Abstract
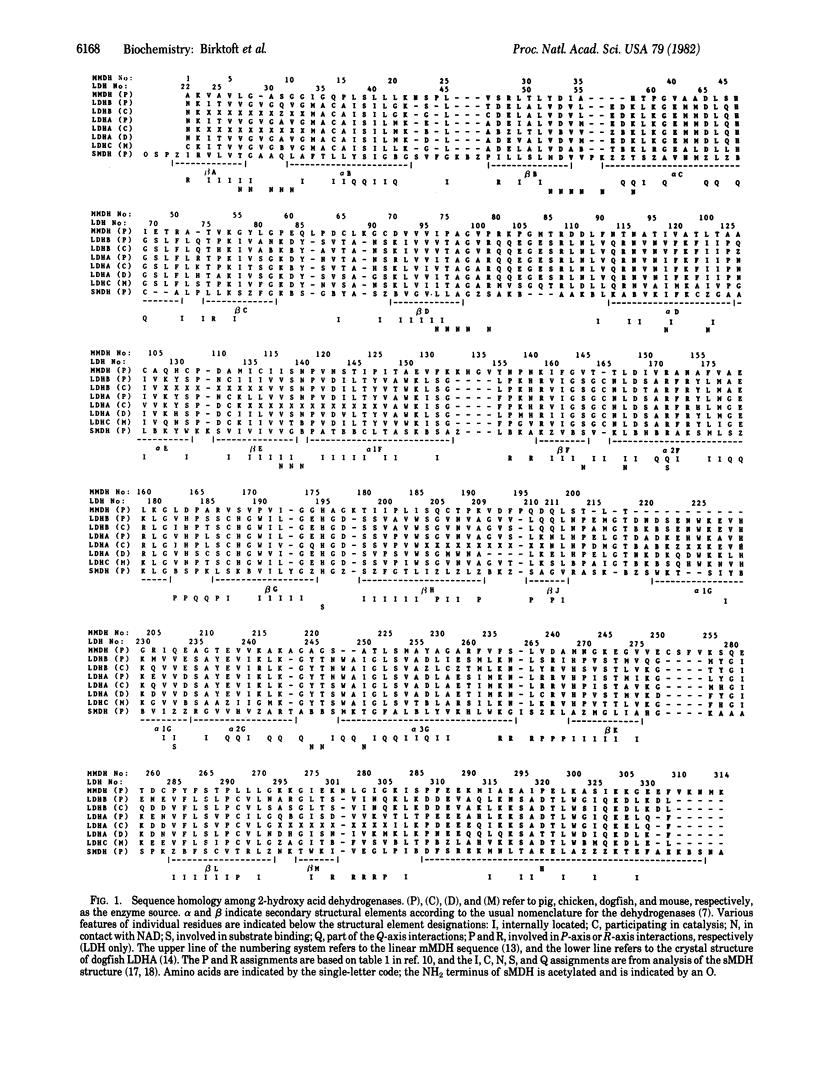
The amino acid sequence of porcine heart mitochondrial malate dehydrogenase (mMDH; L-malate: NAD+ oxidoreductase, EC 1.1.1.37) has been compared with the sequences of six different lactate dehydrogenases (LDH; L-lactate: NAD+ oxidoreductase, EC 1.1.1.27) and with the "x-ray" sequence of cytoplasmic malate dehydrogenase (sMDH). The main points are that (i) all three enzymes are homologous; (ii) invariant residues in the catalytic center of these enzymes include a histidine and an internally located aspartate that function as a proton relay system; (iii) numerous residues important to coenzyme binding are conserved, including several glycines and charged residues; and (iv) amino acid side chains present in the subunit interface common to the MDHs and LDHs appear to be better conserved than those in the protein interior. It is concluded that LDH, sMDH, and mMDH are derived from a common ancestral gene and probably have similar catalytic mechanisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A. A. Separation of rat lactate dehydrogenase isoenzyme C4 from other isoenzymes by affinity and ion-exchange chromatography. Biochem J. 1981 Oct 1;199(1):75–79. doi: 10.1042/bj1990075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz L. E., Chien S. M., Patel H. V., Freeman K. B. A putative precursor of rat liver mitochondrial malate dehydrogenase. FEBS Lett. 1981 Oct 12;133(1):127–129. doi: 10.1016/0014-5793(81)80487-5. [DOI] [PubMed] [Google Scholar]
- Barry C. D., Bosshard H. E., Ellis R. A., Marshall G. R. Evolving macromodular molecular modeling system. Fed Proc. 1974 Dec;33(12):2368–2372. [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Bleile D. M., Schulz R. A., Harrison J. H., Gregory E. M. Investigation of the subunit interactions in malate dehydrogenase. J Biol Chem. 1977 Jan 25;252(2):755–758. [PubMed] [Google Scholar]
- Doolittle R. F. Similar amino acid sequences: chance or common ancestry? Science. 1981 Oct 9;214(4517):149–159. doi: 10.1126/science.7280687. [DOI] [PubMed] [Google Scholar]
- Eventoff W., Rossmann M. G., Taylor S. S., Torff H. J., Meyer H., Keil W., Kiltz H. H. Structural adaptations of lactate dehydrogenase isozymes. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2677–2681. doi: 10.1073/pnas.74.7.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everse J., Kaplan N. O. Lactate dehydrogenases: structure and function. Adv Enzymol Relat Areas Mol Biol. 1973;37:61–133. doi: 10.1002/9780470122822.ch2. [DOI] [PubMed] [Google Scholar]
- Ford G. C., Eichele G., Jansonius J. N. Three-dimensional structure of a pyridoxal-phosphate-dependent enzyme, mitochondrial aspartate aminotransferase. Proc Natl Acad Sci U S A. 1980 May;77(5):2559–2563. doi: 10.1073/pnas.77.5.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau U. M., Trommer W. E., Rossmann M. G. Structure of the active ternary complex of pig heart lactate dehydrogenase with S-lac-NAD at 2.7 A resolution. J Mol Biol. 1981 Sep 15;151(2):289–307. doi: 10.1016/0022-2836(81)90516-7. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A., Love W. E. Structure of lamprey haemoglobin. Nat New Biol. 1971 Aug;232(33):197–203. doi: 10.1038/newbio232197a0. [DOI] [PubMed] [Google Scholar]
