Abstract
Macaques are commonly used in biomedical research as animal models of human disease. The ABO phenotype of donors and recipients plays an important role in the success of transplantation and stem cell research of both human and macaque tissue. Traditional serological methods for ABO phenotyping can be time consuming, provide ambiguous results and/or require tissue that is unavailable or unsuitable. We developed a novel method to detect the A, B, and AB phenotypes of macaques using real-time quantitative PCR. This method enables the simple and rapid screening of these phenotypes in macaques without the need for fresh blood or saliva. This study reports the distribution of the A, B, and AB phenotypes of captive cynomolgus macaques that, while regionally variable, closely resembles that of rhesus macaques. Blood group B, as in rhesus macaques, predominates in cynomolgus macaques and its frequency distribution leads to a probability of major incompatibility of 41%. No silencing mutations have been identified in exons 6 or 7 in macaques that could be responsible for the O phenotype, that, although rare, have been reported. The excess homozygosity of rhesus and cynomolgus macaque genotypes in the present study, that assumes the absence of the O allele, suggests the possibility of some mechanism preventing the expression of the A and B transferases.
Keywords: Macaca fascicularis, Macaca mulatta, long-tailed (cynomolgus) and rhesus macaques, ABO typing
INTRODUCTION
Nonhuman primates, and macaques in particular, have played an important role in the advancement of biomedical science (1, 2). The human and macaque genomes are 93% identical, and the two species exhibit close similarity in their physiology, neurobiology and susceptibility to infectious and metabolic diseases (1). For these reasons, macaques provide good models for the study of many human diseases. Cynomolgus macaques (Macaca fascicularis) are being increasingly used in reproductive medicine and especially transplantation research (3, 4).
Knowledge of the ABO phenotypes of potential donor and recipient macaques involved in transplantation studies is required to determine compatibility and avoid rejection of tissue. ABO typing in macaques has been performed using reverse typing and saliva inhibition protocols because ABH antigens are not found on the surface of macaque red blood cells (RBCs) (5). Unfortunately, fresh blood and saliva of sufficient quantity and quality are not always available and, when available, do not always provide reliable results. For example, researchers sometimes neglect to absorb macaque sera with human type O red blood cells (RBCs) to remove species-specific heteroglutinins before reverse typing them with human A and B RBCs. In addition, some diseases, such as leukemia, can lead to methylation of CpG islands in the ABO promoter region, inhibiting transcription (6). Both circumstances can lead to false positive results and underestimates of the O phenotype. Moreover, the concentration of ABH antigens or antibodies in tissues is highly variable and, in the case of antibodies, decline with age (7), occasionally providing ambiguous agglutination results. These ambiguities are exacerbated by the weak secretor status of most macaques, potentially leading to the erroneous assignment of O phenotypes.
The coupling of sequence-specific priming and real-time quantitative PCR (qPCR) provides a powerful tool for genotyping single-nucleotide polymorphisms (SNPs) (8). These assays are easy to design, can be run quickly, and avoid multi-step procedures like end-point PCR and electrophoresis. Quantitative PCR also can detect genomic DNA at concentrations lower than 1ng (9) or DNA from samples that have been stored for long periods of time, both of which would preclude traditional serological testing of many saliva and serum samples. Elsewhere we have described a novel multiplex PCR method for determining the ABO phenotype of rhesus macaques (10). This multiplex reaction determines the presence of mutations in exon 7 of the ABO gene on macaque chromosome 15 that are required for transcription of the A or B transferase (10). The ABI Custom TaqMan® SNP Genotyping Assay was used to design primers and probes to develop this assay inclusive of the mutations used by Premasuthan et al. (10).
