Abstract
Objective
To test the feasibility and preliminary effectiveness of the SB-PACT program, which includes directly observed therapy of preventive asthma medications in school facilitated by web-based technology for systematic symptom screening, electronic report generation, and medication authorization from providers.
Study design
We conducted a pilot randomized trial of SB-PACT vs. usual care with 100 children (ages 3-10yrs) from 19 inner-city schools in Rochester, NY. Outcomes were assessed longitudinally by blinded interviewers. Analyses included bivariate statistics and linear regression models, adjusting for baseline symptoms.
Results
99 subjects had data for analysis. We screened all children using the web-based system, and 44/49 treatment children received directly observed therapy as authorized by their providers. Treatment children received preventive medications 98% of the time they were in school. Over the school year, children in the treatment group experienced nearly 1 additional symptom-free day/two weeks vs. usual care (11.33 vs. 10.40,p=.13). Treatment children also experienced fewer symptom nights (1.68 vs. 2.20,p=.02), days requiring rescue medications (1.66 vs. 2.44,p=.01) and days absent from school due to asthma (.37 vs. .85,p=.03) compared with usual care. Further, treatment children had a greater decrease in exhaled nitric oxide (−9.62 vs. −.39,p=.03), suggesting reduction in airway inflammation.
Conclusion
The SB-PACT intervention demonstrated feasibility and improved outcomes across multiple measures in this pilot study. Future work will focus on further integration of preventive care delivery across community and primary care systems.
Keywords: asthma, schools, technology, urban, preventive care
Asthma is a chronic disease characterized by inflammation in the airways. Inhaled corticosteroids are the most effective long-term treatment for patients with persistent asthma and the NHLBI Expert Panel guidelines recommend that all patients with persistent asthma receive daily inhaled corticosteroid therapy.1 These medications reduce asthma symptoms, improve pulmonary function, and prevent exacerbations leading to hospitalizations2 when used as recommended. In addition, once medications are prescribed, the guidelines recommend follow-up assessments in 4-6 weeks, with adjustments in therapy as needed, to assure the goals of therapy are met.1
Despite these clear and well-developed guidelines for care, little has been done to assure implementation of the guidelines. Many children in the US with persistent asthma symptoms do not receive preventive medications.3-5 In addition, many children who are prescribed a preventive medication do not achieve optimal control, at least in part due to poor adherence and lack of appropriate follow-up care.6 Importantly, the greatest under-use of preventive medications and lack of appropriate asthma care occurs among poor children living in the inner-city.7
We have developed a unique program of school-based asthma care designed to improve adherence to preventive asthma care guidelines and reduce morbidity for poor and minority children with persistent symptoms.8, 9 Our previous intervention, the School Based Asthma Therapy Trial (SBAT) 2006-2009, included directly observed administration of preventive asthma medications in school, with guideline-based medication dose adjustments for children who continued to have poor control. This program was successful in reducing asthma morbidity;10 however, in its original form the intervention required substantial hands-on participation by the study team to screen children for persistent symptoms and to assure appropriate medications were authorized, prescribed, and delivered to schools for directly observed therapy.
We subsequently developed the School-Based Preventive Asthma Care Technology (SB-PACT) trial, which utilizes a web-based program to overcome key barriers to sustainability identified in the original study. Our goal was to develop a novel mechanism for the implementation of sustainable school-based asthma care in a real-world setting. This paper presents primary outcomes of the SB-PACT pilot study, focusing on the feasibility and preliminary effectiveness of the intervention on asthma morbidity, including symptom-free days, quality of life, absenteeism, and urgent health care use.
METHODS
The University of Rochester Institutional Review Board approved all study procedures. At the beginning of the 2009-2010 school year, children 3-10 years of age attending school in the Rochester (NY) City School District were screened for eligibility. All schools in the RCSD (n=39) agreed to participate. We recruited a convenience sample of 100 children into the study from 19 schools (on average 5 in each school). Children were identified by school medical-alert forms, which are available to school health staff at the start of the school year and include a list of children with an asthma diagnosis. The school nurse or school health aide (with assistance from the study team, as needed) conducted a brief survey with the child’s caregivers using a secure web-based platform to assess the child’s eligibility.