- Hill E., Tsernoglou D., Webb L., Banaszak L. J. Polypeptide conformation of cytoplasmic malate dehydrogenase from an electron density map at 3.0 angstrom resolution. J Mol Biol. 1972 Dec 30;72(3):577–589. doi: 10.1016/0022-2836(72)90176-3. [DOI] [PubMed] [Google Scholar]
- Holbrook J. J., Wolfe R. G. Malate dehydrogenase. X. Fluorescence microtitration studies of D-malate, hydroxymalonate, nicotinamide dinucleotide, and dihydronicotinamide-adenine dinucleotide binding by mitochondrial and supernatant porcine heart enzymes. Biochemistry. 1972 Jun 20;11(13):2499–2502. doi: 10.1021/bi00763a018. [DOI] [PubMed] [Google Scholar]
- James M. N., Delbaere L. T., Brayer G. D. Amino acid sequence alignment of bacterial and mammalian pancreatic serine proteases based on topological equivalences. Can J Biochem. 1978 Jun;56(6):396–402. doi: 10.1139/o78-062. [DOI] [PubMed] [Google Scholar]
- Kun E., Volfin P. Tissue specificity of malate dehydrogenase isozymes. Kinetic discrimination by oxaloacetate and its mono- and difluoro analogues. Biochem Biophys Res Commun. 1966 Jan 24;22(2):187–193. doi: 10.1016/0006-291x(66)90430-x. [DOI] [PubMed] [Google Scholar]
- Lederer F., Glatigny A., Bethge P. H., Bellamy H. D., Matthew F. S. Improvement of the 2.5 A resolution model of cytochrome b562 by redetermining the primary structure and using molecular graphics. J Mol Biol. 1981 Jun 5;148(4):427–448. doi: 10.1016/0022-2836(81)90185-6. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D. Repeating sequences and gene duplication in proteins. J Mol Biol. 1972 Mar 14;64(2):417–437. doi: 10.1016/0022-2836(72)90508-6. [DOI] [PubMed] [Google Scholar]
- Musick W. D., Rossmann M. G. The tentative amino acid sequencing of lactate dehydrogenase C4 by X-ray diffraction analysis. J Biol Chem. 1979 Aug 25;254(16):7621–7623. [PubMed] [Google Scholar]
- Pan Y. C., Huang S., Marciniszyn J. P., Jr, Lee C. Y., Li S. S. The preliminary amino acid sequence of mouse testicular lactate dehydrogenase. Hoppe Seylers Z Physiol Chem. 1980 May;361(5):795–799. [PubMed] [Google Scholar]
- Rudolph R., Heider I., Westhof E., Jaenicke R. Mechanism of refolding and reactivation of lactic dehydrogenase from pig heart after dissociation in various solvent media. Biochemistry. 1977 Jul 26;16(15):3384–3390. doi: 10.1021/bi00634a015. [DOI] [PubMed] [Google Scholar]
- Rupley J. A., Forster L. S., Torikata T., Johnson R. E., O'Neal C. C., Jr Comparison of lactate and malate dehydrogenases: fluorescence and thermodynamic properties. Biochem Biophys Res Commun. 1980 Apr 14;93(3):654–660. doi: 10.1016/0006-291x(80)91128-6. [DOI] [PubMed] [Google Scholar]
- Webb L. E., Hill E. J., Banaszak L. J. Conformation of nicotinamide adenine dinucleotide bound to cytoplasmic malate dehydrogenase. Biochemistry. 1973 Dec 4;12(25):5101–5109. doi: 10.1021/bi00749a013. [DOI] [PubMed] [Google Scholar]
- Wimmer M. J., Mo T., Sawyers D. L., Harrison J. H. Biphasic inactivation of procine heart mitochondrial malate dehydrogenase by pyridoxal 5'-phosphate. J Biol Chem. 1975 Jan 25;250(2):710–715. [PubMed] [Google Scholar]
- Wood D. C., Hodges C. T., Howell S. M., Clary L. G., Harrison J. H. The N-ethylmaleimide-sensitive cysteine residue in the pH-dependent subunit interactions of malate dehydrogenase. J Biol Chem. 1981 Oct 10;256(19):9895–9900. [PubMed] [Google Scholar]



