Abstract
Background
The relationship between compromised immune system and the development of malignancy, generalized dermatitis, and infection after sulfur mustard gas exposure has been established.
Main observation
We introduce a 58-year-old man with an abrupt, de novo and erythrodermic eruption in 2002 that was previously exposed to sulfur mustard during the Iran – Iraq war in 1987. Six weeks after the onset of diffuse eruption, he developed papules on the glans penis and generalized dermatophytosis. A biopsy of his eruption was consistent with cutaneous T-cell lymphoma/Sézary syndrome. A complete blood count demonstrated leukocytosis, eosinophilia and atypical lymphocytosis. Subsequently, Sézary syndrome was confirmed and T-cell count with increased CD4/CD8 in flow cytometry. The biopsy of his penile papules was consistent with Kaposi's sarcoma.
Conclusion
These findings suggest a causative relationship between sulfur mustard gas exposure, cutaneous T cell lymphoma and immune compromised state with opportunistic infections.
Keywords: cutaneous T-cell lymphoma, genotoxins, genital, Kaposi's sarcoma, Sezary syndrome, war
Introduction
Cutaneous T-cell lymphoma (CTCL) comprises a group of clinicopathologic entities that are neoplastic proliferation of T lymphocytes that home to the skin. Eventually the T-cells may lose their dependence on an epidermal environment for growth, and spread to the dermis, lymph nodes, blood, and viscera.[1,2] The cutaneous lesions evolve from patches to plaque and tumors (mycosis fungoides), and Sézary syndrome, where the neoplastic cells circulate in the peripheral blood. The patients present with generalized exfoliation erythroderma, intense pruritus, peripheral lymphadenophaty, and abnormal hyperchromatic mononuclear cells in the skin and peripheral blood.[3] Decreased T-cell function may lead to subsequent immune compromised status and is followed by infection.[4]
Kaposi sarcoma (KS) is a vascular tumor of intermediate malignancy, histologically characterized by proliferation of lymphatic and/or vascular endothelial cells caused by the Kaposi's sarcoma-associated herpesvirus (KSHV), human herpesvirus 8. KS is a systemic disease, which can present with cutaneous lesions with or without internal involvement. Four subtypes have been described: Classic KS, affecting middle aged men of Mediterranean descent, African endemic KS, KS in iatrogenically immunosuppressed patients, and AIDS-related KS.[5]
Sulfur mustard gas is a potent alkylating agent that has a long history of use as a chemical warfare agent, including recent use by Iraq against Iranian soldiers and civilians. The organs most commonly affected by sulfur mustard gas (SM) are skin, eyes, and airways. Skin lesions are seen in more than 90% of the patients exposed to SM. Although the acute systemic and cutaneous effects of SM are well known, few investigations have focused on reporting the long-term carcinogenic effects.[6,7]
Case Report
A 58-year-old farmer developed a generalized, erythrodermic, pruritic eruption of two months duration. His past medical history revealed no prior dermatitis. The lesions started from the lower limbs as erythematous papules and plagues and spread to the whole body within two weeks. During this time he felt chills, fever, malaise, severe itching and lack of appetite. He reported prior exposure to the SM gas 15 years before. After exposure he felt severe pruritus on his skin and burning sensation in the eyes, with coughing and mild respiratory distress accompanied by vomiting. The physicians serving the area managed his acute exposure-related symptoms at the time. After 2 days, his symptoms improved without any lingering untoward effects.
