Abstract
A number of multiresistant bacterial pathogens inactivate antibiotics by producing ZnII-dependent β-lactamases. We show that metal uptake leading to an active dinuclear enzyme in the periplasmic space of Gram-negative bacteria is ensured by a cysteine residue, an unusual metal ligand in oxidizing environments. Kinetic, structural and affinity data show that such ZnII-Cys interaction is an adaptive trait tuning the metal binding affinity, thus enabling antibiotic resistance at restrictive ZnII concentrations.
The efficacy of β-lactam antibiotics is being challenged by the worldwide dissemination of genes encoding metallo-β-lactamases (MβLs).1, 2 These hydrolases are able to confer multiresistance to β-lactam antibiotics in many pathogenic and opportunistic bacteria, leading to an urgent need for MβL inhibitors or new generations of β-lactam antibiotics. Most of the clinically relevant targets are in mobile genetic elements and belong to subclass B1.2 These enzymes bind up to two ZnII equivalents, giving rise to a tetrahedral site (M1), with 3 His and a bridging hydroxide (the nucleophile in the hydrolysis reaction) as metal ligands, and a trigonal-bypiramidal site (M2), with a Cys, His and Asp ligand set, completed by two solvent molecules.3, 4 Assessing the mechanistic role and essentiality of the M1 and M2 sites in B1 lactamases has proven a difficult task. Some reports suggest that the species with one metal ion located at the M1 site (mono-M1) or at the M2 site (mono-M2) are active,5–9 while others conclude that only the dinuclear forms are active.10 Crystal structures obtained for monometallic surrogates disclose a metal ion bound to the M1 site.11–13 However, such models may not reflect the mononuclear enzymes in vivo, since the thiolate of the Cys ligand (Cys221) appears oxidized, precluding metal binding to the M2 site.12,13
Cysteine ligands are uncommon in catalytic zinc sites, even more in aerobic oxidizing environments like the periplasm or the extracellular space.14 Actually, most proteins from the broad MβL Superfamily devoid of β-lactamase activity display a conserved Asp residue in this position, despite exhibiting the same characteristic protein fold.15 Besides, the fact that the Cys221Asp mutation in the B1 lactamase IMP-1 results in a wild-type like catalytic efficiency makes the absence of the Asp ligand even more puzzling.16
We obtained the Cys221Asp mutant of BcII, a prototypical B1 enzyme. The activity of this variant in the presence of excess ZnII paralleled or even surpassed that of wild-type BcII (Supplementary Results, Supplementary Table 1), resembling the situation reported for IMP-1.16 Instead, BcII-Cys221Asp was inactive when the reaction medium was not supplemented with ZnII. The hydrolytic activity of the mutant was recovered upon addition of large amounts of the metal ion, in contrast with the wild-type enzyme, which was fully active at low ZnII concentrations (Supplementary Fig.1).
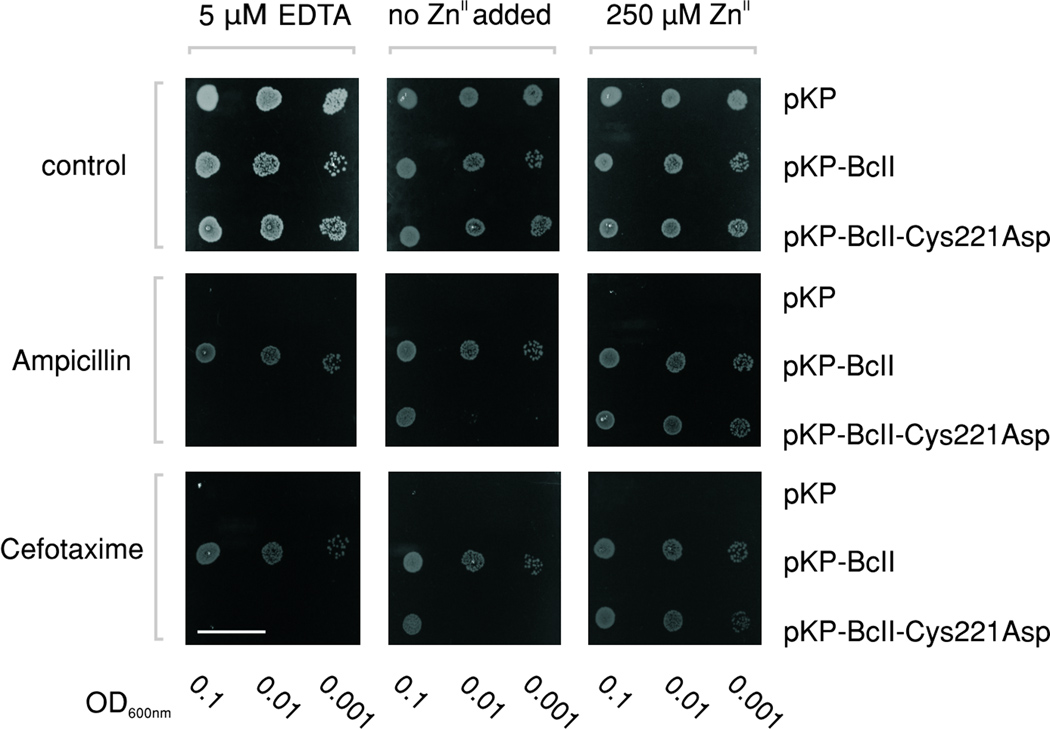