Eligible children had physician-diagnosed asthma with persistent symptoms based on the NHLBI guidelines.1 Children were excluded if their caregiver was unable to speak and understand English, if they had no access to a working phone for follow-up surveys, if they were planning to leave the school district within fewer than 6 months, or if the child had any other significant medical conditions, including congenital heart disease, cystic fibrosis, or other chronic lung disease, that could interfere with the assessment of asthma-related measures.
Once a child was deemed eligible, the study team scheduled a baseline home visit with the family to obtain written informed consent from the parent and assent from children ≥7 years. The baseline evaluation included an assessment of asthma symptoms, standard family and health history variables, and exposure to secondhand smoke. An asthma symptom diary, developed using the school calendar, was given to the caregiver for tracking of asthma symptoms throughout the school year. We also obtained a saliva sample from each child for cotinine concentration to measure secondhand smoke exposure. Lastly, we obtained exhaled nitric oxide measurements from each child using a portable NIOX MINO machine, in order to objectively measure airway inflammation. Enrollment occurred in a rolling fashion, beginning in October of the school year.
Following completion of the baseline assessment, each child was randomly assigned to either the SB-PACT group or the usual care group. Randomization was stratified by the use of a preventive asthma medication at baseline. A permutated block design was used to assure an equal balance of children in each group over time. The randomization scheme was independently developed by the Biostatistics Center; the interviewer called the Study Coordinator who provided the subject’s ID number and treatment assignment.
SB-PACT Group
Program Overview
The SB-PACT intervention includes several key steps: (1) systematic web-based screening to assess children’s asthma using guideline-based symptom questions along with an algorithm to compute an NHLBI severity or control classification; (2) report generation and electronic communication with PCPs for authorization of directly observed therapy of preventive asthma medications through school; (3) prescription of guideline-based preventive medications which are purchased through the child’s health insurance and delivered to schools and children’s homes by a local pharmacy; (4) directly observed administration of medications at school by a school nurse or health aide; and (5) systematic reassessment of symptoms using the same system, with guideline-based adjustments in therapy as needed. We also incorporated 0.3 FTE support from an Asthma Care Coordinator (ACC) to facilitate communication between school health staff, healthcare providers and caregivers. The ACC is a registered nurse with additional training in childhood asthma. Further details of the program are presented elsewhere.11
Study Processes
The ACC reviewed the screening data and transmitted an electronic asthma report to the PCP which included a recommendation for directly observed therapy at school. The PCP was then prompted to approve a prescription for a preventive asthma medication that was ordered through one of a number of pharmacies that provide delivery services, and agree to monitor the child for potential side effects. One canister of preventive medication, with a spacer and mask (if indicated), was delivered to the family at home. The family used this inhaler for medication doses on weekend days and other days in which the child did not attend school. A second medication canister with a spacer and mask was delivered to the child’s school for use on school days. School health staff administered one dose of medication to the child during the school day. The school nurse showed children how to use medications properly and instructed them to rinse their mouth with water after each dose. We also provided written instructions on inhaler technique to families, with demonstration when requested. Even though adherence to medication administration was assured by school health staff on the days the child attended school, adherence was encouraged but not assured on days the child did not attend school.
All children in the study had persistent asthma symptoms and/or poor asthma control upon enrollment, and thus warranted the use of a daily preventive asthma medication according to the NHLBI guidelines. The starting medication administered through the study varied depending on the child’s baseline asthma therapy; some children began a new preventive medication, others continued with a previously prescribed medication or were stepped-up in their therapy. The Asthma Care Coordinator reviewed all caregiver reported preventive medications (if any) prior to the start of the study, and made a recommendation to the PCP. The PCP then authorized the recommended medication (or could provide authorization for an alternate medication) to be administered as directly observed therapy through school. Most children received once-daily dosing because it is effective12 and allows for medication administration during school hours. If more frequent dosing was needed, additional doses were taken at home.
Assessment for possible medication dose adjustments occurred during the school year approximately 1 and 2 months after the start of directly observed therapy. Symptom information was collected by the same web-based mechanism, which was relayed electronically to the Asthma Care Coordinator, and medication adjustments were made for children who continued to have poor control. Information regarding possible changes in the child’s regimen was relayed to the child’s PCP and the family, and both parties had to agree prior to implementation of the adjusted dose.
