Abstract
Bleomycin (BLM) is an example of an anticancer drug that should be delivered into cytosol for its efficient therapeutic action. With this in mind, we developed octaarginine (R8)-modified fusogenic DOPE-liposomes (R8-DOPE-BLM). R8-modification dramatically increased (up to 50-fold) the cell-liposome interaction. R8-DOPE-liposomes were internalized via macropinocytosis and did not end up in the lysosomes. R8-DOPE-BLM led to a significantly stronger cell death and DNA damage in vitro relative to all controls. R8-DOPE-BLM demonstrated a prominent anticancer effect in the BALB/c mice bearing 4T1 tumors. Thus, R8-DOPE-BLM provided efficient intracellular delivery of BLM leading to strong tumor growth inhibition in vivo.
Keywords: Bleomycin, octaarginine, liposomes
1. Introduction
The therapeutic application of many anticancer drugs is limited, or not effective, because of their poor cellular uptake, lack of specifity toward tumor tissues and the ability of cancer cells to develop resistance to chemotherapeutical agents. So far, multiple attempts have been made to develop a tumor-targeted pharmaceutical carrier with the ability to provide an effective cellular internalization of a delivered anticancer drug directly into the cell cytoplasm, bypassing the endocytic pathways, while protecting drugs from lysosomal degradation, thus enhancing the drug efficacy [1]. Liposomes, artificial phospholipid vesicles, have been considered a promising platform for development of such delivery system for well over two decades [2]. Still, the problem of liposomal drug delivery inside cancer cells to intracellular targets such as mitochondria or nuclei remains to be resolved.
One of the well-established anticancer drugs used in chemotherapy against lymphomas, squamous-cell carcinomas and germ-cell tumors is bleomycin (BLM), a glycopeptide antibiotic produced by the bacterium Streptomyces verticillus [3]. Numerous data indicate that DNA damage is the major contributor to the cytotoxicity of BLM [4; 5]. BLM causes 43-oxidation exclusively in single- and double-strand DNA due to the formation of complexes with iron (Fe II) that reduce molecular oxygen to superoxide and hydroxyl radicals, which cause single- and double-stranded breaks in DNA [6]. BLM is a relatively large (1.5 kDa) and hydrophilic molecule unable to diffuse efficiently across cell membranes resulting in its low intracellular concentrations [7]. The treatment with increased concentrations of BLM, however, leads to lung fibrosis, which occurs in up to 46% of the patients [8].
Previously, many attempts have been made to increase therapeutical efficacy of BLM using liposomal encapsulation [9; 10; 11; 12]. However, the most obvious problem in using liposomes for the delivery of anticancer compounds such as BLM, which causes DNA damage, and thus should first delivered to the cytosol, is that liposomes are internalized by cells via endocytosis and destined mostly to lysosomes for degradation [13]. Thus, an effective liposome-based delivery system should provide not only effective intracellular transport of a drug, but also bypass the endosome-lysosomal compartment.
Fusogenic liposomes containing pH-sensitive polyanionic polymers and lipids, such as dioleoyl phosphatidylethanolamine (DOPE) and cholesterylhemisuccinate (CHEMS) are frequently used to provide liposome-mediated drug delivery into a cytoplasmic compartment due to their endosomal escape [14]. Liposomes composed of fusogenic lipids are stable at physiological pH but undergo certain structural transitions under acidic conditions leading to disruption of the endosomal membrane followed by the release of the aqueous liposomal contents into the cytoplasm [15; 16].
Another well-known approach to facilitate cell-interaction and intracellular delivery is the modification of drugs or drug carriers with cell-penetrating peptides (CPPs). CPPs, such as octaarginine (R8), are a large group of positively charged cationic peptides known to facilitate cell interaction and internalization [17; 18]. The R8-modification of fusogenic liposomes has already been shown to be a promising strategy for the intracellular delivery of DNA [19], siRNA [20] and some proteins [21]. However, the potential anticancer properties of R8-modified DOPE-liposomes loaded with a chemotherapeutic agent are still in need of study and evaluation.
The aim of this paper was to provide an effective cytoplasmic delivery vehicle for a chemotherapeutic agent, bleomycin (BLM), to facilitate its interaction with nuclear material using a delivery system based on PEGylated octaarginine (R8)-modified fusogenic DOPE-liposomes (R8-DOPE-BLM). We demonstrated that R8-modification of fusogenic DOPE-based liposomes dramatically increased the liposomal uptake by cells. R8-DOPE-liposomes were internalized via macropinocytosis and bypassed the endosome-lysosomal compartment. We also demonstrated that BLM-loaded R8-modified fusogenic DOPE-liposomes can be very effective in killing cancer cells both in vitro and in vivo.
2. Materials and methods
2.1. Materials
Egg phosphatidylcholine (ePC), egg phosphatidylethanolamine (ePE), cholesterol (Chol), dioleoyl phosphatidylethanolamine (DOPE), cholesterylhemisuccinate (CHEMS), DSPE-PEG1k, DSPE-PEG2k, and DOPE-PEG2k were purchased from Avanti Polar Lipids (Alabaster, AL, USA) and used without further purification. p-Nitrophenylcarbonyl-poly(ethylene glycol)-nitrophenyl carbonate (pNP-PEG2k-pNP) was purchased from Laysan Bio Inc. (Arab, AL), PE-rhodamine B (Rh) were purchased from Sigma (St. Louis, MO, USA). Mouse monoclonal (H4B4) anti-lysosome-associated membrane protein (Lamp-2) antibody (CTD-19) were purchased from Abcam (Cambridge, MA, USA). Mounting medium Fluormount-G® was from SouthernBiotech (Birmingham, AL, USA). The FragEl™ DNA fragmentation Detection Kit was purchased from Merck KGaA (Darmstadt, Germany). Octaarginine was synthesized using the ABI 431 Peptide Synthesizer at Tufts University (Boston, USA). Human epithelial cervical cancer CCL-2 (HeLa) and a murine mammary carcinoma (4T1) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cell culture media and supplements were from CellGro (Kansas City, MO, USA). All other chemicals and buffer components were analytical grade preparations.
2.2. Preparation of ePE-PEG2k-R8 conjugate
DOPE was first conjugated to pNP-PEG2k-pNP at only one end of the PEG molecule in chloroform, using triethylamine (TEA) as a catalyst. For this purpose, pNP-PEG2k-pNP reagent in 5-fold excess over ePE was used to reduce bis-product formation. pNP-PEG2k-pNP (64.6 μmol) was dissolved in 1 ml dry chloroform and then ePE (12.9 μmol) and TEA (38.8 μmol) were added to the solution. The mixture was incubated overnight at room temperature (RT) in an argon atmosphere with stirring. The reaction was monitored with TLC (Silica Gel 60 F254) in a mixture of chloroform:methanol:water (an 80:20:2 volume ratio) as an eluent. Dragendorff reagent, molybdenum blue and ninhydrin were used to visualize PEG, phospholipids and amine spots respectively. After the completion of the reaction, the solvent was evaporated by freeze-drying on a Freeze Dry System Freezone 4.5 (Labconco, Kansas City, MO). The product was dissolved in 1ml HCl (0.001 M, pH = 3) and separated on a column loaded with Sepharose CL-4B and eluted with 0.001 M HCl. The 2 ml fractions were collected and analyzed by TLC for the presence of the final product. The combined fractions were freeze-dried and the white solid product (4.6 mol, yield 36%) was dissolved in dry chloroform and stored at −80°C.