These results suggest that MβL variants with an Asp ligand might not be able to withstand restrictive ZnII concentrations. To test this hypothesis, we examined the in vivo performance of wild-type BcII and BcII-Cys221Asp in E. coli cells expressing and secreting these proteins to the periplasmic space. Both proteins accumulated to comparable levels in the periplasm, as revealed by Western blot analysis (Supplementary Fig.2). However, the minimum inhibitory concentrations of ampicillin and cefotaxime were substantially lower for the mutant (cf. 32 and 2 µg/ml respectively, whereas wild-type values were 512 and 32 µg/ml). Addition of EDTA to the growth medium abolished antibiotic resistance in cells expressing BcII-Cys221Asp, while cells expressing the wild-type enzyme proved to be less sensitive to metal limiting conditions (Fig. 1). The antibiotic-sensitive phenotype in cells expressing BcII-Cys221Asp was reverted by addition of extra ZnII to the growth medium, thus enhancing resistance (Fig.1). In line with these results, the lactamase activity measured with periplasmic extracts was more sensitive to ZnII availability in the case of BcII-Cys221Asp (Supplementary Fig. 3). We conclude that the in vivo performance of BcII-Cys221Asp is conditioned by ZnII availability, indicating a decreased metal binding affinity in this mutant.
Figure 1. The capacity to confer resistance of BcII-Cys221Asp enzyme to E. coli cells is impaired under low ZnII conditions.
The antibiotic sensitivity of E. coli JM109 expressing BcII-Cys221Asp and BcII in the periplasmic space were measured as a function of the ZnII availability in the growth medium. Colony spots result from serial dilutions of cells transformed with the pKP vector (negative control), pKP-BcII (expressing wild-type BcII), and pKP-BcII- Cys221Asp (expressing the Cys221Asp mutant). Each strain was challenged with ampicillin (32 µg/ml) and cefotaxime (2 µg/ml), under different ZnII concentrations; namely, low ZnII (5 µM EDTA), no added ZnII, and excess ZnII (250 µM ZnSO4). These experiments were repeated three times with independent cultures for each condition tested. The length of the white scale bar corresponds to 20 mm.
Dissociation constants for ZnII were estimated by competition with the chromophoric chelator 4-(2-pyridylazo)-resorcinol (PAR) (Supplementary Fig. 4). For the wild type enzyme, two binding events with similar Kd values in the low-nanomolar range were evident (Supplementary Table 2). Instead, BcII-Cys221Asp displayed markedly different affinities for the two binding sites (Kd1= (9 ± 1.7) nM and Kd2= (267 ± 71) nM), i.e., binding of the second ZnII equivalent is impaired in the mutant. On the other hand, recovery of the lactamase activity of the apoenzymes upon addition of ZnII depends on a single binding event, with Kact values of (88 ± 7) nM for wild type BcII, and (7305 ± 365) nM for BcII-Cys221Asp (Supplementary Fig. 1 and Supplementary Methods). These values represent ZnII dissociation constants in the presence of substrate. Although for wild type and Cys221Asp mutant the affinity for the second metal equivalent is slightly impaired in the presence of substrate compared to the affinity in the resting state, this effect is much more drastic in the Cys221Asp mutant.