His physical examination revealed multiple, erythematous annular plaques with fine scales on the trunk and the lower and upper limbs [Fig. 1A]. We identified bilateral adenopathy in groin and axilla was identified. His hands and feet showed mild scaling, fissures, bullae and nail dystrophy. The histopathology of biopsied lesions on the extremities showed significant epidermotropism and Pautrier's microabscesses in the absence of any notable spongiosis overlying dermal fibrosis and an atypical lymphocytic infiltrate [Fig. 1B]. Microscopic examination of his peripheral blood showed 10% atypical lymphocytes and Sézary cells [Fig. 1C]. Peripheral blood examination showed 16x10 WBC, Polymorphic cells 29%, lymphocytes 51% and eosinophil cells 20%; all the liver and kidney function tests were normal. The CT scans of the chest, abdomen and pelvic revealed slight splanomegaly and confirmed our exam findings of bilateral lymphadenopathy in groin and axilla. Furthermore, the histological examination his annular plaques scarping and microbiological culture indicated Trichophyton rubrum. Flow cytometry of his peripheral blood revealed CD8 = 2.6%, CD4 = 96.1%, CD3 = 19.5%, CD2 = 78.9%, CD45 = 97.7% and CD7 = 10.2%, CD19 = 1%, CD14 = 4.7%, CD4/CD8 = 45%. All virology test results were negative for HIV, HTLVI and HTLV-II. No antibody titers were identified for either HCV or HBV; HB antigens were negative in two stage.
Figure 1.
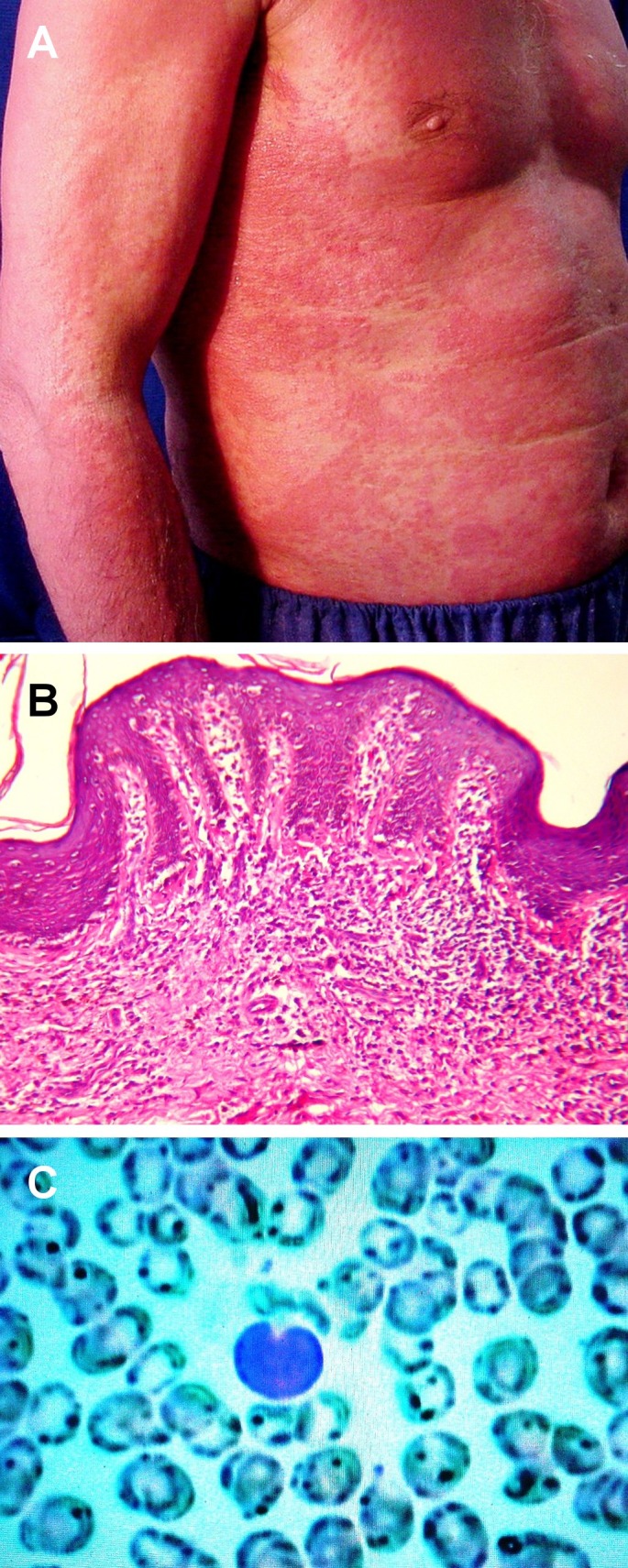
(A) Erythematous annular plaques with fine scales on the trunk and the upper limb. (B) Histological view of epidermotropism and Pautrier's microabscesses in the absence of any notable spongiosis overlying dermal fibrosis and an atypical lymphocytic infiltrate. (H&E 40). (C) Peripheral blood shows atypical lymphocytes and Sézary cells.