MATERIALS AND METHODS
Eighty-nine blood samples from cynomolgus macaques (Macaca fascicularis) obtained from Primate Products International (Immokalee, FL) were analyzed. The blood samples were collected and shipped to the Molecular Anthropology Laboratory (MAL), University of California, Davis, complying with the standard operating procedures for handling and transporting biological hazardous agents at the University of California, Davis. The 89 blood samples tested included: 10 from southern Sumatra, 20 from Vietnam, 20 from Mauritius, 19 from southwestern Mindanao, Philippines, and 20 from Cambodia. DNA was extracted from these blood samples using the QIAamp DNA Blood Mini Kit following the manufacturer’s protocol. The extracted DNA was amplified using the exon 6 and 7 forward and reverse primers, cloned and sequenced using the same steps described in Premasuthan et al. (10). Reference sequences of cynomolgus macaques used to design primers and probes were those published on the National Center for Biotechnology Information (NCBI-http://www.ncbi.nlm.nih.gov), GenBank accession numbers- AH008220.0 (Cy*O101), AF052078.1 (Cy*A102), AF052079.1 (Cy*A103), AF052082.1 (Cy*B101), and AF052083.1 (CyB*102). Reference sequences of rhesus macaques were taken from GenBank accession numbers AF094693.1, AF052084.1 (Rh*B101), AF052086.1 (Rh*B103), AF052085.1 (Rh*B102), and AF052080.1 (Rh*A101). The reference sequence of the complete rhesus macaque ABO gene was obtained from the annotated rhesus genome assembly (build rheMac2) and human ABO reference sequences were from the annotated human genome (build hg19). The reference sequences and sequenced data obtained from Premasuthan et al. (10) were aligned using Seaview 4.0 (11) and Sequencher 4.9 (Gene Codes corporation, Ann Arbor, MI) to identify the mutations associated with the A, B, and AB phenotypes.
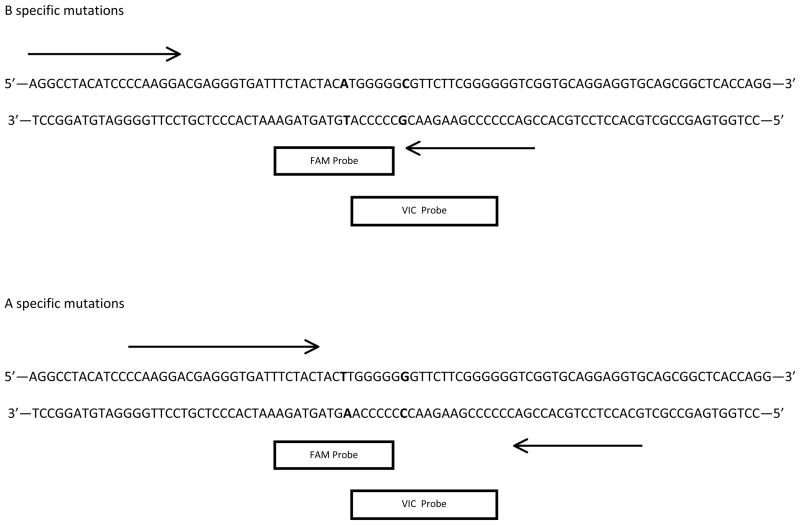
Once the functional mutations were identified on exon 7, the Applied Biosystems (Foster City, CA) online Custom TaqMan ® Assay Design tool- TaqMan® SNP Genotyping Assay was used to design primers and probes similar to the method described in Premasuthan et al. (10). The forward and reverse primers (Table 1) were identical for both rhesus and cynomolgus macaques. The probe sequences were specific to the cynomolgus macaque A- and B-specific coding regions and were ordered as MGB probes in specific fluorescent dye colors (FAM and VIC) with no fluorescent quencher. An illustration of the primer and probe locations on the ABO locus is provided in Figure 1. The amino acids at these two positions are required for expression of the A and B transferases in all cercopithecoid primates (12). The A-specific and B-specific primers and probes target these mutations. The A-specific probe is complementary to the CTT and GGG codons for leucine and glycine at amino acid positions 266 and 268, respectively, while the B-specific primer targets the ATG and CGG codons at those positions that code for methionine and alanine.
TABLE 1.
Primers and probes in qPCR reagent mixtures used to detect the A and B phenotypes
| B-specific Oligos | ||
| Forward primer | AGGCCTACATCCCCAAGGA | |
| Reverse primer | CCGACCCCCCGAAGAAC | |
| Probe_VIC | VIC | CCCCCATGTAGTAGAA |
| Probe_FAM | FAM | CCGAAGAACGCCCCCA |
| A-specific Oligos | ||
| Forward primer | CAAGGACGAGGGTGATTTCTACTAC | |
| Reverse primer | GCACCTCCTGCACCGA | |
| Probe_VIC | VIC | CCCGAAGAACCCCCCCA |
| Probe_FAM | FAM | CCCCCAAGTAGTAGAA |
Figure 1.
Annealing sites of primers (denoted by arrows) and probes of the B and A specific mutations (denoted by boxes) at the ABO locus.