The different Kd values in BcII-Cys221Asp allow us to assess the effect of each metal binding site on the enzyme activity. For this purpose, we analyzed the activity of BcII-Cys221Asp at different metal/enzyme ratios at enzyme concentrations high enough to warrant stoichiometric conditions. We employed a rapid mixing device coupled to a spectrophotometer, which allows detection of reaction rates spanning several orders of magnitude. No activity was detected at metal/enzyme ratios ≤ 1, while addition of excess ZnII gave rise to a fully active enzyme (Supplementary Fig. 5). We conclude that the mononuclear species is not active in BcII-Cys221Asp, and thus the impaired resistance at limiting ZnII conditions is due to an inability to assemble a functional dinuclear site.
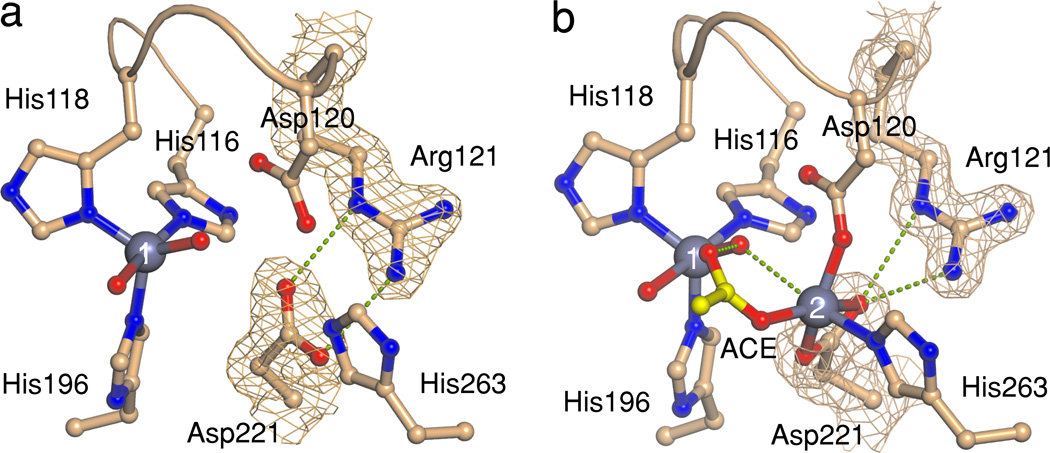
In order to characterize the mono- and dinuclear species at the molecular level, we obtained crystal structures for the mono- and di-ZnII forms of BcII-Cys221Asp, solved at 1.71 Å and 1.58 Å resolution, respectively (Fig. 2 and Supplementary Table 3). The di-ZnII species could be obtained only upon soaking the crystals with 20 mM ZnII. In both cases, the M1 site shows a distorted tetrahedral geometry analogous to those witnessed for available BcII structures, whereas the Asp221 residue conformation depends on the M2 site metal occupancy. In mono-ZnII BcII-Cys221Asp (Fig. 2a), Asp221 appears salt bridged to the Arg121 guanidinium group, whereas the carboxylate sidechain is rotated 64° in the di-ZnII variant, coordinated in a bidentate-chelating fashion to the M2 site ZnII (Fig. 2b). The strong salt-bridge interaction in the mono-ZnII variant traps Asp221 in a conformation disfavoring metal binding to the M2 site, and is expected to elicit the sequential metal binding mode. On the other hand, the structure of the fully loaded BcII-Cys221Asp metal site closely resembles those of native di-ZnII B1 enzymes (Supplementary Fig. 6), in line with the high activity determined for this enzyme species. These structures are consistent with the otherwise counterintuitive lower binding affinity provided by an Asp ligand, and reveal that the inactive mononuclear species of BcII-Cys221Asp accumulating at low ZnII concentrations is mono-M1. Therefore, we conclude that metal binding to the M2 site acts as an activity switch.
Figure 2. Structures of the mono-ZnII and di-ZnII binding sites of BcII-Cys221Asp enzyme.
The metal-binding sites of mono-ZnII (a), and di-ZnII (b) BcII-Cys221Asp were solved at 1.71 Å and 1.58 Å resolution, respectively. Electron density maps (2FO – FC) are shown for Asp221 and Arg121 sidechains, contoured at 1.5 σ. Numbers indicate ZnII ions. Dotted lines indicate relevant electrostatic interactions. An acetate ion (ACE) was modeled bound to the di-ZnII form. Red spheres depict metal-bound water molecules.