The DOPE-PEG2k-pNP product formed was used for further conjugation with the amine group of octaarginine via its remaining activated pNP end. For this, 0.48 μmol DOPE-PEG-pNP (after evaporation of chloroform) was mixed with 0.48 μmol octaarginine in 0.5 ml phosphate buffered saline (PBS: 137 mM NaCl, 8 mM Na2HPO4, 2.7 mM KCl, 1.5 mM K2HPO4, pH 8.4) and stirred at room temperature (RT) overnight. The conjugation product (DOPE-PEG2k-R18) was purified from non-reacted octaarginine by dialysis against water (Spectra/Por Dialysis Membrane, cutoff 1,500).
2.3. Liposome preparation
Four different liposomal formulations (Table 1) were obtained by the hydration of lipid films. Briefly, the lipid films were obtained from a lipid mixture in chloroform. Chloroform was removed on a rotary evaporator followed by freeze-drying on a Freeze Dry System Freezone 4.5 (Labconco, Kansas City, MO). The lipid films were hydrated with vigorous vortexing with PBS (pH 7.4), without, or in the presence of 2 mg/ml bleomycin (BLM). Liposomes were sized by an extrusion of the hydrated lipid through 200 nm pore sized Nuclepore polycarbonate membranes (Whatman, Clifton, NJ, USA) using an Avanti handed extrusion device (Avanti Polar Lipids, Alabaster, AL, USA). The liposomes were separated from non-encapsulated BLM by dialysis. For microscopic experiments, BLM-free liposomal formulations were labeled with the lipophilic fluorescent marker, rhodamine-B-phosphoethanolamine (PE-RhB, ex/em: 530/590 nm). Briefly, the chloroform solution of PE-RhB (1 mol. %) was introduced to the lipid mixture in chloroform. The lipid films were hydrated with either PBS (pH 7.4) or PBS supplemented with 10 mg/ml FITC-Dextran4kDa. The liposomes were prepared as described above and then separated from free FITC-Dextran4kD by gel-filtration (Column 0.7×25 cm, BioGel A1.5M, PBS).
Table 1.
Composition and properties of R8-modified and non-modified liposomes.
| Abbreviation | Composition (mol %) | Size ± SD (nm) | Zeta, mV | BLM content mg/ml | Encapsulation efficiency* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ePC | Chol | DOPE | CHEMS | DSPE-PEG 1k | DOPE- PEG 1k | R8-PEG 2k | |||||
| P-PC | 60 | 30 | - | - | 7.5 | - | 2.5 | 170 ± 6 | −37 ± 3 | 0.155 ± 0.03 | 7.7 ± 1.5 |
| R8-PC | 60 | 30 | - | - | 7.5 | - | 2.5 | 142 ± 17 | − 12 ± 9 | 0.163 ± 0.04 | 8.1 ± 1.9 |
| P-DOPE | - | - | 80 | 10 | - | 7.5 | 2.5 | 171 ± 13 | −45 ± 4 | 0.157 ± 0.07 | 7.8 ± 0.8 |
| R8-DOPE | - | - | 80 | 10 | - | 7.5 | 2.5 | 136 ± 32 | −27 ± 6 | 0.164 ± 0.05 | 8.2 ± 2.4 |
Encapsulation efficiency of BLM was calculated as a percent to the initial BLM content (2 mg/ml)
2.4. BLM content by HPLC
BLM content in liposomal formulations was measured by HPLC [22]. Briefly, 100 μl of a liposomal sample was frozen at −80°C overnight and then freeze-dried on a Freeze Dry System Freezone 4.5. The samples were re-dissolved in 100 μl of MeOH and non-dissolved buffer salts were removed by centrifugation at 10,000 g for 15 min. The supernatant (50 μl) was applied for HPLC analysis on a Hitachi Elite Lachrom system (Tokyo, Japan) composed of the following units: a quaternary pump, model L-2130; an automatic sample injector model L-2200; a diode-array detection system L2466. EZChrom Elite (Agilent, Pleasanton, CA) data software was used for the system operation and data processing. The chromatographic separation was performed on a reverse-phase column (Symmetry C18 5 μm, 4.6 × 150 mm, Waters, Milford, MA) using a binary gradient elution. Mobile phase A consisted of 10 mM sodium perchlorate and 0.1 % phosphoric acid (pH 4.3) in deionized water. Mobile phase B consisted of acetonitrile. A constant flow rate of 1 mL/min was used to increase phase A from 5% to 25% within 20 min; this composition (25% solvent A) was held for 5 min and then changed to 100% solvent B in 2 min with a 2 min hold and followed by a post-time of 5 min under initial conditions (5% solvent A). A detection wavelength of 240 nm and a reference wavelength of 540 nm were used. The peaks of BLM A2 and BLM B5 forms were appeared at ~ 11 and 13 min respectively. The BLM content was calculated using a calibration curve of standard BLM solutions in MeOH (0.25–10 μg/ml, R2 = 0.993). Encapsulation efficiency of BLM was calculated as a percent of the liposomal BLM to the initial BLM content (2 mg/ml) in the buffer.
2.5. In vitro stability of liposomes in the presence of plasma proteins
To measure the stability of liposomes during the prolonged incubation with the culture medium, liposomes were prepared by hydration of lipid films in PBS supplemented with calcein at self-quenching concentration (100 mM). Liposomes were formed by extrusion as described above and then separated from the encapsulated calcein by gel-filtration (Column 0.7×25 cm, BioGel A1.5M, PBS). In vitro liposomal stability was examined by the incubation of calcein-loaded liposomes (50 g/ml) with either PBS or PBS supplemented with fetal bovine serum (10% v/v) and 0.1 % NaN3 (to prevent bacterial contamination) at 37°C. Equal volumes of the liposomal samples were taken after the incubation for 0, 4, 8, 24 and 48 hours and then solubilized by Triton-X100 treatment. The high concentration of the encapsulated calcein leads to self-quenching of its fluorescence, resulting in a low background fluorescence intensity of the liposomes. The calcein released from leaked liposomes increases the background fluorescence allowing monitoring of the stability of the liposomal membrane. To measure release of calcein, the percent increase in fluorescent intensity was measured by the fluorescence spectroscopy (ex/em: 488/520) and calculated according to the formula: FI= 100×[FImax−FIo]/FImax, where FI0 is the fluorescence intensity of liposomes before treatment with Triton-X100; FImax is the fluorescence intensity of liposomes after treatment with Triton-X100. The results are presented as percent of calcein encapsulation normalized to the calcein encapsulation level at 0 hrs.
2.6. Physicochemical characterization of liposomes
Liposome size and size distribution were determined by a Coulter N4 MD Submicron Particle Size Analyzer (Beckman-Coulter, Fullerton, CA). The z-potential values of the various liposomal preparations were measured using the z-potential analyzer Zeta-Plus (Brookhaven Instruments, Holtsville, NY). BLM content in liposomal formulations was measured by HPLC (see SI Methods) [22].
2.7. Cell growth
HeLa and 4T1 cells were grown at 37°C in 5% CO2 and 95% humidity in DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mM glutamine. The cell cultures were detached by trypsinization with 0.5% trypsin in PBS containing 0.025% EDTA.
2.8. Cell liposome interaction in vitro
HeLa or 4T1 cells were seeded in 6-well plates at 200,000 cells per well for 24 hrs. The cells were incubated with liposomes in complete DMEM for 1, 4 or 4 + 20 hrs (post-incubation with liposome-free medium), washed three times with DMEM to remove unbound liposomes and used for FACS experiments.
2.9. FACS analysis
The fluorescence of liposome-treated cells was measured in a Becton Dickinson FACScan™ (Becton Dickinson, San Jose, CA), and data analysis performed using CellQuest software (Becton Dickinson). The green fluorescence was determined at the emission wavelength of 520 nm (channel FL-1), and the red fluorescence was recorded at the emission wavelength of 580 nm (channel FL-2). A total of 10,000 events were acquired for each sample.