The examination of genitalia was remarkable for multiple red, violaceous papules coalescing to an impetigenized plaque with yellow-brown scale crust on his glans penis and sulcus corona. These lesions arose two weeks prior to the examination [Fig. 2A]. The biopsy of such lesion demonstrated a dermal nodular vascular proliferation with spindle slit-like lumina, dilated blood vessels and hemorrhage [Fig. 2B-C]. Ultimately we diagnosed the patient with cutaneous T-cell lymphoma/Sézary syndrome, Kaposi's sarcoma, compromised T-cell immunity and generalized dermatophytosis. The patient was managed accordingly: 1) Ciprofloxacin and clindamycin for 10 days for sepsis, and symptoms of skin infection; 2) Terbinafine tablets and topical clotrimazole for one month to treat dermatophytosis and 3) I.V. Epirubicin and I.M. interferon alpha for three weeks for erythrodermic CTCL and Kaposi sarcoma. The patient died of sepsis and severe cachexia weight loss one year later.
Figure 2.
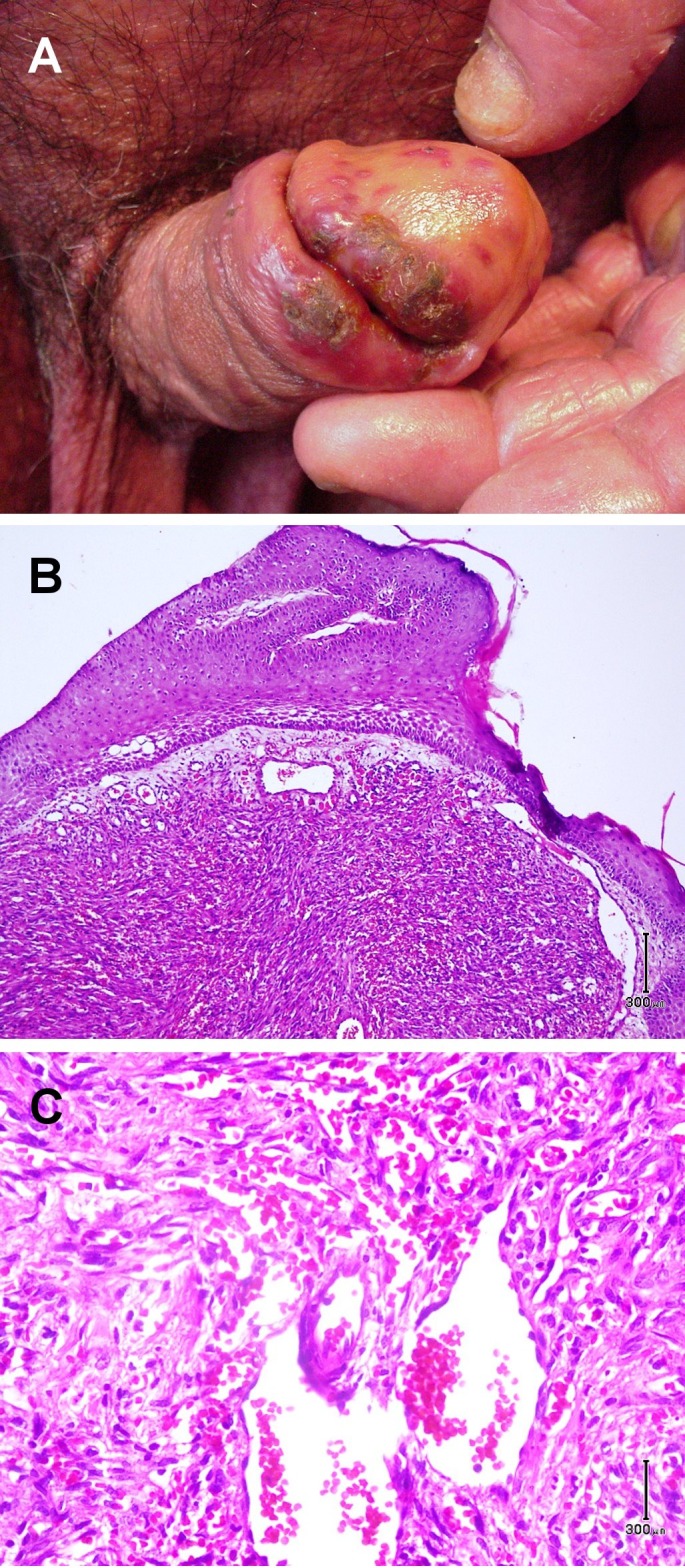
(A) Multiple red, violaceous papules coalescing to an impetigenized plaque with yellow-brown scale crust on the glans of penis and sulcus corona. (B-C) Histological view of nodular vascular proliferation with spindle slit-like lumina, dilated blood vessels and hemorrhage. (H&E 40 and 100).