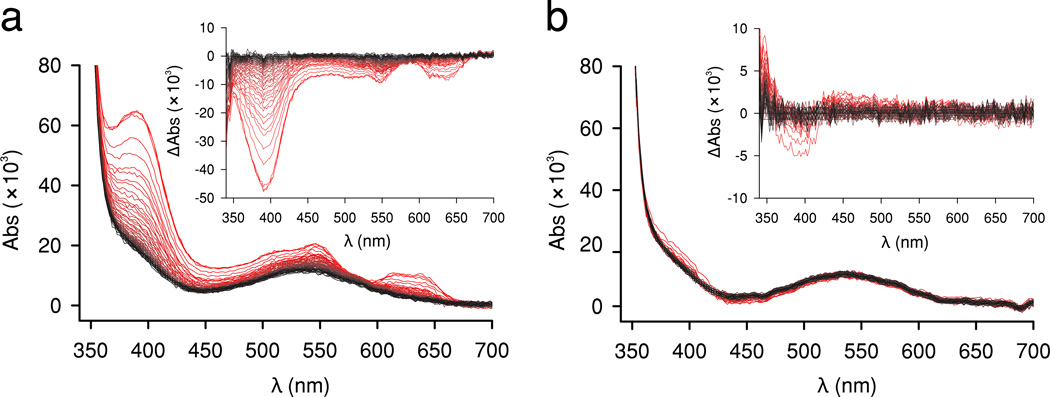
The different binding affinities in BcII-Cys221Asp enable the study of the M1 mononuclear species and to assess its mechanistic behavior, in contrast to wild-type BcII.17 Coordination changes in the metal site during enzymatic turnover can be followed via a photodiode array coupled to a stopped-flow device in the CoII-substituted enzyme. Imipenem hydrolysis by di-CoII BcII-Cys221Asp proceeds with the accumulation of an intermediate with a strong absorption band at 390 nm (Fig. 3a), which resembles that reported for the same reaction mediated by CoII-wild type BcII.9 When the same experiment was performed in the presence of 0.7 equivalents of CoII, no imipenem hydrolysis occurred and the spectral features of the mono-M1 species remained unperturbed, revealing that the mononuclear variant, aside from being inactive, is unable to bind imipenem (Fig. 3b).The final spectrum of the reaction catalyzed by the dinuclear enzyme is identical to that of the mono-M1 species (Fig. 3b and Supplementary Fig. 7), suggesting that the M2 site is depleted during catalysis, giving rise to the inactive mono-CoII variant. These mechanistic studies reveal that the metal ion at the M2 site is essential to stabilize the reaction intermediate, and therefore to render an active enzyme.
Figure 3. The monoCoII BcII-Cys221Asp is inactive and unable to bind the imipenen substrate.
The hydrolysis of imipenem mediated by di-CoII BcII-Cys221Asp (a), and mono-CoII BcII-Cys221Asp (b) was detected via photodiode-array stopped-flow kinetic measurements. Spectra represent absorbance for a time span of 100 milliseconds (from pink to black). Insets show the same data as difference spectra (ΔA) taking the spectrum obtained after 100 milliseconds as reference. The initial spectrum in (a) exhibits an absorption band at 390 nm due to formation of a reaction intermediate, which decays in time together with ligand-field bands in the 600–650 nm region. The spectrum of the mono-CoII form (peak at 540 nm) (b) remained unchanged after 50 seconds. Reaction medium was 100 mM Hepes pH 7.5, 200 mM NaCl, at 7 °C, with 163 µM for both mono- and di-CoII species and an initial imipenem concentration of 2.5 mM.
Some authors have suggested that the role of the M2 site is essential for lowering the pKa of the bridging water molecule.10 Both mono- and di-ZnII Cys221Asp display ZnII-H2O distances consistent with a bound hydroxide, suggesting that the M1 site suffices for nucleophile activation. This evidence further supports the proposal that the M2 site is essential for substrate binding, C-N bond cleavage and intermediate stabilization in the mechanism of MβLs.9, 18 B2 lactamases are active as mononuclear enzymes, with the only metal ion located at the M2 site, further supporting its essentiality for catalysis and antibiotic resistance.19
Though we were unable to definitively discriminate between apo-, mono-, and dimetallic proteins as directly purified from cells (Supplementary Figure 8), the multiple lines of evidence reported herein support the conclusion that BcII and the clinically relevant B1 MβLs are likely to be active in vivo as dimetallic enzymes. An Asp221 residue decreases the M2 site metal-binding affinity, precluding the accumulation of the functional dinuclear species at sub-stoichiometric metal concentrations. Instead, the native Cys221 residue warrants an active dinuclear enzyme in these conditions, enabling activation of B1 lactamases even at low ZnIIconcentrations.20
Bacteria are known to concentrate ZnII ions in the cytosol even under metal limiting conditions.21 However, ZnII bioavailability in the periplasm of Gram-negative bacteria depends largely on its extracellular levels, being particularly challenged by the presence of glutathione and selective ion pumps which deplete the ZnII content in the periplasm.22 Because MβLs are translocated to the periplasmic space in a Zn(II)-free unfolded form23, they are expected to have evolved molecular mechanisms to refold in Zn(II)-deficient environments, ensuring survival of the host when exposed to β-lactam antibiotics. This study shows that natural selection has favored a Cys221 ligand in B1 lactamases to warrant formation of the dinuclear species in these conditions, despite being an unusual metal ligand for such a catalytic zinc site functional in aerobic environments. In addition, this work suggests that the active form of B1 lactamases contains a ZnII ion in the M2 site, and inhibitor design should target this metallated species.