2.10. Confocal laser scanning microscopy (CLSM)
To evaluate the intracellular trafficking of R8-liposomes, HeLa cells grown on glass coverslips to 60–70% confluence were treated with 20 μg/ml liposomes labeled with PE-RhB for 1, 4 or 4 + 20 hrs. The cells were washed three times with DMEM to remove the unbound liposomes and then fixed with 4% paraformaldehyde (15 min at RT). The nuclei were visualized by the cell-staining with Hoechst 33342 (10 μg/ml) for 15 min in PBS. The coverslips were mounted on glass slides with Fluormount-G® medium and sealed using a nail polish. The slides were observed with a Zeiss LSM 700 inverted confocal microscope (Carl Zeiss Co. Ltd., Jena, Germany) equipped with a 63×, 1.4-numerical aperture plan-apochromat oil-immersion objective. The images were analyzed using the ImageJ 1.42 software (NIH).
To confirm the internalization of R8-liposome via macropinocytosis, HeLa cells were pre-incubated with DMEM supplemented with FITC-Dextran70kDa, a macropinocytic marker (0.35 mg/ml) for 30 min. Then 20 μg/ml of P-DOPE- or R8-DOPE-liposomes were added to the medium and incubated with the cells for 4 hrs. The cells were fixed, and the co-localization of the liposomal RhB (red) with FITC-Dextran70kDa (green) was evaluated by CLSM. Pearson’s correlation coefficient (PCC) and Mander’s overlap coefficient (MOP) were calculated by ImageJ for a least 20 cells from 6 images obtained from two different experiments.
To follow the lysosomal accumulation of R8-liposomes, HeLa cells were incubated with R8-PC- or R8-DOPE-liposomes for 4+20 hrs. The cells were fixed as described above and then permeabilized by incubation with 0.2% saponin and 1% BSA in PBS for 10 min at RT, washed three times with a blocking solution (1% BSA in PBS, pH 7.4), and kept for 30 min in the same buffer. Next, the cells were stained with a mouse anti-human Lamp-2 mAb (1:50) as a lysosomal marker for 60 min at RT, and then washed with the blocking solution. Visualization was done by cell incubation with Alexa488-conjugated goat anti-mouse IgG (1:1000 dilution) for 60 min at RT followed by three washes with the blocking solution. The colocalization of liposomal RhB and a lysosomal marker Lamp-2 was observed by CLSM.
To investigate the endosomal escape of water-soluble intra-liposomal loads, HeLa cells were treated with 20 μg/ml R8-PC- or R8-DOPE-liposomes (both loaded with a model compound, FITC-Dextran4kDa in DMEM for 4 hrs. In the case of R8-DOPE treatment, the cells were co-incubated additionally with 50 μM chloroquine. Chloroquine, a lysosomotropic agent, accumulates inside the acidic compartments and because of its buffering capacity, prevents endosomal acidification [23]. The cells were then washed and incubated with liposome/chloroquine-free DMEM for an additional 20 hrs. Colocalization of the liposomal lipophilic marker PE-RhB (red) and hydrophilic FITC-Dextran4kDa (green) loaded into the internal lysosomal compartment was evaluated by CLSM as described above.
2.11. Cytotoxicity assay
The cytotoxic effect of BLM-loaded R8-liposomes was assessed using CellTiter-Blue® Assay (Promega). This assay is based on the ability of living cells to convert a redox dye (resazurin) into the fluorescent end product (resorufin). Briefly, cells were seeded in 96-well plates at 10,000 cells per a well for 24 hrs. The medium was removed and replaced with 100 μl of fresh culture medium supplemented with either liposomes or free BLM at different concentrations and incubated for 24 hrs. The cells were washed with fresh medium, and 100 μl DMEM supplemented with 10 μl CellTiter-Blue® kit was added. The cells were incubated for 1 h at 37°C in a 5% CO2 incubator. The fluorescence was measured with a Synergy™ microplate reader (em/ex: 530/590 nm). The percentage of live cells was calculated relative to control (untreated) cells.
2.12. 4T1 tumor xenografts
The experiments were performed with 6–8 week old female BALB/c mice (Charles River Laboratories, Wilmington, MA) under a protocol approved by the Institutional Animal Care and Use Committee in accordance with Principles of Laboratory Animal Care (NIH publication #85–23, revised in 1985). 4T1 cells (2×106 in 0.2 ml PBS) were injected subcutaneously into the left hind flank and allowed to develop until tumors were approximately 100 mm3. The animals were randomized into five groups of 8–9 animals and injected i.v. via the tail vein five times at two days intervals with 200 μl of PBS, liposomal formulations (40 mg/kg) or free BLM (0.63 mg/kg, a dose equal to BLM-content in the liposomal formulations) Tumor sizes were measured in two dimensions daily using calipers and volume calculated: volume = (width squared × length)/2. At day 22 after tumor initiation, the mice were sacrificed and tumor weights were taken.
2.13. TUNEL assay
To measure DNA damage by the BLM-loaded liposomes in vitro, 4T1 cells were seeded in 6-well plates at 100,000 cells per well and grown for 24 hrs. The cells were incubated with BLM-loaded liposomes (0.5 mg/ml) or free BLM (at the concentration equal to BLM content in the liposomal formulations) for 24 hrs in complete DMEM. The cells were harvested by gentle trypsinization, fixed with 4% paraformaldehyde for 10 min at RT, permeabilized with proteinase K (20 μg/ml) for 5 min at RT, and then subjected to TUNEL assay using a FragEl™ DNA fragmentation Detection Kit (Merck) following the manufacturer’s protocol for in vitro cultured cells. The TUNEL-positive cells were examined by FACS analysis. A total of 10,000 events were acquired for each sample.
The pro-apoptotic effect of BLM-loaded R8-DOPE-liposomes in vivo was measured on the frozen tumor sections by the TUNEL assay. For this, tumor samples were frozen immediately after the extraction by immersing in isopentane pre-cooled in liquid nitrogen and then stored at −80°C. The thick frozen tumor sections (8-μm) were cut, mounted on superfrost plus slides, fixed in 4% paraformaldehyde for 10 min at RT, permeabilized with proteinase K (20 μg/ml) for 15 min at RT, and then subjected to TUNEL assay using the FragEl™ DNA fragmentation Detection Kit following the manufacturer’s protocol for frozen sections. The TUNEL-positive cells were examined by fluorescence microscopy. Four random images obtained from two different tumors for each treatment group were analyzed using ImageJ software.
2.14. Statistical analysis
The data were tested for statistical significance using the paired Student’s t-test. P values, calculated with the SPSS 10.00 software package, were considered significant at p < 0.05.
3. Results
3.1. Characterization of liposomes
Liposomal formulations were prepared and characterized in terms of size, surface charge and BLM-loading (Table 1). PEGylated with PE-PEG1k non-fusogenic liposomes based on PC/Chol (R8-PC-liposomes) and fusogenic liposomes composed of pH-sensitive lipids DOPE/CHEMS (R8-DOPE-liposomes) were prepared by hydration of lipid films with multiple extrusions to form unilamellar liposomal structure. The both liposomal formulations were modified with 2.5 mol. % of octaarginine (R8)-conjugated to DOPE-PEG2K. “Plain” non-modified, P-PC- and P-DOPE-liposomes (2.5% DOPE-PEG2K) were used as control formulations. The average size of plain liposomes was ~170 nm; the size of liposomes modified with R8-DOPE-PEG2K was slightly (non- significantly) smaller (~ 140 nm). Both R8-PC- and R8-DOPE-liposomes modified with strongly positive R8-DOPE-PEG2K conjugate demonstrated a significant increase in the surface charge in contrast to the plain liposomal compositions (Table 1). R8-PC-liposomes had a zeta potential of −12 ± 9 mV, while R8-DOPE-liposomes were more negatively charged (−27 ± 6 mV). This is explained by the initial difference in the surface charge of these two formulations (P-PC-liposomes, −37 ± 9 vs P-DOPE-liposomes, −45 ± 4 mV).
