7. doi: 10.1542/peds.2004-1697. Epub 2004/11/03. [DOI] [PubMed] [Google Scholar]
- 16.The Cryotherapy for Retinopathy of Prematurity Cooperative Group Multicenter trial of cryotherapy for retinopathy of prematurity. Snellen visual acuity and structural outcome at 5 1/2 years after randomization. Archives of ophthalmology. 1996;114(4):417–24. doi: 10.1001/archopht.1996.01100130413008. Epub 1996/04/01. [DOI] [PubMed] [Google Scholar]
- 17.The Cryotherapy for Retinopathy of Prematurity Cooperative Group. McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstetrics and gynecology. 2008;111(1):35–41. doi: 10.1097/01.AOG.0000297311.33046.73. Epub 2008/01/01. [DOI] [PubMed] [Google Scholar]
- 18.The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Marret S, Ancel PY, Marpeau L, Marchand L, Pierrat V, Larroque B, et al. Neonatal and 5-year outcomes after birth at 30-34 weeks of gestation. Obstetrics and gynecology. 2007;110(1):72–80. doi: 10.1097/01.AOG.0000267498.95402.bd. Epub 2007/07/03. [DOI] [PubMed] [Google Scholar]
- 19.Joseph KS, Nette F, Scott H, Vincer MJ. Prenatal corticosteroid prophylaxis for women delivering at late preterm gestation. Pediatrics. 2009;124(5):e835–43. doi: 10.1542/peds.2009-0905. Epub 2009/10/28. [DOI] [PubMed] [Google Scholar]
- 20.Saunders RA, Donahue ML, Christmann LM, Pakalnis AV, Tung B, Hardy RJ, et al. Racial variation in retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Archives of ophthalmology. 1997;115(5):604–8. doi: 10.1001/archopht.1997.01100150606005. Epub 1997/05/01. [DOI] [PubMed] [Google Scholar]
- 21.Schaffer DB, Palmer EA, Plotsky DF, Metz HS, Flynn JT, Tung B, et al. Prognostic factors in the natural course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1993;100(2):230–7. doi: 10.1016/s0161-6420(93)31665-9. Epub 1993/02/01. [DOI] [PubMed] [Google Scholar]
- 22.Aralikatti AK, Mitra A, Denniston AK, Haque MS, Ewer AK, Butler L. Is ethnicity a risk factor for severe retinopathy of prematurity? Archives of disease in childhood Fetal and neonatal edition. 2010;95(3):F174–6. doi: 10.1136/adc.2009.160366. Epub 2009/12/02. [DOI] [PubMed] [Google Scholar]
- 23.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. The New England journal of medicine. 2002;346(15):1128–37. doi: 10.1056/NEJMsa012337. Epub 2002/04/12. [DOI] [PubMed] [Google Scholar]
- 24.Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. Jama. 1998;280(20):1747–51. doi: 10.1001/jama.280.20.1747. Epub 1998/12/08. [DOI] [PubMed] [Google Scholar]
- 25.Swisher SG, Deford L, Merriman KW, Walsh GL, Smythe R, Vaporicyan A, et al. Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. The Journal of thoracic and cardiovascular surgery. 2000;119(6):1126–32. doi: 10.1067/mtc.2000.105644. Epub 2000/06/06. [DOI] [PubMed] [Google Scholar]
- 26.Chung JH, Phibbs CS, Boscardin WJ, Kominski GF, Ortega AN, Needleman J. The effect of neonatal intensive care level and hospital volume on mortality of very low birth weight infants. Medical care. 2010;48(7):635–44. doi: 10.1097/MLR.0b013e3181dbe887. Epub 2010/06/16. [DOI] [PubMed] [Google Scholar]
- 27.Phibbs CS, Baker LC, Caughey AB, Danielsen B, Schmitt SK, Phibbs RH. Level and volume of neonatal intensive care and mortality in very-low-birth-weight infants. The New England journal of medicine. 2007;356(21):2165–75. doi: 10.1056/NEJMsa065029. Epub 2007/05/25. [DOI] [PubMed] [Google Scholar]