Supplementary Material
Acknowledgements
This work has been supported by grants from Howard Hughes Medical Institute (HHMI) ANPCyT and NIH (1R01AI100560) to AJV; and Laboratório Nacional de Luz Sincrotron (LNLS), Campinas, Brazil. AJV is fellow of the John Simon Guggenheim Foundation. AJV, PET and JAC are staff members of CONICET. MRM is recipient of a doctoral fellowship from CONICET.
Abbreviations
- MβL
metallo-β-lactamase
- LMCT
ligand-to-metal charge-transfer
- EDTA
ethylene-diamine-tetraacetic acid
- PAR
chelator 4-(2-pyridylazo)-resorcinol.
Footnotes
Author Contributions
JMG, JAC, MRM, PET and AJV designed experiments and analyzed results. JMG, MRM, PET and AJV wrote the manuscript. JMG, JAC and MRM expressed and purified proteins. JMG and JAC determined kinetic parameters, and performed cobalt substitution. JMG performed stopped-flow measurements. MRM determined the dissociation constants for ZnII by competition experiments and the activity dependence on ZnII concentration. JMG and FJMM determined the crystal structures. JAC designed and made plasmid constructs, determined minimum inhibitory concentrations, and performed in vivo antibiotic sensitivity tests. JAC and MRM performed periplasmic extracts and Western blot assays.
The authors have no competing financial interests as defined by Nature Publishing Group.
Reference List
- 1.Crowder MW, Spencer J, Vila AJ. Metallo-beta-lactamases: Novel Weaponry for Antibiotic Resistance in Bacteria. Acc. Chem. Res. 2006;39:721–728. doi: 10.1021/ar0400241. [DOI] [PubMed] [Google Scholar]
- 2.Fisher JF, Meroueh SO, Mobashery S. Bacterial resistance to beta-lactam antibiotics: compelling opportunism, compelling opportunity. Chem Rev. 2005;105:395–424. doi: 10.1021/cr030102i. [DOI] [PubMed] [Google Scholar]
- 3.Fabiane SM, et al. Crystal structure of the zinc-dependent beta lactamase from Bacillus cereus at 1.9 A resolution: binuclear active site with features of a mononuclear enzyme. Biochemistry. 1998;37:12404–12411. doi: 10.1021/bi980506i. [DOI] [PubMed] [Google Scholar]
- 4.Orellano EG, Girardini JE, Cricco JA, Ceccarelli EA, Vila AJ. Spectroscopic characterization of a binuclear metal site in Bacillus cereus beta-lactamase II. Biochemistry. 1998;37:10173–10180. doi: 10.1021/bi980309j. [DOI] [PubMed] [Google Scholar]
- 5.Hu Z, Peryannan G, Bennett B, Crowder M. Role of the Zn-1 and Zn-2 sites in Metallo-beta-lactamase L1. J. Am. Chem Soc. 2008;130:14207–14216. doi: 10.1021/ja8035916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawk MJ, et al. Differential binding of Co(II) and Zn(II) to metallo-beta-lactamase Bla2 from Bacillus anthracis. J. Am. Chem. Soc. 2009;131:10753–10762. doi: 10.1021/ja900296u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llarrull LI, Tioni MF, Vila AJ. Metal Content and Localization during Turnover in B.cereus Metallo-beta -Lactamase. J. Am. Chem. Soc. 2008;130:15842–15851. doi: 10.1021/ja801168r. [DOI] [PubMed] [Google Scholar]
- 8.Wommer S, et al. Substrate-activated zinc binding of metallo-beta -lactamases: physiological importance of mononuclear enzymes. J. Biol. Chem. 2002;277:24142–24147. doi: 10.1074/jbc.M202467200. [DOI] [PubMed] [Google Scholar]
- 9.Tioni MF, et al. Trapping and Characterization of a Reaction Intermediate in Carbapenem Hydrolysis by B. cereus Metallo-beta -lactamase. J. Am. Chem. Soc. 2008;130:15852–15863. doi: 10.1021/ja801169j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badarau A, Page MI. Enzyme Deactivation Due to Metal-Ion Dissociation during Turnover of the Cobalt-beta-Lactamase Catalyzed Hydrolysis of beta-Lactams. Biochemistry. 2006;45:11012–11020. doi: 10.1021/bi0610146. [DOI] [PubMed] [Google Scholar]