Each reaction included 1 ng of template DNA, 12.5 μL of TaqMan® Universal PCR Master Mix, No AmpEraseR UNG (Applied Biosystems), 1.25μL of 20X Custom TaqMan® SNP Genotyping Assay (Applied Biosystems; Assay IDs: AHD1TXQ and AHFAR3Y for positions 266 and 268, respectively), and ddH2O was added for a total reaction volume of 25μL. Each sample was run in duplicate for each position for a total of 4 reactions per sample. The qPCR assay was run on a 7300 Real Time PCR System (Applied Biosystems) following the Allelic Discrimination assay run and analysis protocols in the Applied Biosystems Allelic Discrimination Getting Started Guide (13). The amplification conditions are as follows: Pre-read hold at 60°C for 1 minute, a hold at 95°C for 15 minutes followed by 40 cycles of 92°C for 15 seconds and 60°C for 1 minute, and a post-read hold at 60°C for 1 minute.
The ABO phenotypes of the cynomolgus macaques reported here were compared with those for rhesus macaques previously reported by Premasuthan et al. (10). Phenotype frequencies were estimated for each species and regional population by allele counting, assuming the absence of the O allele. Each population was tested for Hardy-Weinberg (HW) equilibrium at the 0.05 level of probability using a chi-squared test for goodness of fit of the observed and expected numbers of A, B, and AB phenotypes [with two degrees of freedom (df) and Yate’s correction for continuity (14)]. The distributions of ABO phenotypes in rhesus and cynomolgus macaques were compared with each other using a contingency chi-square test for homogeneity with two degrees of freedom at the 0.05 level of probability. The probability of randomly selecting a donor/recipient pair exhibiting major incompatibility (IC) was computed for both the rhesus and cynomolgus macaque populations as IC = 2 (fAfB) + fAB(fA + fB) + fO(fA + fB + fAB), where fA, fB, fAB and fO are the frequencies of the phenotypes A, B, AB and O, respectively.
RESULTS
Of the 89 samples analyzed, 18 were type A, 43 were type B, and 28 were type AB (Table 2). The frequency distributions of the two species were not statistically significantly different from each other (χ2=0.22). However, the observed phenotypes of both all rhesus (χ2= 9.83) and all cynomolgus (χ2 =7.92) macaques differed from those expected under HW equilibrium conditions at the 0.01 level of probability (Table 2). Most populations and all populations combined for both species exhibited excesses of both homozygous genotypes (AA and BB).
TABLE 2.
Origin, sample size, number of individuals with each phenotype, and the estimates of probability of randomly selecting incompatible donor/recipient pairs (IC) among rhesus and cynomogus macaques.
| Origin | Phenotype Frequency | IC | χ2 | ||
|---|---|---|---|---|---|
| A | B | AB | |||
| Indian (N = 31) | 3 | 16 | 12 | 0.34 | 0.01 |
| Chinese (N = 47) | 13 | 24 | 10 | 0.45 | 12.50* |
| All Rhesus Macaques (N = 78) | 16 | 40 | 22 | 0.40 | 9.83* |
| Sumatra (N=10) | 3 | 4 | 3 | 0.45 | 0.60 |
| Vietnam (N=20) | 3 | 7 | 10 | 0.46 | 0.04 |
| Philippines (N=19) | 2 | 14 | 3 | 0.17 | 2.08 |
| Cambodia (N=20) | 4 | 10 | 6 | 0.41 | 1.32 |
| Mauritius (N=20) | 6 | 8 | 6 | 0.45 | 2.04 |
| All Cynomolgus Macaques (N = 89) | 18 | 43 | 28 | 0.41 | 7.92* |
An asterisk marks χ2 distributions that exceed the critical value for significance at 0.05 level of probability.
The mean frequencies of the A and B alleles were very similar in the two species, assuming the absence of the O allele: the frequencies of the A allele in rhesus and cynomolgus macaques were 0.35 and 0.37 and those of the B allele were 0.65 and 0.63, respectively. The A allele in rhesus macaques reached its highest frequency in China (0.38) while in cynomolgus macaques the A allele was most common in Sumatra and Mauritius (0.45 in both). The B allele was highest in frequency in Indian rhesus macaques (0.71) and Philippine cynomolgus macaques (0.79). The random probability of major incompatibility (IC) was highest for Chinese rhesus (0.45) and Sumatran (0.45) and Vietnamese (0.46) cynomolgus macaques and lowest for Indian rhesus (0.34) and Philippine cynomolgus (0.17) macaques (Table 2).