Medication recommendations were based on the NHLBI guidelines for asthma care with the assessments for adjustments in therapy corresponding to peak asthma season. The schema did not include any step-down in therapy because all of the children had persistent symptoms at the start of the trial and could benefit from several months of anti-inflammatory therapy. A natural time for discontinuing or stepping-down therapy occurred at the end of the school year when the children no longer were receiving medications through school. Two weeks prior to the close of the study, we notified physicians and families that children who were receiving preventive medications at school would no longer be receiving these medications through the study. PCPs were encouraged to contact families to manage medicines directly.
Usual Care Group
Similar to children receiving the SB-PACT intervention, children in the usual care group were screened for eligibility using the online screening tool at the beginning of the school year, but reports were not sent to their PCPs and directly observed therapy was not implemented in school. At the time of the baseline visit, we encouraged parents of children in the usual care group to contact their child’s provider to further discuss their child’s symptoms and need for enhanced preventive care. Families were responsible for bringing their child to the provider’s office for a visit and for filling prescriptions and administering medications as prescribed. We provided families of children in both groups with written educational handouts on asthma triggers, treatments, and local asthma resources.
Outcomes Assessment
The intervention continued until the end of the school year, which varied from 6-8 months depending on the timing of enrollment for each child. We assessed feasibility by the success in: 1) enrolling and maintaining study participants (% agreeing to participate, % completing intervention), and 2) the implementation of the program in schools (efficiency of the delivery of medication canisters to the schools, % days children receive medications in school). We obtained medication administration records from school nurses to assess consistency of medication delivery through school.
Clinical outcomes were assessed at 1, 2, and 4 months post-baseline via telephone interviews, and an in-home visit at the end of school year (approx. 6-8 months). All follow-up data were collected by a research group blinded to the child’s group allocation. The primary outcome was mean symptom-free days over two weeks, averaged over the study period. Caregivers reported the number of days their child experienced no symptoms of asthma (24 hour period with no coughing, wheezing, shortness of breath, or need for rescue medications) over the past 2 weeks. They were referred to their symptom diaries at the time of the interview to assist with symptom recollection.
We also measured the number of days and nights with symptoms, activity limitation, rescue medication use, school absenteeism, parent sleep interruption, and change in family plans due to the child’s asthma over the prior 2 weeks. Caregiver quality of life was assessed using the previously validated Pediatric Asthma Caregiver’s Quality of Life Questionnaire (PACQLQ)13, with higher mean scores indicating better quality of life (range 1-7). Healthcare utilization was obtained during each survey by asking caregivers about the child’s urgent (office and ED visits, hospitalizations) and non-urgent visits for asthma care since the previous interview. Exhaled nitric oxide was obtained as an objective measure of airway inflammation, during the baseline assessment and final follow-up assessments. We used a portable NIOX MINO Airway Inflammation Monitor, which measures forced exhaled nitric oxide using the electrochemistry method. Children exhale into the device at a steady rate for 6-10 seconds (range 5-300 ppb).
In addition, we measured standard variables known to influence asthma outcomes, including demographic variables (race, sex, ethnicity, insurance, caregiver’s age and education level), medical variables (allergy), and caregiver depression using the Kessler Psychological Distress scale.14 We also measured smoke exposure by caregiver interview,15 and obtained salivary cotinine (at baseline and the final assessment) using a standardized technique.16, 17
At the end of the school year, we administered a structured survey with Likert-scale questions to assess feasibility and acceptability of this program from caregivers, school nurses and providers. For caregivers in the treatment group, we asked about their comfort with asthma care delivered through school and their impressions of the school nurse’s role in the program. Physicians provided feedback on the feasibility of authorizing medications through the web-based SB-PACT system and the value of directly observed therapy in schools. School nurses expressed their opinions on the feasibility of providing asthma care through the school system and whether they perceived that this program provided a health benefit for the children in the treatment group.
Analyses
Because this was designed as a pilot study with limited power, we consider analyses exploratory. All randomized subjects were kept in their originally assigned groups for analysis. Demographic variables and baseline outcomes were compared with confirm balance between randomized groups. We calculated mean values over the 4 asssessment points. To test for differences between the groups on clinical and functional outcomes, we used two-sample t-tests or the Mann-Whitney tests for continuous outcome variables (e.g.; symptom-free days, symptom nights), and chi-square tests for discrete response variables (acute visits, hospitalizations). We adjusted for baseline symptoms in the primary data analysis using linear regression models.