Discussion
In this case report, we present clinics, histology, serology, virology and flow cytometry results in a patient exposed to SM during the Iran – Iraq war in 1987. This patient showed early and extensive erythrodermic CTCL phase after his documented exposure without prior patches and plaques. Moreover Sézary cells, atypical lymphocytes in peripheral blood and ratio of CD4/CD8 = 45 provide support for his rapid progression of diffuse erythroderma and Sézary syndrome.[8] Sych and Kalamkarian first reported the coexistence of Kaposi sarcoma and mycosis fungoides in 1968.[9] Similarly the coexistence of malignant lymphoma and Kaposi sarcoma has been documented and attributed to immune suppressive drugs.[10] Kaposi sarcoma, previously common among patients with AIDS prior to highly active antiretroviral therapy (HAART), has been rarely documented with T-cell lymphoma.[11,12] In addition, there have been reported cases of mycosis fungoides with fungal infections.[13,14]
Sulfur mustard is an alkylating agent capable of damaging DNA. Thus the carcinogenic and radiomimetic effects seen in victims of SM exposure are not surprising. To date, the number of cutaneous cancers reported subsequent to acute and chronic SM exposure is low, and it is unclear whether some of these cases are related to the carcinogenic effects of SM or are related to the presence of chronic skin ulcers and scars.[5] Previous studies have reported multiple skin tumors (34%), even in unexposed skin, and numerous painful ulcerations (45%) in 53 workers of a World War II German factory after 2 decades of occupational exposure to sulfur mustard gas.[15] In another study occupational exposure to hazardous chemicals was reported in 30% of the patients with mycosis fungoides by a co-operative study group.[16]
Our group has been treating patients exposed to SM during and subsequent to the Iran – Iraq war in 1987 and has documented the long-term follow up of such cutaneous exposures. These include cutaneous malignant neoplasms in 9 patients: 5 cases of basal cell carcinoma and 1 case each of squamous cell carcinoma, Bowen disease, dermatofibrosarcoma protuberans, and mycosis fungoides (tumoral). In all of these patients except 2 cases of basal cell carcinoma, the skin neoplasm developed on the scar of previous SM induced lesions.[6]
We report herein a unique case of de novo erythrodermic CTCL subsequent to SM, a known genotoxin, exposure accompanied by compromised T-cell function leading to the development of Kaposi sarcoma and an opportunistic generalized dermatophytosis.
Conclusion
This case provides circumstantial relationship between sulfur mustard exposure and the development of CTCL.
Acknowledgments
We acknowledge Soheil Sam Dadras to review this article.
