Abstract
Background
Solid organ transplant recipients are at an increased risk for infections because of long-term immunosuppression to prevent graft rejection. Fungal infections with dermatophytes are a common cause of cutaneous infections seen in organ transplant recipients and cutaneous dermatophyte infections may progress to Majocchi’s granuloma. Itraconazole is an anti-fungal compound used for the treatment of infections of the skin, nails and mucous membranes.
Main observation
We report on a heart transplant recipient who developed widespread Trichophyton rubrum infection presenting as Majocchi’s granuloma. Itraconazole treatment was complicated by drug interactions. Trichophyton rubrum infection progressed, while itraconazole treatment was varied in dose and delivery form.
Conclusions
In patients with Trichophyton rubrum infections, refractory to itraconazole treatment, altered drug absorption or drug interactions has to be considered. Careful monitoring and adjustment of itraconazole is of vital importance.
Keywords: adverse event, fungal infection, itraconazole, Majocchi's Granuloma, mycosis, Trichophyton rubrum
Introduction
Solid organ transplant recipients (OTRs) are exposed to severe infections because of immunosuppressive drug regimens to prevent graft rejection. Fungal infections with dermatophytes are common seen in OTRs.[1]
Due to impaired cell-mediated immunity, cutaneous fungal infection may penetrate deep into the skin and induce a pronounced inflammatory reaction resulting in granulomatous skin disease, or in systemic spread.
Case Report
A 49-year-old male underwent heart transplantation two years ago because of cardiomyopathy. Immunosuppression consisted of Prednisone, Azathioprine and Tacrolimus. The patient was referred to the department of dermatology with newly diagnosed papulonodular lesions of the dorsal aspects of both feet [Fig. 1].
Figure 1.
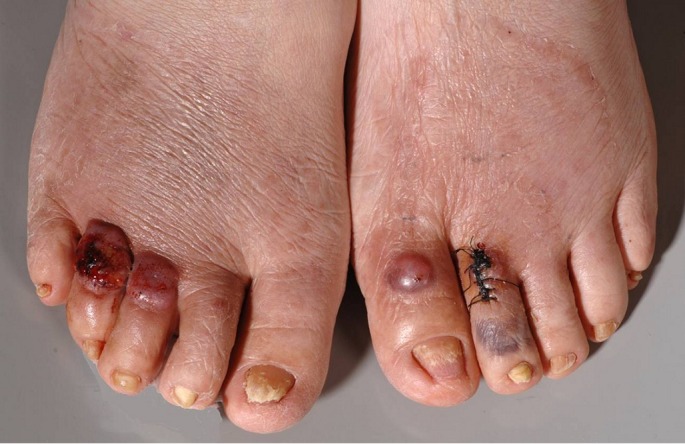
The back of both feet with brownish-red plaques with an infiltrated peripheral rim and peripheral scaling. In the central parts of these plaques, atrophic wrinkling of the epidermis becomes apparent. On the dorsal aspects of certain toes, dark red to brown nodules with or without erosions are seen. Nails are dystrophic and onycholytic.
Clinical examination, histology and mycology of the lesional biopsy revealed a Trichophytum rubrum induced Majocchi’s granuloma [Fig. 2].
Figure 2.
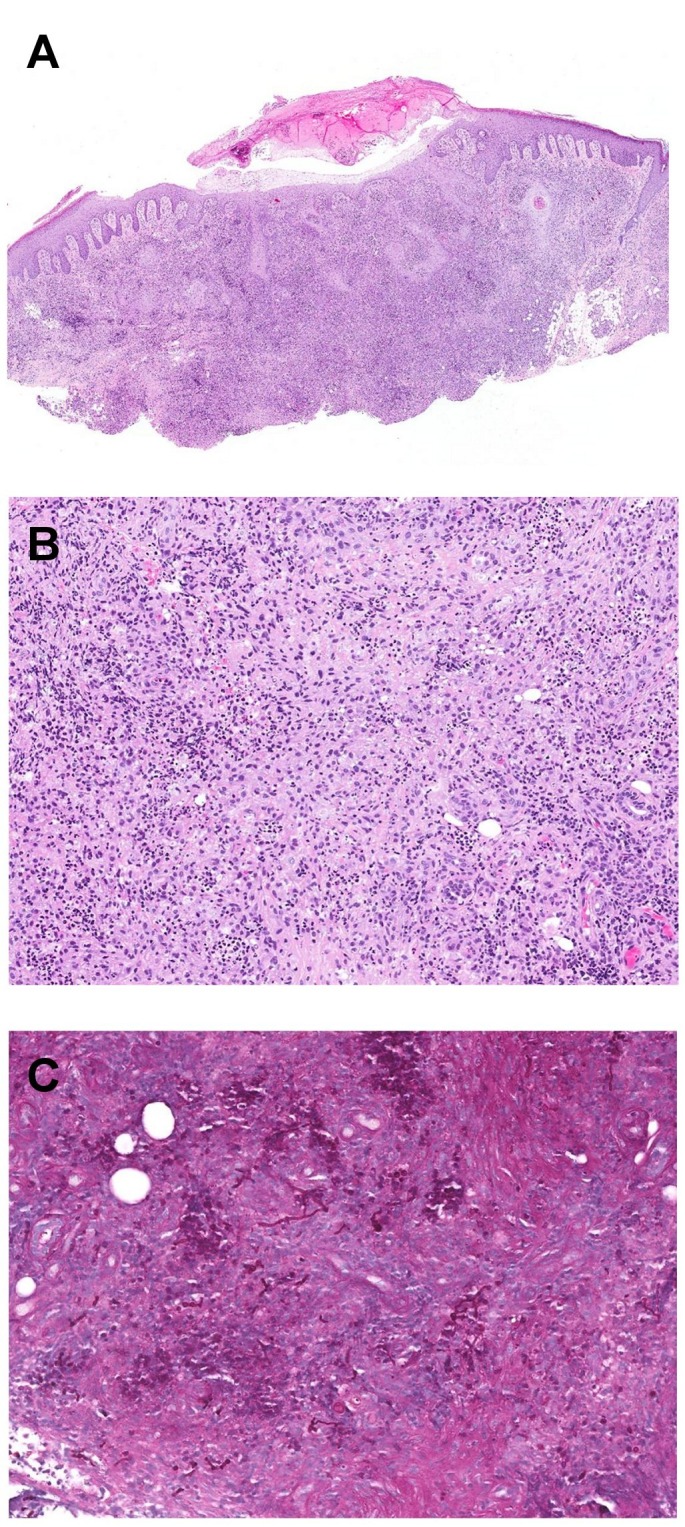
Pseudoepitheliomatous hyperplasia of the epidermis and extensive, poorly formed granulomas (A). Lymphohistiocytic cells with focal collections of neutrophils (B). The PAS stain reveals an abundance of strikingly pleomorphic segmented hyphae and arthrospores (C).
Treatment with itraconazole capsules 100 mg bid was started. After 8 weeks new sporotrichoid skin lesions developed. As serum levels of itraconazole were found below 100 ng/ml, the dose was increased to 300 mg daily. The formulation was switched from capsules to an intravenous preparation and then to the peroral solution with higher bioavailability. Within 10 days, a clinical improvement was seen, but Tacrolimus plasma levels rose from 5.9 ug/l to 21.8 ug/l, enhancing immunosuppression. Dose reduction reestablished the therapeutic range (8.7 ug/l).