RESULTS
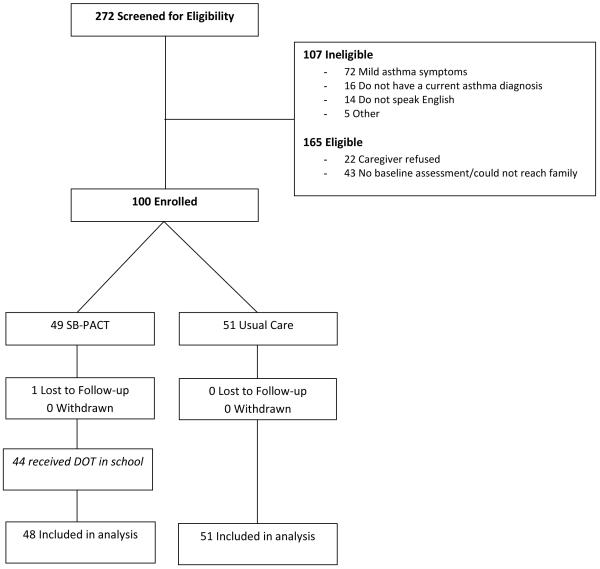
We identified 165 eligible children with asthma and 100 were enrolled (response rate: 61%). No subjects withdrew from the study; one subject in the treatment group was lost to follow-up prior to any follow-up data collection (Figure; available at www.jpeds.com). As previously described, we were able to successfully screen all children using the web-based system. Most (44/49) children randomly assigned to the intervention group began directly observed therapy in school as authorized by their providers (1 child was lost to follow-up, 3 providers did not authorize directly observed therapy at school, and 1 parent chose to administer all medication doses at home). Initial medications were purchased by the study team for 2 families who were underinsured at the start of the study. The majority of providers (82%) used the electronic communication system and the remainder requested documents by facsimile. Medications were delivered to the schools by the pharmacy for most subjects (65%), or were either delivered by the asthma coordinator or picked up by the caregiver. Directly observed therapy was successfully initiated for all of these children, and children received their medications 98% of the time they were in school.
Figure.
Consort Diagram
There were no differences in demographic characteristics, asthma symptoms or exhaled nitric oxide between study groups at baseline (Tables I and II). Overall, 58% of children were male, 57% were African American, 26% Hispanic, and the mean age was 7.2 years. The majority of children (70%) were covered by Medicaid insurance, 42% of caregivers had less than a high school education, and 58% of the children lived with one or more smoker. The mean number of symptom free-days at baseline, over two weeks, was 7.32 days.
Table 1. Demographics.
| N (%) | Overall N=99 |
Treatment N=48 |
Control N=51 |
P-value |
|---|---|---|---|---|
| Child sex: Male | 57 (58%) | 25 (52%) | 32 (63%) | .314 |
|
| ||||
| Child age, Mean (SD) | 7.20 (1.8) | 7.48 (1.7) | 6.98 (1.8) | .157 |
|
| ||||
| Child race: | ||||
| White | 7 (7%) | 3 (6%) | 4 (8%) | .512 |
| African American | 56 (57 %) | 30 (62%) | 26 (51 %) | |
| Other | 36 (36%) | 15 (31%) | 21 (41%) | |
|
| ||||
| Child ethnicity: Hispanic | 26 (26%) | 12 (25%) | 14 (28%) | .823 |
|
| ||||
| Medicaid Insurance | 69 (70%) | 33 (69%) | 36 (71%) | 1.000 |
|
| ||||
| Child has allergies | 51 (52%) | 27 (56%) | 24 (47%) | .423 |
|
| ||||
| Caregiver age: <30yrs | 39 (39%) | 21 (44%) | 18 (35%) | .417 |
|
| ||||
| Caregiver: Single | 71 (72 %) | 37 (77%) | 34 (67%) | .273 |
|
| ||||
| Caregiver education: Less than high school | 42 (42%) | 21 (44%) | 21 (41%) | .841 |
|
| ||||
| Caregiver is depressed | 37 (37%) | 13 (27%) | 24 (47%) | .061 |
|
| ||||
| Caregiver smokes | 43 (43%) | 20 (41%) | 23 (45%) | .691 |
|
| ||||
| Smokers in home: Yes | 58 (58%) | 27 (55%) | 31 (61%) | .686 |
|
| ||||
| Cotinine (ng/ml), Mean (SD) | 2.72 (3.0) | 2.54 (2.6) | 2.89 (3.4) | .580 |
Table 2. Baseline Asthma Symptoms, Quality of Life, and Exhaled Nitric Oxide.