HPLC analysis [22] of bleomycin (BLM) content in the liposomes showed that about 0.16 mg/ml of BLM loading can be achieved at a 10 mg/ml lipid concentration. Stability of the liposomal preparations in the presence of serum proteins was measured by calcein release (see Fig. S1). The incubation of liposomes for 24 hrs in the presence of plasma did not lead to a significant release of the liposomal calcein and did not affect the liposome size (data not shown).
3.2. Cellular uptake of R8-liposomes
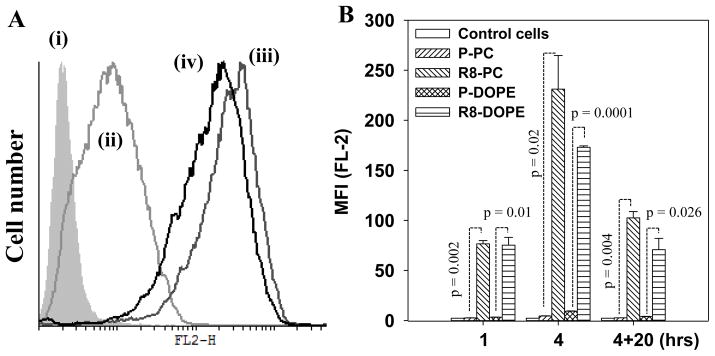
To estimate cellular internalization of R8-liposomes, HeLa cells were treated with BLM-free RhB-labeled R8-liposomes for 1, 4 or 4 + 20 hrs. The fluorescence of liposome-treated cells was measured by FACS analysis (Fig. 1A, B). The modification of the liposomes with R8-conjugate led to a sharp increase in the liposome-cell interaction. R8-PC-liposomes had 30-fold increased uptake after 1 hr incubation with cells relative to the non-modified P-PC-liposomes. The extension of the incubation time to 4 hrs, elevated the uptake of R8-PC up to 50-fold (Fig. 1B). In contrast to non-fusogenic R8-PC-liposomes, fusogenic R8-DOPE-liposomes demonstrated a somewhat lower cellular uptake. This can be explained by the difference in the surface charge of R8-PC and R8-DOPE liposomes with more negative charge of R8-DOPE-liposome (Table 1) that may affect the cell-liposome interaction.
Fig. 1. Interaction of R8-Liposomes with HeLa cells by FACS.
HeLa cells were treated with liposomes (50 μg/ml) for 1, 4 or 4+20 hrs in complete DMEM. The fluorescence of cells was measured with a FACS. (A) A typical histogram of the cells treated with R8-liposomes for 4 hrs: (i) untreated cells and the cells treated with (ii) P-DOPE-liposomes; (iii) R8-PC-liposomes; (iv) R8-DOPE-liposomes. (B) The mean fluorescence intensity of the liposome-treated cells. The data are a mean for 3 experiments ± SEM.
3.3. Intracellular trafficking R8-DOPE liposomes by confocal laser scanning microscopy (CLSM)
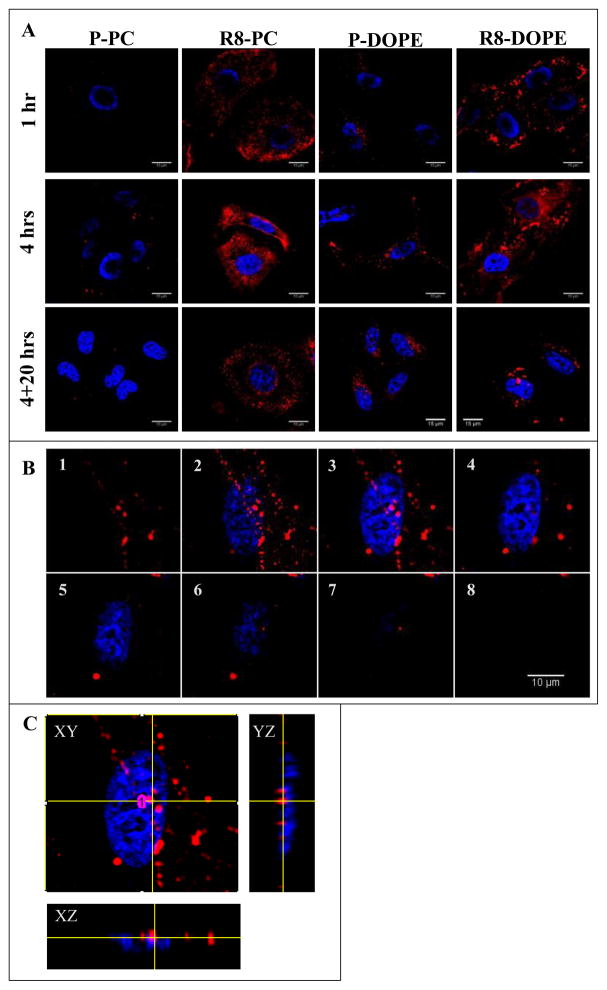
The results of FACS analysis were supported by CLSM of HeLa cells treated with R8-liposomes (Fig. 2). As shown on microphotographs obtained by CLSM, after 1 h of the co-incubation with cells, R8-PC- and R8-DOPE-liposomes showed association primarily with the cell’s surface. Both the R8-PC- and R8-DOPE-liposomes had a significantly greater level of cell-liposome interaction relative to plain liposomes. Extension of the incubation time to 4 hrs led to higher liposome internalization, with the complete intracellular localization of R8-liposomes after an additional 20 hrs of incubation in liposome-free medium.
Fig. 2. Intracellular localization of R8-liposomes.
HeLa cells were treated with liposomes (50 μg/ml) for 1, 4 or 4+20 hrs in complete DMEM. (A) The intracellular localization of liposomal Rh (red) evaluated by confocal microscopy. (B) Z-stacked images of a cell treated with R8-DOPE-liposomes for 4+20 hrs (1–8: from bottom to top, section intervals 0.7 μm). (C) Orthogonal projections reconstructed from Z-stacked images of the cells treated with R8-DOPE-liposomes for 4+20 hrs. The nuclei were visualized by staining with Hoechst 33342 (blue).
It should be mentioned that R8-PC- and R8-DOPE-liposomes presented a different morphological pattern upon their cell association and internalization (Fig. 2A). Thus, in contrast to R8-PC-liposomes, fusogenic R8-DOPE-liposomes were associated with the cell surface in the form of larger aggregates. Such enlarged structures were preserved as further endocytosis proceeded. The analysis of the orthogonal projections reconstructed from Z-stacked images (Fig. 2B, C) revealed that, in contrast to P-DOPE-liposomes and R8-PC-liposomes, R8-DOPE-liposomes were partially associated with the cell’s nuclei after being internalized.