DISCUSSION
The qPCR-based ABO phenotyping assay reported here detects the presence of the DNA coding sequence specific to A and B transferases in both rhesus and cynomolgus macaques. The B phenotype was more common than the A phenotype in both the rhesus and cynomolgus macaques but varied by region of origin within species. Yamamoto et al. (15) reported the presence of a mutation responsible for the O blood group in exon 6 of rhesus chromosome 15, but did not specify its exact nucleotide position. Others have reported the presence of blood group O in macaques based on serological tests described above (16, 17). However, DNA sequences generated by our laboratory for rhesus and cynomolgus macaques alleged to possess phenotype O in other studies lack any mutations in exon 6 or exon 7 that could be responsible for an O (null) phenotype (10, 12, 18).
As reported elsewhere (10) and by others (12, 18), the DNA sequences generated in this study did not reveal any mutations in either exon 6 or 7 that could potentially lead to an O phenotype. If such a mutation exists, it lies outside exons 6 and 7, which comprise the great majority of the locus, and might represent mutations at the donor or receptor splice sites of the last intron or epigenetic mechanisms such as methylation of the promoter region. While the systematic excess of homozygous genotypes in both species could have resulted from sampling alone, it suggests the possible presence of an O allele. However, if the allele is relatively rare (19), it would influence the presence or absence of an A or B transferase only in the even rarer homozygous form mooting its relevance for transplantation research.
The data presented in this study differ from serological data reported by Malaivijitnond et al. (16) in that our rhesus macaque sample exhibited a higher frequency of the B phenotype and no population exhibited evidence of an O phenotype. Doxiadis et al. (12) demonstrated that individuals with A, B, and AB phenotypes identified through reverse typing also possessed the appropriate diagnostic mutations targeted in the present study when sequenced. Phenotypes based on the saliva inhibitory test (SIT) were more frequently discordant with DNA sequences at the ABO locus, presumably because of the insensitivity of the SIT to small amounts of antigen. As in our own study, Doxiadis et al. (12) found no mutations associated with the O allele or phenotype in exons 6 or 7. Because previous studies have often not been able to confirm the presence of O phenotypes assigned using SIT by performing reverse typing, it is possible that reports of the O phenotype in macaques result from the insensitivity of the SIT to relatively small quantities of the A and/or B antigens in the saliva. This hypothesis is supported by the fact that most macaques are only weak secretors of the ABH antigens, expressing both the Lewis a and the Lewis b antigens (20). While SNPs that identify the O allele in macaques with the O phenotype were reported to have been found in exon 6 (15), the functional mutations responsible for the phenotype, which in humans create a stop codon that prevents the addition of polysaccharides to the H antigen (5, 15), have not been identified in subsequent studies, including the present one.
The ABO phenotyping assay described above is a rapid, reliable, and inexpensive method that is also robust and sensitive. The method uses DNA rather than blood, eliminating the requirement of fresh samples and complications involved in the storage and handling of blood. As observed in the rhesus macaques, the B phenotype is prevalent in cynomolgus macaques. Because the frequency distributions of both rhesus and cynomolgus macaques lead to a high probability of major incompatibility, studies of transplantation involving either species should ensure compatibility of ABO phenotypes of donor and recipient research subjects. Our conclusions regarding regional variation in phenotype frequencies need to be confirmed using larger population samples of both rhesus macaques and cynomolgus macaques. Further studies should focus on identifying the presence of one or more mutations responsible for an O allele outside the coding region.
Acknowledgments
We are grateful to all the facilities listed in Kanthaswamy et al. (21) that have contributed samples used in this study. This study was supported by National Institutes of Health grants R24RR005090 and R24RR025871 to DGS and National Institute of Justice grant 2008-DN-BX-K288 to SK. We also thank two anonymous reviewers for their insightful comments.
Footnotes
The authors declare no conflict of interests.