| Overall N=99 |
Treatment N=48 |
Control N=51 |
P-value | |
|---|---|---|---|---|
| Symptom Free Days ^ | 7.32 (4.8) | 7.58 (4.9) | 7.14 (4.7) | .683 |
| Symptom Days ^ | 4.31 (4.3) | 4.54 (4.4) | 4.10 (4.3) | .559 |
| Symptom Nights ^ | 4.33 (4.6) | 4.58 (5.0) | 3.98 (4.2) | .744 |
| Days of Activity Limitation ^ | 2.82 (3.7) | 2.88 (3.7) | 2.82 (3.7) | .934 |
| Days of Rescue Med Use ^ | 3.58 (4.4) | 4.27 (4.9) | 2.90 (3.8) | .246 |
| Days Parent Lost Sleep ^ | 2.10 (3.5) | 2.42 (3.9) | 1.84 (3.2) | .901 |
| Days Family Changed Plans ^ | .64 (1.6) | .90 (2.2) | .41 (0.8) | .760 |
| Days Absent from School due to asthma ^ | .52 (1.1) | .46 (1.0) | .55 (1.2) | .877 |
| Quality of Life (range 1-7) | 6.03 (1.0) | 6.25 (0.8) | 5.82 (1.2) | .085 |
| Exhaled Nitric Oxide (ppb) | 22.34 (22.1) | 25.33 (26.0) | 19.66 (17.8) | .222 |
Mann-Whitney Test for non-parametric data, shown as Mean (SD)
Number of days reported over 2 weeks (range 0-14)
Table III summarizes the primary outcomes by group, controlling for baseline asthma symptoms. Over the school year, children in the treatment group experienced nearly 1 additional symptom free day every two weeks compared with the usual care group. In addition, children in the treatment group experienced fewer nights with symptoms, fewer days requiring rescue medication use and fewer days absent from school due to asthma. Parents of children in the treatment group reported fewer days requiring the family to change their plans to accommodate the child’s asthma and fewer nights that they lost sleep due to the child’s asthma. Further, children in the treatment group had a greater decrease in exhaled nitric oxide from baseline to the final assessment compared with children in the usual care group.
Table 3. Primary Outcomes: Asthma Symptoms, Quality of Life, and Exhaled Nitric Oxide.
| Treatment N=48 Mean (SD) |
Control N=51 Mean (SD) |
95% CI of Beta | *P-value | |
|---|---|---|---|---|
| Symptom Free Days ^ | 11.33 (2.6) | 10.40 (3.4) | −.283, 2.035 | .137 |
| Symptom Days ^ | 1.68 (2.0) | 2.20 (2.2) | −1.374, .261 | .180 |
| Symptom Nights ^ | 1.52 (1.8) | 2.34 (2.2) | −1.675, −.126 | .023 |
| Days of Activity Limitation ^ | 1.21 (1.6) | 2.04 (2.5) | −1.679, −.028 | .043 |
| Days of Rescue Med Use ^ | 1.66 (2.0) | 2.44 (2.6) | −1.950, −.252 | .012 |
| Days Parent Lost Sleep ^ | .59 (1.0) | 1.29 (1.8) | −1.302, −.185 | .010 |
| Days Family Changed Plans ^ | .12 (0.4) | .39 (0.8) | −.542, −.047 | .020 |
| Days Absent from School due to asthma ^ | .37 (0.7) | .85 (1.3) | −.901, −.036 | .034 |
| Quality of Life (range 1-7) | 6.46 (0.7) | 6.31 (0.9) | −.304, .326 | .945 |
| Change in Exhaled Nitric Oxide (ppb) | −9.62 (22.2) | −.39 (14.9) | −17.690, −.773 | .033 |
Individual linear regression analyses control for symptoms reported at baseline.
Number of days reported over 2 weeks (range 0-14)
Healthcare utilization throughout the school year is shown in Table IV. There were no differences between the proportion of children presenting for healthcare visits between treatment groups. Throughout the study period, nearly 40% in both groups discussed asthma at a visit with their physician, and 20% went to the emergency room or their doctor’s office for an acute asthma exacerbation. One child in each group was hospitalized during the school year. There were no reports of adverse events in either group.