References
- Braun-Falco O, Plewig G, Wolff HH, Burgdorf W. Dermatology: with 281 tables. 2th, completely rev. ed. Berlin: Springer; 2000. pp. 1617–1620. [Google Scholar]
- Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH. et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- Arndt KA, LeBoitt PE, Robinson JK, Cutaneous medicine and surgery. Philadelphia: WB Saunders Company; 1996. pp. 1639–1641. [Google Scholar]
- Vonderheid EC, Bernengo MG, Burg G, Duvic M, Heald P, Laroche L. et al. Update on erythrodermic cutaneous T-cell lymphoma: report of the International Society for Cutaneous Lymphomas. J Am Acad Dermatol. 2002;46:95–106. doi: 10.1067/mjd.2002.118538. [DOI] [PubMed] [Google Scholar]
- Lemlich G, Schwam L, Lebwohl M. Kaposi's sarcoma and acquired immunodeficiency syndrome. Postmortem findings in twenty-four cases. J Am Acad Dermatol. 1987;16:319–325. doi: 10.1016/s0190-9622(87)70043-7. [DOI] [PubMed] [Google Scholar]
- Emadi SN, Mortazavi M, Mortazavi H. Late cutaneous manifestations 14 to 20 years after wartime exposure to sulfur mustard gas: a long-term investigation. Arch Dermatol. 2008;144:1059–1061. doi: 10.1001/archderm.144.8.1059. [DOI] [PubMed] [Google Scholar]
- Emadi SN, Moeineddin F, Sorush MR. Urinary and cutaneous complications of sulphur mustard poisoning preceding pulmonary and ocular involvement: an unusual sequence of symptoms. Clin Exp Dermatol. 2009;34:e7–10. doi: 10.1111/j.1365-2230.2008.02965.x. [DOI] [PubMed] [Google Scholar]
- Harmon CB, Witzig TE, Katzmann JA, Pittelkow MR. Detection of circulating T cells with CD4+CD7- immunophenotype in patients with benign and malignant lymphoproliferative dermatoses. J Am Acad Dermatol. 1996;35(3 Pt 1):404–410. doi: 10.1016/s0190-9622(96)90605-2. [DOI] [PubMed] [Google Scholar]
- Kalamkarian AA, Sych LI. A case of coexistence of mycosis fungoides and Kaposi's sarcoma. Vestn Dermatol Venerol. 1968;42:12–16. [PubMed] [Google Scholar]
- Beylot C, Beylot J, Veyret V, Bioulac P, Broustet A, Bauduceau B, Moretti G. Kaposi sarcoma and malignant lymphoma. Ann Dermatol Venereol. 1977;104:817–823. [PubMed] [Google Scholar]
- Longacre TA, Foucar K, Koster F, Burgdorf W. Atypical cutaneous lymphoproliferative disorder resembling mycosis fungoides in AIDS. Report of a case with concurrent Kaposi's sarcoma. Am J Dermatopathol. 1989;11:451–456. doi: 10.1097/00000372-198910000-00007. [DOI] [PubMed] [Google Scholar]
- Waiters RW, Soler AP, Selim MA. Kaposi sarcoma presenting as yellow-green penile plaques in a black man with HIV. Cutis. 2011;88:14–16. [PubMed] [Google Scholar]
- Chave TA, Graham-Brown RA. Mycosis fungoides masquerading as tinea of the axilla. Clin Exp Dermatol. 2002;27:66–67. doi: 10.1046/j.0307-6938.2001.00939.x. [DOI] [PubMed] [Google Scholar]
- Hull PR, Saxena A. Mycosis fungoides and chronic lymphocytic leukaemia--composite T-cell and B-cell lymphomas presenting in the skin. Br J Dermatol. 2000;143:439–444. doi: 10.1046/j.1365-2133.2000.03679.x. [DOI] [PubMed] [Google Scholar]
- Pearson GS. Veterans at Risk: The Health Effects of Mustard Gas and Lewisite, edited by Constance M. Pechura and David P. Rall. Nature. 1993;365:218. doi: 10.1038/365218a0. [DOI] [PubMed] [Google Scholar]
- Fischmann AB, Bunn PA Jr, Guccion JG, Matthews MJ, Minna JD. Exposure to chemicals, physical agents, and biologic agents in mycosis fungoides and the Sézary syndrome. Cancer Treat Rep. 1979;63:591–596. [PubMed] [Google Scholar]