- 11.Carfi A, et al. The 3-D structure of a zinc metallo-beta-lactamase from Bacillus cereus reveals a new type of protein fold. EMBO J. 1995;14:4914–4921. doi: 10.1002/j.1460-2075.1995.tb00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy TA, et al. Crystal structure of Pseudomonas aeruginosa SPM-1 provides insights into variable zinc affinity of metallo-beta-lactamases. J. Mol. Biol. 2006;357:890–903. doi: 10.1016/j.jmb.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez JM, Buschiazzo A, Vila AJ. Evidence of adaptability in metal coordination geometry and active-site loop conformation among B1 metallo-beta-lactamases. Biochemistry. 2010;49:7930–7938. doi: 10.1021/bi100894r. [DOI] [PubMed] [Google Scholar]
- 14.Davis AV, O'Halloran TV. A place for thioether chemistry in cellular copper ion recognition and trafficking. Nat. Chem. Biol. 2008;4:148–151. doi: 10.1038/nchembio0308-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daiyasu H, Osaka K, Ishino Y, Toh H. Expansion of the zinc metallo-hydrolase family of the beta-lactamase fold. FEBS Lett. 2001;503:1–6. doi: 10.1016/s0014-5793(01)02686-2. [DOI] [PubMed] [Google Scholar]
- 16.Haruta S, Yamaguchi H, Yamamoto ET, Eriguchi Y, Nukaga M, O'Hara K, Sawai T. Functional analysis of the active site of a metallo-beta-lactamase proliferating in Japan. Antimicrob. Agents Chemother. 2000;44:2304–2309. doi: 10.1128/aac.44.9.2304-2309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llarrull LI, Tioni MF, Kowalski J, Bennett B, Vila AJ. Evidence for a dinuclear active site in the metallo-beta-lactamase BcII with substoichiometric Co(II). A new model for metal uptake. J Biol. Chem. 2007;282:30586–30595. doi: 10.1074/jbc.M704613200. [DOI] [PubMed] [Google Scholar]
- 18.Rasia RM, Vila AJ. Structural determinants of substrate binding to Bacillus cereus metallo-beta-lactamase. J. Biol. Chem. 2004;279:26046–26051. doi: 10.1074/jbc.M311373200. [DOI] [PubMed] [Google Scholar]
- 19.Simona F, Magistrato A, Dal Peraro M, Cavalli A, Vila AJ, Carloni P. Common mechanistic features among metallo-beta-lactamases: a computational study of Aeromonas hydrophila CphA enzyme. J Biol Chem. 2009;284:28164–28171. doi: 10.1074/jbc.M109.049502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacquin O, et al. Positively cooperative binding of zinc ions to Bacillus cereus 569/H/9 beta-lactamase II suggests that the binuclear enzyme is the only relevant form for catalysis. J. Mol. Biol. 2009;392:1278–1291. doi: 10.1016/j.jmb.2009.07.092. [DOI] [PubMed] [Google Scholar]
- 21.Outten CE, O'Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 22.Ma Z, Jacobsen FE, Giedroc DP. Coordination chemistry of bacterial metal transport and sensing. Chem. Rev. 2009;109:4644–4681. doi: 10.1021/cr900077w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moran-Barrio J, Limansky AS, Viale AM. Secretion of GOB metallo-beta-lactamase in Escherichia coli depends strictly on the cooperation between the cytoplasmic DnaK chaperone system and the Sec machinery: completion of folding and Zn(II) ion acquisition occur in the bacterial periplasm. Antimicrob. Agents Chemother. 2009;53:2908–2917. doi: 10.1128/AAC.01637-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.