At the same time the patient became septic due to Staphylococcus aureus. Treatment with Floxacillin and Rifampicin was initiated affecting antifungal and immunosuppressive therapy, keeping itraconazole plasma levels below 100 ng/ml and reducing plasma levels of Tacrolimus < 2.5 ug/l.
Discussion
The common clinical presentation of cutaneous fungal infection is tinea corporis with superficial dermal inflammation. Deep skin- penetration of fungal agents may induce an inflammatory granulomatous skin reaction first described by Majocchi.[2] From two forms distinguished, the deep subcutaneous form is seen in immunosuppressed individuals. Trichophyton rubrum is commonly associated with Majocchi’s granuloma.[3] Diagnosis is confirmed by skin histology with PAS staining and tissue culture. Systemical treatment is mandatory. The preferred drug is itraconazole because it accumulates in skin appendages.[4]
In the described patient clinical symptoms were refractory to itraconazole for several reasons: Initially the dose of itraconazole was too low and absorption was diminished as itraconazole needs a low gastric pH and a high-fat meal for optimal absorption.[5] Our patient was intermittently on proton- pump inhibitors and was unable to follow dietary recommendations.
As itraconazole inhibits CYP3A4, plasma levels of Tacrolimus rose, enhancing immunosuppression and susceptibility for infections.
In sepsis, treatment with Rifampicin, a strong inducer of CYP3A4, kept itraconazole plasma concentrations below 100 ng/ml in spite of rising the dose and induced a decrease of Tacrolimus plasma levels.
Conclusion
In severely ill patients on multiple drug regimens, careful monitoring and adjustment of prescribed drugs is of vital importance because of drug interactions and different absorption and bioavaiability depending on the application form.
No definitive correlation exists between itraconazole serum levels and efficacy or toxicity.[5] Determination of itraconazole serum levels may provide more information of the threshold level for successful therapy.
References
- Marik PE. Fungal infections in solid organ transplantation. Expert Opin Pharmacother. 2006;7:297–305. doi: 10.1517/14656566.7.3.297. [DOI] [PubMed] [Google Scholar]
- Majocchi D. Sepra una nuova tricofizia (granuloma tricofitico), studi clinici micologici. Bull R Accad Roma. 1883:9220–9223. [Google Scholar]
- Sequeira M, Burdick AE, Elgart GW, Berman B. New-onset Majocchi's granuloma in two kidney transplant recipients under tacrolimus treatment. J Am Acad Dermatol. 1998;38:486–488. doi: 10.1016/s0190-9622(98)70511-0. [DOI] [PubMed] [Google Scholar]
- Stoppie P, Borghgraef P, Borgers M. Cutaneous distribution of orally administered itraconazole in guinea pigs, studied by autoradiography. J Eur Acad Dermatol Venereol. 1994;3:475–478. [Google Scholar]
- Buchkowsky SS, Partovi N, Ensom MH. Clinical pharmacokinetic monitoring of itraconazole is warranted in only a subset of patients. Ther Drug Monit. 2005;27:322–333. doi: 10.1097/01.ftd.0000150135.22645.ea. [DOI] [PubMed] [Google Scholar]