Table 4. Health Care Utilization.
| Overall N=99 |
Treatment N=48 |
Control N=51 |
P-value | |
|---|---|---|---|---|
| Any Doctor Visit where Asthma was discussed | 40 (40%) | 17 (35%) | 23 (45%) | .314 |
| Any Doctor Visit for an Asthma Follow-up | 15 (15%) | 4 (8%) | 11 (22%) | .092 |
| Any Doctor Visit for an Asthma Exacerbation | 14 (14%) | 6 (12%) | 8 (16%) | .775 |
| Any ER visit related to Asthma | 7 (7%) | 4 (8%) | 3 (6%) | 1.00 |
| Any visit for an Acute Asthma Exacerbation | 20 (20%) | 9 (19%) | 11 (22%) | .804 |
| Any Hospitalization | 2 (2%) | 1 (2%) | 1 (2%) | 1.000 |
At the end of the study, all of the responding providers (n=25) reported that they felt directly observed therapy of preventive asthma medications was beneficial for urban children with persistent asthma, and all providers with children in the treatment group (n=16) reported feeling comfortable authorizing preventive asthma therapy through this program. Among caregivers whose children participated in the treatment group, most felt that it was easy to work with the school system (91%), were comfortable with the school nurse providing daily preventive asthma therapy (94%), and felt that the school nurse did a good job helping to manage their children’s asthma (91%). The majority of responding school nurses (12 of 13) stated that they would like to see the SB-PACT program continue in their schools, 85% felt that it was feasible to continue this program, and none felt it was a burden to administer daily preventive medicine to their students.
The majority of school nurses also felt that the program improved communication between school nurses and parents, and that students participating in this program were healthier and missed less school. Many nurses stated the program helped the children to consistently receive their daily medications through directly observed therapy and prevented interruption in therapy by having the pharmacy deliver the medications to school instead of requiring parents to pick up medications and deliver them.
DISCUSSION
This pilot study of school-based asthma care demonstrated feasibility as well as preliminary effectiveness in reducing morbidity for high-risk children with asthma. As intended, the web-based screening mechanism worked efficiently for most participants, the majority of PCPs used the electronic communication system, and medications were delivered systematically. Importantly, children receiving the intervention experienced fewer symptoms, less absenteeism from asthma, and reduced airway inflammation. Although the small sample size limited the power of the analyses, the improvements seen were similar in magnitude to the findings from our prior studies and differences in several outcomes reached statistical significance. These results suggest that this integrated model of care, designed to promote sustainability, can effectively reduce morbidity among high risk inner-city children with asthma.
This study tests an integrated system of preventive medication delivery and asthma assessment in schools. It is clear from prior studies that poor adherence to preventive care guidelines is common, particularly for underserved children. The Chronic Care Model18 suggests that interventions incorporating new mechanisms of care based in community settings are particularly effective for vulnerable populations of patients. Schools represent the ideal location to target children to improve the delivery of care for chronic illness because of the potential to reach large numbers of children and optimize their care in the setting where they routinely spend many of their days. Further, collaborations with schools provide the opportunity to reach high-risk children and target those in greatest need of assistance, regardless of whether they receive regular health care.8
Several prior studies have tested school-based programs for urban children with asthma. The majority have focused on asthma self-management education for students and their caregivers.19-22 In addition, education programs have also been implemented in Head Start settings, targeting both staff and parents to improve care for underserved children with asthma.23, 24 A few studies have specifically tested directly observed therapy in schools, and have shown positive effects.25,26 We are not aware of any other studies testing the implementation of a school-based asthma care intervention using secure web-based technology for communication and medication authorization, which links to directly observed therapy through school.
This study’s strengths lie in its ability to target a traditionally underserved group and work within an existing system of care to improve preventive medication adherence. We found that many of the barriers in the original study were overcome with a web-based mechanism for asthma screening, control monitoring, report generation, and medication authorization. Although some challenges in implementation were encountered,11 in general we found the system to be feasible and efficient. Caregivers, providers, and school health staff expressed general satisfaction with the program and the majority stated that this program should continue within the school district.