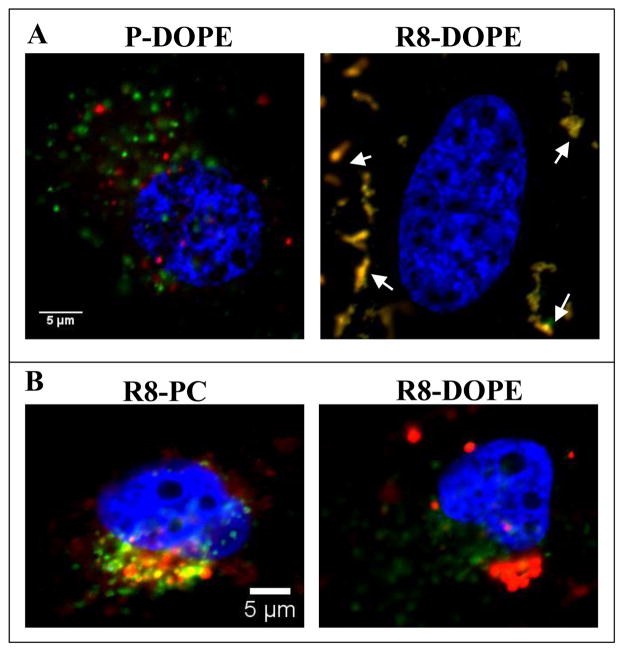
To evaluate the endocytotic mechanism of R8-DOPE-liposomes, we used FITC-Dextran70kDa as a marker of macropinocytosis (Fig. 3A). R8-DOPE-liposomes were highly co-localized with FITC-Dextran70kDa (PCC = 0.91 ± 0.02) in contrast to non-modified “plain” P-DOPE-liposomes that showed no colocalization with FITC-Dextran70kDa (PCC = 0.26 ± 0.04).
Fig. 3. Colocalization of the liposomal RhB with macropinocytotic (A) and lysosomal (B) markers.
(A) HeLa cells were treated with either P-DOPE- or R8-DOPE-liposomes in DMEM complemented with FITC-Dextran70kD (0.35 mg/ml) for 4 hrs. (B) HeLa cells treated with R8-PC- or R8-DOPE- liposomes in complete DMEM for 4+20 hrs were stained with Lamp-2 mAb. The nuclei were visualized by staining with Hoechst 33342 (blue). Colocalization (yellow) of liposomal RhB-PE (red) with the organelle’s markers (green) was evaluated by confocal microscopy.
To estimate the lysosomal accumulation of R8-modified liposomes, we compared the colocalization of non-fusogenic R8-PC- and fusogenic R8-DOPE liposomes with a lysosomal marker, Lamp-2 (Fig. 3B). In contrast to R8-PC-liposomes (PCC = 0.72 ± 0.04), R8-DOPE-liposomes (PCC = −0.15 ± 0.04) were not localized in the lysosomes after 4+20 hrs of their co-incubation with cells (Fig. 3B).
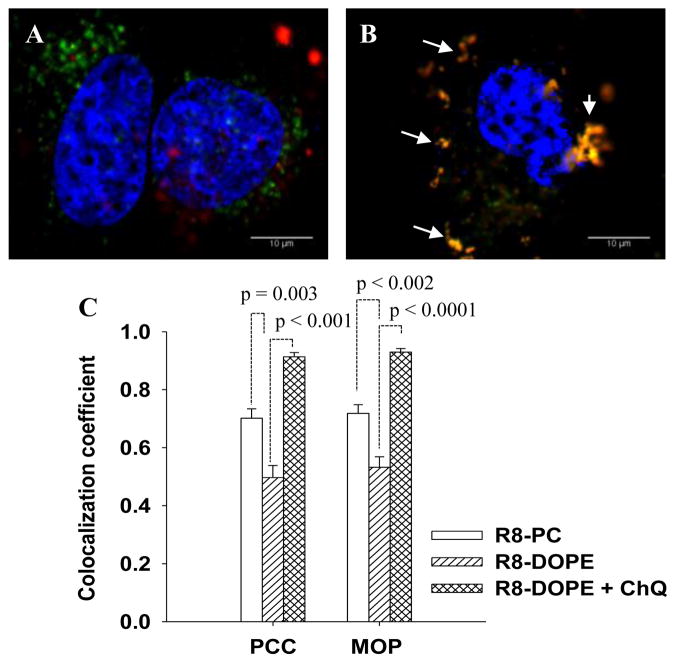
To evaluate the intracellular trafficking of the liposomal lipophilic PE-RhB and hydrophilic marker, FITC-Dextran4kDa, HeLa cells were treated with R8-PC- and R8-DOPE-liposomes (both loaded with FITC-Dextran4kDa) for 4+20 hrs. The cells treated with R8-DOPE-liposomes were additionally co-incubated with chloroquine (ChQ) to prevent the endosomal acidification effects on the ability of DOPE-liposomes to fuse with the endosomal membrane. Fig. 4(A-B) shows that PE-RhB and FITC- Dextran4kDa were poorly co-localized in the cells treated with R8-DOPE-liposomes, while their colocalization was significantly elevated for cells treated with non-fusogenic R8-PC-liposomes. The treatment of cells with R8-DOPE-liposomes in the presence of ChQ significantly increased colocalization of the liposomal PE-RhB and FITC-Dextran4kDa.
Fig. 4. Intracellular distribution of the lipophilic RhB and hydrophilic FITC-Dextran4kD liposomal markers without (A) or in the presence (B) of chloroquine.
HeLa cells were treated with FITC-Dextran4kDa-loaded R8-DOPE-liposomes in a medium without (A) or in the presence of 50 μM chloroquine (B) for 4 hrs. The cells were washed and incubated in liposome/chloroquine-free medium for an additional 20 hrs. Colocalization (yellow) of the liposomal RhB-PE (red) and FITC-Dextran4kD(green) was evaluated by confocal microscopy. (C) Mean values of Pearson’s correlation coefficient (PCC) and Mander’s overlap coefficient (MOP) were calculated by ImageJ software for 6 images from two different experiments ± SEM.
3.4. Cytotoxicity and DNA damage by BLM-loaded R8-liposomes in vitro
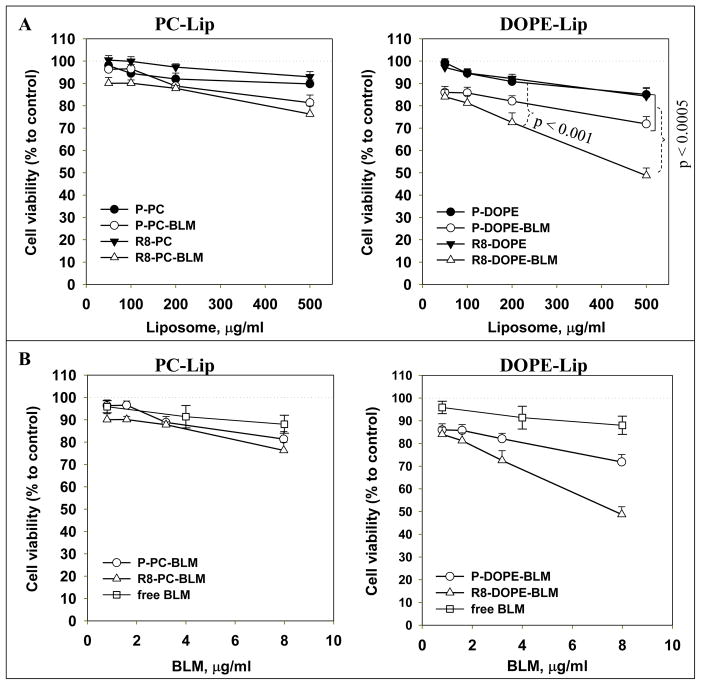
The effect of BLM-loaded R8-liposomes on the cell viability (Fig. 5) and DNA damage (Fig. 6) was investigated using the 4T1 murine mammary carcinoma cell line. BLM-loaded R8-PC-liposomes showed no significant cytotoxic effect in comparison to non-modified P-PC-BLM-liposomes; while R8-DOPE-BLM-liposomes caused significantly increased cell death relative to all control formulations (R-PC-BLM-, P-DOPE-BLM- and R-DOPE-liposomes). A similar cytotoxic effect was observed with HeLa cells (see SI, Fig. S2). Free BLM, at the range of concentrations equivalent to the concentration of BLM in R8-DOPE-liposomes, had a very minor cytotoxic effect (see Fig. 5 and Fig. S2).