References
- 1.Gibbs RA, Rogers J, et al. Rhesus Macaque Genome Sequencing Analysis Consortium. Evolutionary and Biomedical Insights from the Rhesus Macaque Genome. Science. 2007;316:222–34. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- 2.Fultz PN. Nonhuman Primate Models for AIDS. Clinical Infectious Diseases. 1993;17:S230–S5. doi: 10.1093/clinids/17.supplement_1.s230. [DOI] [PubMed] [Google Scholar]
- 3.West NB, Brenner RM. Estrogen receptor levels in the oviducts and endometria of cynomolgus macaques during the menstrual cycle. Biology of Reproduction. 1983;29:1303–12. doi: 10.1095/biolreprod29.5.1303. [DOI] [PubMed] [Google Scholar]
- 4.Karl J, Wiseman R, Campbell K, et al. Identification of MHC class I sequences in Chinese-origin rhesus macaques. Immunogenetics. 2008;60:37–46. doi: 10.1007/s00251-007-0267-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Socha WW, Ruffie J. Blood groups of primates: Theory, practice, evolutionary meaning. New York: Alan R. Liss, Inc; 1983. [Google Scholar]
- 6.Bianco-Miotto T, Hussey DJ, Day TK, O’Keefe DS, Dobrovic A. DNA Methylation of the ABO Promoter Underlies Loss of ABO Allelic Expression in a Significant Proportion of Leukemic Patients. PLoS ONE. 2009;4:e4788. doi: 10.1371/journal.pone.0004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terao K, Fujimoto K, Cho F, Honjo S. Anti-A and anti-B blood group antibody levels in relation to age in cynomolgus monkeys. Japanese journal of medical science & biology. 1983;36:289–93. doi: 10.7883/yoken1952.36.289. [DOI] [PubMed] [Google Scholar]
- 8.Tobler AR, Short S, Andersen MR, et al. The SNPlex genotyping system: a flexible and scalable platform for SNP genotyping. Journal of biomolecular techniques : JBT. 2005;16:398–406. [PMC free article] [PubMed] [Google Scholar]
- 9.Barr A, Premasuthan A, Satkoski J, Smith DG, George D, Kanthaswamy S. A Rapid Quantitative Real-Time PCR-Based DNA Quantification Assay Coupled with Species Assignment Capabilities for Two Hybridizing Macaca Species. Folia Primatologica. 2011;82:71–80. doi: 10.1159/000328124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Premasuthan A, Kanthaswamy S, Satkoski J, Smith DG. A simple multiplex polymerase chain reaction to determine ABO blood types of rhesus macaques (Macaca mulatta) Tissue Antigens. 2011;77:584–8. doi: 10.1111/j.1399-0039.2010.01602.x. [DOI] [PubMed] [Google Scholar]
- 11.Gouy M, Guindon S, Gascuel O. SeaView Version 4: A Multiplatform Graphical User Interface for Sequence Alignment and Phylogenetic Tree Building. Molecular Biology and Evolution. 2010;27:221–4. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- 12.Doxiadis GGM, Otting N, Antunes SGM, et al. Characterization of the ABO blood group genes in macaques: evidence for convergent evolution. Tissue Antigens. 1998;51:321–6. doi: 10.1111/j.1399-0039.1998.tb02970.x. [DOI] [PubMed] [Google Scholar]
- 13.Applied Biosystems. Allelic Discrimination Getting Started Guide for 7300/7500/7500 Fast Systems. Applied Biosystems; 2010. [Google Scholar]
- 14.Cassens BJ. NMS Preventive Medicine and Public Health. 2. USA: Lippincott Williams & Wilkins; 1992. [Google Scholar]
- 15.Yamamoto F-i, Clausen H, White T, Marken J, Hakomori S-i. Molecular genetic basis of the histo-blood group ABO system. Nature. 1990;345:229–33. doi: 10.1038/345229a0. [DOI] [PubMed] [Google Scholar]
- 16.Malaivijitnond S, Sae-Low W, Hamada Y. The human-ABO blood groups of free-ranging long-tailed macaques (Macaca fascicularis) and parapatric rhesus macaques (M. mulatta) in Thailand. Journal of Medical Primatology. 2008;37:31–7. doi: 10.1111/j.1600-0684.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 17.Farkas T, Cross RW, Hargitt E, Lerche NW, Morrow AL, Sestak K. Genetic Diversity and Histo-Blood Group Antigen Interactions of Rhesus Enteric Caliciviruses. Journal of Virology. 2010;84:8617–25. doi: 10.1128/JVI.00630-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kermarrec N, Roubinet F, Apoil P-A, Blancher A. Comparison of allele O sequences of the human and non-human primate ABO system. Immunogenetics. 1999;49:517–26. doi: 10.1007/s002510050529. [DOI] [PubMed] [Google Scholar]
- 19.Kimura M. Rare variant alleles in the light of the neutral theory. Molecular Biology and Evolution. 1983;1:84–93. doi: 10.1093/oxfordjournals.molbev.a040305. [DOI] [PubMed] [Google Scholar]
- 20.Linden S, Mahdavi J, Semino-Mora C, et al. Role of ABO Secretor Status in Mucosal Innate Immunity and H. pylori Infection. PLoS Pathog. 2008;4:e2. doi: 10.1371/journal.ppat.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanthaswamy S, Satkoski J, George D, Kou A, Erickson B, Smith D. Hybridization and Stratification of Nuclear Genetic Variation in Macaca mulatta and M. fascicularis. International Journal of Primatology. 2008;29:1295–311. doi: 10.1007/s10764-008-9295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]