There are some limitations to this study. First, because this was designed as a pilot study, we had limited power to detect differences between groups, particularly for less common events such as emergency visits and hospitalizations. We did not have adequate power for subgroup analyses. Children in the control group may have had improved outcomes simply from their participation in the trial, creating a conservative bias. Further, findings from this work can only be generalized to similar target populations and school districts. Many school districts in the US are facing significant financial strains and have limited resources, thus additional responsibilities for school personnel to implement a program like this may not be feasible in some settings. However, even in schools that do not have full-time health personnel, daily medication administration for other conditions (e.g. attention deficit disorder) occurs regularly. Thus, the provision of daily preventive asthma medications could be a simple and logical system change to improve adherence. Further, the web-based system for asthma screening was specifically designed to promote sustainability by being user-friendly and efficient, and most schools have internet capabilities.
In conclusion, we found that the SB-PACT intervention was feasible, acceptable, and improved outcomes across multiple measures in this pilot study. The ultimate goal is to promote diffusion of an efficient system of care throughout schools, optimize access to effective healthcare, and reduce morbidity among high-risk children in urban communities. Because partnering with schools represents an ideal means to reach impoverished, underserved children with asthma and improve preventive care, future work will focus on further refinement of the program with full integration across community and primary care systems and evaluation of cost-effectiveness.
ACKNOWLEDGMENTS
We would like to thank Andrew MacGowan, Donna Hill, PhD, Flora McEntee, RN, the school-nurse program and the Rochester City School District for their ongoing partnership and support of our work. We would also like to acknowledge SophiTEC, Inc for their partnership in the development of the web-based technology used in this study. Lastly, we would like to thank Alison Yee for her assistance in preparing this manuscript and the SB-PACT study team for their limitless energy to help children with asthma.
Funded by the National Heart, Lung and Blood Institute of the National Institutes of Health (RC1HL099432).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Registered at ClinicalTrials.gov: NCT01175434
The authors declare no conflicts of interest.
REFERENCES
- 1.National Asthma Education and Prevention Program . Expert panel report III: guidelines for the diagnosis and management of asthma. National Institute of Health, National Heart, Lung, and Blood Institute; Bethesda, MD: 2007. publication No. 07-4051. [Google Scholar]
- 2.Wennergren G, Kristjansson S, Strannegard IL. Decrease in hospitalization for treatment of childhood asthma with increased use of antiinflammatory treatment, despite an increase in prevalence of asthma. J Allergy Clin Immunol. 1996 Mar;97(3):742–748. doi: 10.1016/s0091-6749(96)80150-3. [DOI] [PubMed] [Google Scholar]
- 3.Halterman JS, Aligne CA, Auinger P, McBride JT, Szilagyi PG. Inadequate therapy for asthma among children in the United States. Pediatrics. 2000 Jan;105(1 Pt 3):272–276. [PubMed] [Google Scholar]
- 4.Bauman LJ, Wright E, Leickly FE, et al. Relationship of adherence to pediatric asthma morbidity among inner-city children. Pediatrics. 2002 Jul;110:e6–e6. doi: 10.1542/peds.110.1.e6. [DOI] [PubMed] [Google Scholar]
- 5.Vargas PA, Rand CS. A pilot study of electronic adherence monitoring in low-income, minority children with asthma. Am J Respir Crit Care Med. 1999;159:A260. [Google Scholar]
- 6.Halterman JS, Auinger P, Conn KM, Lynch K, Yoos HL, Szilagyi PG. Inadequate therapy and poor symptom control among children with asthma: findings from a multistate sample. Ambul Pediatr. 2007 Mar-Apr;7(2):153–159. doi: 10.1016/j.ambp.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Akinbami LJ, LaFleur BJ, Schoendorf KC. Racial and income disparities in childhood asthma in the United States. Ambul Pediatr. 2002 Sep-Oct;2(5):382–387. doi: 10.1367/1539-4409(2002)002<0382:raidic>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Halterman JS, Borrelli B, Fisher S, Szilagyi P, Yoos L. Improving care for urban children with asthma: design and methods of the School-Based Asthma Therapy (SBAT) trial. J Asthma. 2008 May;45(4):279–286. doi: 10.1080/02770900701854908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halterman JS, Szilagyi PG, Yoos HL, et al. Benefits of a school-based asthma treatment program in the absence of secondhand smoke exposure: results of a randomized clinical trial. Arch Pediatr Adolesc Med. 2004 May;158(5):460–467. doi: 10.1001/archpedi.158.5.460. [DOI] [PubMed] [Google Scholar]