Fig. 5. Effect of BLM-loaded R8-liposomes on cell viability.
4T1 cells were treated with BLM-loaded liposomes or free BLM for 24 hrs in complete DMEM. The cell viability was evaluated by the Cell-Titer Blue assay. The data are presented as liposomal lipid (A) or encapsulated BLM concentrations (B). The mean values are for 7 experiments ± SEM.
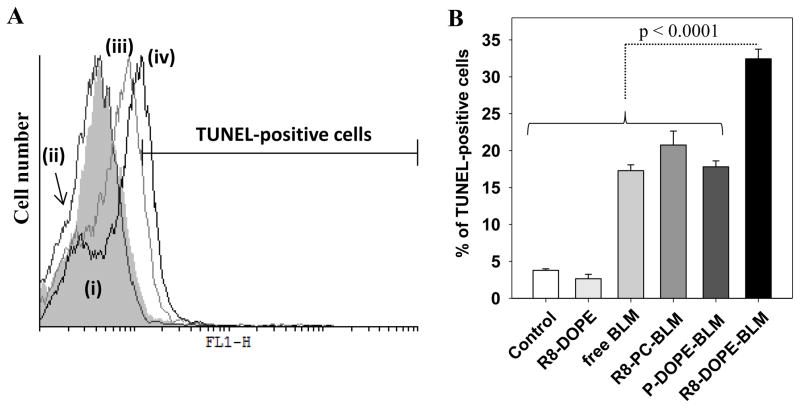
Fig. 6.
DNA damage by BLM-loaded R8-liposomes. 4T1 cells were treated with BLM-loaded liposomes (0.5 mg/ml) or free BLM (at concentration equal to BLM content in the liposomal formulations) for 24 hrs in complete DMEM. The cells were trypsinized, fixed with 4% paraformaldehyde, permeabilized with proteinase K and then subjected to TUNEL assay. The TUNEL-positive cells (FL-1 channel) were examined by FACS analysis. (A) A typical histogram of the cells treated with liposomes or free BLM and subjected to TUNEL assay: (i) untreated and cells treated with: (ii) R8-DOPE-liposomes; (iii) P-DOPE-BLM-liposomes; (iv) R8-DOPE-BLM-liposomes. (B) The percent of TUNEL-positive cells. The data are a mean for 5 experiments ± SEM.
Since the cytotoxic effect of BLM is known to be due to single- and double-stranded DNA breaks [6], we exploited the TUNEL assay, which is based on binding terminal deoxynucleotidyl transferase (TdT) to exposed 33-OH ends of DNA fragments that catalyzes the addition of the fluorescein-labeled deoxynucleotides. BLM delivered by R8-DOPE-liposomes provoked significantly increased DNA damage relative to all controls as indicated by the increased percentage of TUNEL-positive cells (Fig. 6B). Thus, we can conclude that fusogenic DOPE-liposomes modified with R8-conjugates allow for effective cytoplasmic and eventually nuclear accumulation of BLM to cause the cell death via the damage of the cell’s DNA.
3.5. Effect of BLM-loaded R8-liposomes on the tumor growth and apoptosis in vivo
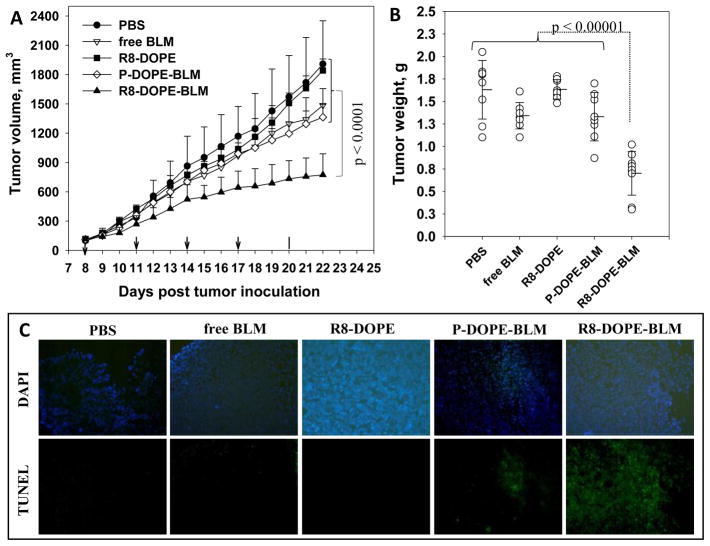
The anti-tumor effect of BLM delivered by R8-DOPE-liposomes has been demonstrated in BALB/c mice bearing 4T1 murine mammary carcinomas. Tumor-bearing mice were randomly separated into five groups of 8–9 animals, and R8-DOPE-, P-DOPE-BLM-, R8-DOPE-BLM-liposomes (at 40 mg lipids per kg of mouse weight), free BLM (0.63 mg/kg) or PBS were injected at two day intervals via the tail vein. The treatment with R8-DOPE-BLM-liposomes strongly inhibited the tumor growth (up to 60%) relative to all control groups (p < 0.0001, Fig. 7A). It should be noted that free BLM and BLM loaded in non-modified P-DOPE-liposome still demonstrated minor, but definitely statistically significant inhibition (p = 0.025 and p = 0.005 respectively) of the tumor growth in comparison to the buffer treatment only. The final tumor weights in animals treated with R8-DOPE-BLM-liposomes were significantly lower (up to 2-fold) in comparison to the groups treated with control liposomal formulations (P-DOPE-BLM- and R8-DOPE-liposomes), free BLM and PBS (Fig. 7B).
Fig. 7. Anti-tumor and pro-apoptotic effect of R8-DOPE-BLM in vivo.
The BALb/c mice bearing 4T1 breast cancer tumor were tail vein administrated with PBS, free BLM, R8-DOPE-liposomes, P-DOPE-BLM-liposomes or R8-DOPE-BLM-liposomes five times with two day interval. (A) Tumor volumes. (B) The tumor weights after removal at day 22. The data are the mean for 8–9 animals ± S.D. (C) The frozen tumor sections were subjected to TUNEL staining and examined by fluorescence microscopy. The upper line shows the sections stained with DAPI and the lower line indicates the TUNEL staining.
The TUNEL assay was used with frozen tumor sections to measure the pro-apoptotic effect of BLM-loaded R8-DOPE-liposomes (Figure 7C). The cell nuclei of tumors treated with PBS or BLM-free P-DOPE-liposomes exhibited no green fluorescence attributable to FITC-labeled TdT. Few TUNEL-positive cells were observed in the tumors treated with free BLM and BLM loaded in P-DOPE-liposomes, while the tumors treated with R8-DOPE-BLM-liposomes had a massive amount of apoptotic cells. Thus, fusogenic R8-modified DOPE-liposomes allowed for the strong anti-tumor and proapoptotic effect of delivered BLM in an in vivo mouse tumor model.
4. Discussion
For this study, we hypothesized that by entrapment of BLM in liposomes composed of the fusogenic lipids DOPE/CHEMS and with the liposome surface modified by a cell-penetrating peptide R8, we would achieve an increased anticancer effect of the drug due to its improved cytosolic (and eventually nuclear) delivery.