- 10.Halterman J, Szilagyi P, Fisher S, et al. A randomized controlled trial to improve care for urban children with asthma: results of the School-Based Asthma Therapy trial. Arch Pediatr Adolesc Med. 2011;165:262–268. doi: 10.1001/archpediatrics.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halterman J, Sauer J, Fagnano M, et al. Working toward a sustainable system of asthma care: development of the School-Based Preventive Asthma Care Technology (SB-PACT) Trial. J Asthma. doi: 10.3109/02770903.2012.669441. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaForce CF, Pearlman DS, Ruff ME, et al. Efficacy and safety of dry powder fluticasone propionate in children with persistent asthma. Ann Allergy Asthma Immunol. 2000 Nov;85(5):407–415. doi: 10.1016/S1081-1206(10)62556-2. [DOI] [PubMed] [Google Scholar]
- 13.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in the parents of children with asthma. Qual Life Res. 1996 Feb;5(1):27–34. doi: 10.1007/BF00435966. [DOI] [PubMed] [Google Scholar]
- 14.Kessler RC, Barker PR, Colpe LJ, et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry. 2003 Feb;60(2):184–189. doi: 10.1001/archpsyc.60.2.184. [DOI] [PubMed] [Google Scholar]
- 15.Matt GE, Wahlgren DR, Hovell MF, et al. Measuring environmental tobacco smoke exposure in infants and young children through urine cotinine and memory-based parental reports: empirical findings and discussion. Tob Control. 1999 Autumn;8(3):282–289. doi: 10.1136/tc.8.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernert JT, Jr., McGuffey JE, Morrison MA, Pirkle JL. Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. J Anal Toxicol. 2000 Jul-Aug;24(5):333–339. doi: 10.1093/jat/24.5.333. [DOI] [PubMed] [Google Scholar]
- 17.Willers S, Axmon A, Feyerabend C, Nielsen J, Skarping G, Skerfving S. Assessment of environmental tobacco smoke exposure in children with asthmatic symptoms by questionnaire and cotinine concentrations in plasma, saliva, and urine. J Clin Epidemiol. 2000 Jul;53(7):715–721. doi: 10.1016/s0895-4356(99)00212-7. [DOI] [PubMed] [Google Scholar]
- 18.Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998 Aug-Sep;1(1):2–4. [PubMed] [Google Scholar]
- 19.Clark NM, Brown R, Joseph CL, Anderson EW, Liu M, Valerio MA. Effects of a comprehensive school-based asthma program on symptoms, parent management, grades, and absenteeism. Chest. 2004 May;125(5):1674–1679. doi: 10.1378/chest.125.5.1674. [DOI] [PubMed] [Google Scholar]
- 20.Evans D, Clark NM, Feldman CH, et al. A school health education program for children with asthma aged 8-11 years. Health Educ Q. 1987;14:267–279. doi: 10.1177/109019818701400302. [DOI] [PubMed] [Google Scholar]
- 21.Shah S, Peat JK, Mazurski EJ, et al. Effect of peer led programme for asthma education in adolescents: cluster randomized controlled trial. Bmj. 2001;322:583. doi: 10.1136/bmj.322.7286.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tinkelman DG, Schwartz A. School-Based Asthma Disease Management. J Asthma. 2004;41:455–462. doi: 10.1081/jas-120033988. [DOI] [PubMed] [Google Scholar]
- 23.Huss K, Winkelstein M, Calabrese B, et al. Asthma management practices and education needs of head start directors and staff. J Sch Health. 2002 Oct;72(8):329–333. doi: 10.1111/j.1746-1561.2002.tb07918.x. [DOI] [PubMed] [Google Scholar]
- 24.Nelson BW, Clark NM, Valerio MA, Houle CR, Brown RW, Brown C. Working with a Head Start population with asthma: lessons learned. J Sch Health. 2006 Aug;76(6):273–275. doi: 10.1111/j.1746-1561.2006.00111.x. [DOI] [PubMed] [Google Scholar]
- 25.Gerald L, McClure L, Mangan JM, et al. Increasing adherence to inhaled steroid therapy among schoolchildren: Randomized, controlled trial of school-based supervised ashtma therapy. Pediatrics. 2009;123:466–474. doi: 10.1542/peds.2008-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McEwen M, Johnson P, Neatherlin J, Millard M, Lawrence G. School-based management of chronic asthma among inner-city African-American school children in Dallas, Texas. J Sch Health. 1998;68(5):196–201. doi: 10.1111/j.1746-1561.1998.tb01300.x. [DOI] [PubMed] [Google Scholar]