Conventional PEGylated liposomes, despite their benefits, including a prolonged circulation time in blood, are known to have poor intracellular uptake that together with a tendency to accumulate in lysosomes makes their application as a delivery carrier less effective. On the other hand, cytosolic delivery with subsequent nuclear localization of an anticancer drug, such as BLM, with cytotoxicity based on DNA damage, can be critical for its chemotherapeutical potency.
To provide effective cellular internalization and endosomal escape of the liposome-encapsulated BLM, we modified the surface of DOPE/CHEMS/DOPE-PEG1k (80/10/7.5 mol. %)-based liposomes with R8-DOPE-PEG2k conjugates (2.5 mol. %). The physicochemical and anticancer properties of R8-DOPE-liposomes were compared with the effect of R8-modified non-fusogenic phosphatidylcholine/cholesterol (PC)-based liposomes as well as with non-modified “plain” PC- and DOPE-liposomes.
As expected, the modification of the liposomal surface with a cationic peptide such as R8 significantly elevated the surface charge (Table 1). Interestingly, the R8-modification of PC-based liposomes increased their zeta potential more strongly compared to fusogenic DOPE-based liposomes. Obviously, the lower initial zeta potential of liposomes composed of anionic lipids DOPE and CHEMS was not compensated for by introduction of the positively charged R8-conjugates.
The differences in the surface charge of R8-modified PC- and DOPE-based liposomes affected their interaction with the treated cells. As shown by FACS analysis (Fig. 1), in contrast to R8-PC-liposomes, fusogenic R8-DOPE-liposomes have relatively less cellular internalization. Still, the R8-modification of fusogenic DOPE-liposomes dramatically enhanced their cellular uptake compared to non-modified liposomes (up to 30-fold). Importantly, despite the lesser cellular association/uptake, BLM-loaded R8-DOPE-liposomes demonstrated a significantly stronger cytotoxic effect (Fig. 5) and DNA damage (Fig. 6) relative to the non-fusogenic R8-PC-liposomes. Since R8-DOPE-liposomes show high stability during the incubation in the presence of serum proteins (Fig. S1), their improved cytotoxicity cannot be explained by the leakage of BLM from the liposomes into the medium.
Thus, by comparing the improved cytotoxicity and DNA damaging by fusogenic R8-DOPE-BLM liposomes to non-fusogenic R8-PC-BLM liposomes, it can be concluded that the modification of the liposomal surface with R8-conjugates alone is not enough to provide the effective cytosolic/nuclear relocation of BLM; the presence of fusogenic lipids in the liposomal composition is essential for the BLM release from the endosomal compartment. Indeed, with confocal microscopy comparing the intracellular trafficking of R8-modified PC- and DOPE-liposomes, we observed that non-fusogenic R8-PC-liposomes were highly colocalized with the lysosomal marker, Lamp-2, while R8-DOPE-liposomes did not appear in the lysosomal compartment (Fig. 3B).
To provide an additional confirmation of the ability of fusogenic R8-DOPE-liposomes to deliver their hydrophilic loads to the cytoplasm, we traced lipophilic PE-RhB and hydrophilic FITC-Dextran4kDa markers of the lipid and inner liposomal compartments, respectively (Fig. 4). We expected that if the fusogenic R8-DOPE-liposomes disrupt the endosomal membrane, the intra-liposomal marker, FITC-Dextran4kDa, will be relocated to the cytosol and resulting in its reduced colocalization with the lipophilic PE-RhB. Since the fusogenicity of DOPE-liposomes is pH-sensitive, we used chloroquine, which increases the intra-endosomal pH, to prevent the fusion of the liposomal lipids with the endosomal membrane. Indeed, we observed that the treatment of the cells with R8-DOPE-liposomes in the presence of chloroquine prevented the release of FITC-Dextran4kDa from endosomes resulting in its higher colocalization with the lipophilic marker PE-RhB relative to the cells treated with only R8-DOPE-liposomes and non-fusogenic R8-PC-liposomes. Thus, we demonstrated that R8-DOPE liposomes endocytosed by cancer cells not only avoid accumulation in the lysosomes but also release their hydrophilic loads into the cytoplasm.
The cell surface heparan sulfate proteoglycans (HSPGs) are known to be a non-specific site for binding CPPs [24; 25]. As reported previously, liposomes modified with a high density of R8 are internalized via the macropinocytosis [24; 26]. It was proposed that while R8-liposomes are bound to the cell surface HSPGs, they promote proteoglycan clustering to initiate a significant rearrangement in the cytoskeleton thus stimulates macropinocytosis [26].
Similarly, in agreement with the previous findings [26], we observed that R8-DOPE-liposomes co-localized strongly with FITC-Dextran70kDa, a marker of macropinosomes (Fig. 3A). Notably, in contrast to R8-PC-liposomes, R8-DOPE-liposomes formed enlarged structures associated with the cell surface and, after being internalized, were associated partially with the cell nuclei (Fig. 2C). We speculate that after the internalization via macropinocytosis, the combination of positively charged octaarginine and fusogenic lipids modifies the macropinosome surface, leading to fusion/association of R8-DOPE-containing endosomes with the cell nuclei. The nuclear accumulation of R8-conjugates has been previously demonstrated [27]. However, a more precise conclusion regarding the nuclear association of R8-DOPE-liposomes can be made only after additional microscopy experiments, preferably on non-fixed live cells.
Numerous attempts have been made recently to improve the intracellular delivery of BLM especially in solid tumors such as breast carcinomas [28]. Thus, electroporation was used to increase the intracellular internalization of BLM co-administrated with IL-12 in 4T1-breast cancer xenograft mouse model [29]. BLM-based electrochemotherapy has been successfully applied in patients with metastatic melanoma and breast cancer [30]. In this study, we have chosen the 4T1-xenograft mouse model to estimate the antitumor potency of the BLM encapsulated in R8-modified fusogenic liposomes towards the breast cancer, which is known to be less responsible to the BLM-treatment. We demonstrated that the treatment of 4T1 xenograft-bearing mice with R8-DOPE-BLM strongly inhibited the tumor growth (Fig. 7A–B) and initiated a massive apoptosis in the threated tumors (Fig. 7C). The significant improvement in inhibition of the tumor growth by BLM in R8-DOPE-liposomes compared to free BLM and BLM-encapsulated in “plain” non-modified liposomes suggests that the modification of the liposomal surface with octaarginine peptide, which increase dramatically the intracellular delivery of liposomes as was shown in vitro (Fig. 1), is critically important to achieve the desire antitumor effect of the liposomal BLM towards solid tumors, such as breast carcinoma.
As known, long-circulating PEGylated liposomes have a tendency to accumulate in tumor’s area via the enhanced permeability and retention (EPR) effect [31]. We may expect that our R8-DOPE-liposomes, which also have a PEG layer, would be accumulated increasingly in the tumor due to the EPR effect. As we showed, the strongly elevated liposomal internalization via macropinocytosis was promoted by octaarginine peptides and associated enhanced fusion/association with the nuclear membrane. The liposomal DOPE/CHEMS lipids fused with the endosomal membrane, initiating escape of the liposomal BLM into cytosol leading to drug accumulation in the cell’s nuclei followed by DNA lesions and cell death.
We conclude that the modification of fusogenic DOPE-based liposomes with the cell-penetrated peptide, octaarginine, facilitates nuclear delivery of the anticancer drug, BLM and a strong anticancer effect both in vitro and in vivo. The use of such a drug delivery system can significantly increase the chemotherapeutic efficiency of many anticancer drugs by improving their cellular uptake and specific intracellular targeting.
Supplementary Material
Acknowledgments
This research was funded by the NIH grant RO1 CA128486 to Vladimir P. Torchilin.
Footnotes
Conflict of Interest Statement
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Torchilin VP. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu Rev Biomed Eng. 2006;8:343–375. doi: 10.1146/annurev.bioeng.8.061505.095735. [DOI] [PubMed] [Google Scholar]
- 2.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Stubbe J. Bleomycins: towards better therapeutics. Nat Rev Cancer. 2005;5:102–112. doi: 10.1038/nrc1547. [DOI] [PubMed] [Google Scholar]
- 4.Iqbal ZM, Kohn KW, Ewig RA, Fornace AJ., Jr Single-strand scission and repair of DNA in mammalian cells by bleomycin. Cancer Res. 1976;36:3834–3838. [PubMed] [Google Scholar]
- 5.Kuo MT. Preferential damage of active chromatin by bleomycin. Cancer Res. 1981;41:2439–2443. [PubMed] [Google Scholar]
- 6.Giloni L, Takeshita M, Johnson F, Iden C, Grollman AP. Bleomycin-induced strand-scission of DNA. Mechanism of deoxyribose cleavage. J Biol Chem. 1981;256:8608–8615. [PubMed] [Google Scholar]
- 7.Roy SN, Horwitz SB. Characterization of the association of radiolabeled bleomycin A2 with HeLa cells. Cancer Res. 1984;44:1541–1546. [PubMed] [Google Scholar]
- 8.Sleijfer S. Bleomycin–induced pneumonitis. Chest. 2001;120:617–624. doi: 10.1378/chest.120.2.617. [DOI] [PubMed] [Google Scholar]
- 9.Arndt D, Zeisig R, Bechtel D, Fichtner I. Liposomal bleomycin: increased therapeutic activity and decreased pulmonary toxicity in mice. Drug Deliv. 2001;8:1–7. doi: 10.1080/107175401300002685. [DOI] [PubMed] [Google Scholar]
- 10.Gabizon A, Price DC, Huberty J, Bresalier RS, Papahadjopoulos D. Effect of liposome composition and other factors on the targeting of liposomes to experimental tumors: biodistribution and imaging studies. Cancer Res. 1990;50:6371–6378. [PubMed] [Google Scholar]
- 11.Maekawa S, Sugimachi K, Kitamura M. Selective treatment of metastatic lymph nodes with combination of local hyperthermia and temperature-sensitive liposomes containing bleomycin. Cancer Treat Rep. 1987;71:1053–1059. [PubMed] [Google Scholar]
- 12.Sur P, Hazra B, Roy DK, Sur B. Enhancement of the activity of bleomycin on Ehrlich ascites carcinoma cells by liposomal encapsulation. Indian J Exp Biol. 1984;22:115–119. [PubMed] [Google Scholar]
- 13.Straubinger RM, Duzgunes N, Papahadjopoulos D. pH-sensitive liposomes mediate cytoplasmic delivery of encapsulated macromolecules. FEBS Lett. 1985;179:148–154. doi: 10.1016/0014-5793(85)80210-6. [DOI] [PubMed] [Google Scholar]
- 14.Torchilin VP, Zhou F, Huang L. pH-Sensitive liposomes. 1993;3:201–255. [Google Scholar]
- 15.Chu CJ, Dijkstra J, Lai MZ, Hong K, Szoka FC. Efficiency of cytoplasmic delivery by pH-sensitive liposomes to cells in culture. Pharm Res. 1990;7:824–834. doi: 10.1023/a:1015908831507. [DOI] [PubMed] [Google Scholar]
- 16.Slepushkin VA, Simoes S, Dazin P, Newman MS, Guo LS, Pedroso de Lima MC, Duzgunes N. Sterically stabilized pH-sensitive liposomes. Intracellular delivery of aqueous contents and prolonged circulation in vivo. J Biol Chem. 1997;272:2382–2388. doi: 10.1074/jbc.272.4.2382. [DOI] [PubMed] [Google Scholar]
- 17.Sawant R, Torchilin V. Intracellular delivery of nanoparticles with CPPs. Methods Mol Biol. 2011;683:431–451. doi: 10.1007/978-1-60761-919-2_31. [DOI] [PubMed] [Google Scholar]
- 18.Torchilin VP. Cell penetrating peptide-modified pharmaceutical nanocarriers for intracellular drug and gene delivery. Biopolymers. 2008;90:604–610. doi: 10.1002/bip.20989. [DOI] [PubMed] [Google Scholar]
- 19.Khalil IA, Kogure K, Futaki S, Hama S, Akita H, Ueno M, Kishida H, Kudoh M, Mishina Y, Kataoka K, Yamada M, Harashima H. Octaarginine-modified multifunctional envelope-type nanoparticles for gene delivery. Gene Ther. 2007;14:682–689. doi: 10.1038/sj.gt.3302910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C, Tang N, Liu X, Liang W, Xu W, Torchilin VP. siRNA-containing liposomes modified with polyarginine effectively silence the targeted gene. J Control Release. 2006;112:229–239. doi: 10.1016/j.jconrel.2006.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furukawa R, Yamada Y, Takenaga M, Igarashi R, Harashima H. Octaarginine-modified liposomes enhance the anti-oxidant effect of Lecithinized superoxide dismutase by increasing its cellular uptake. Biochem Biophys Res Commun. 2011;404:796–801. doi: 10.1016/j.bbrc.2010.12.062. [DOI] [PubMed] [Google Scholar]
- 22.Fiedler HP, Wachter J. High-performance liquid chromatographic determination of bleomycins. J Chromatogr. 1991;536:343–347. doi: 10.1016/s0021-9673(01)89268-2. [DOI] [PubMed] [Google Scholar]
- 23.Steinman RM, Mellman IS, Muller WA, Cohn ZA. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983;96:1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khalil IA, Kogure K, Futaki S, Harashima H. High density of octaarginine stimulates macropinocytosis leading to efficient intracellular trafficking for gene expression. J Biol Chem. 2006;281:3544–3551. doi: 10.1074/jbc.M503202200. [DOI] [PubMed] [Google Scholar]
- 25.Tyagi M, Rusnati M, Presta M, Giacca M. Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J Biol Chem. 2001;276:3254–3261. doi: 10.1074/jbc.M006701200. [DOI] [PubMed] [Google Scholar]
- 26.Khalil IA, Kogure K, Futaki S, Harashima H. Octaarginine-modified liposomes: enhanced cellular uptake and controlled intracellular trafficking. Int J Pharm. 2008;354:39–48. doi: 10.1016/j.ijpharm.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Brunner J, Barton JK. Targeting DNA mismatches with rhodium intercalators functionalized with a cell-penetrating peptide. Biochemistry. 2006;45:12295–12302. doi: 10.1021/bi061198o. [DOI] [PubMed] [Google Scholar]
- 28.Gothelf A, Mir LM, Gehl J. Electrochemotherapy: results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat Rev. 2003;29:371–387. doi: 10.1016/s0305-7372(03)00073-2. [DOI] [PubMed] [Google Scholar]
- 29.Torrero MN, Henk WG, Li S. Regression of high-grade malignancy in mice by bleomycin and interleukin-12 electrochemogenetherapy. Clin Cancer Res. 2006;12:257–263. doi: 10.1158/1078-0432.CCR-05-1514. [DOI] [PubMed] [Google Scholar]
- 30.Campana LG, Mocellin S, Basso M, Puccetti O, De Salvo GL, Chiarion-Sileni V, Vecchiato A, Corti L, Rossi CR, Nitti D. Bleomycin-based electrochemotherapy: clinical outcome from a single institution’s experience with 52 patients. Ann Surg Oncol. 2009;16:191–199. doi: 10.1245/s10434-008-0204-8. [DOI] [PubMed] [Google Scholar]
- 31.Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011;63:131–135. doi: 10.1016/j.addr.2010.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.