Abstract
Background
The shortage in organ donation is a major limiting factor for patients with end-stage lung disease. Expanding the donor pool would be beneficial. We investigated the importance of geographic distance between the donor and recipient and hypothesized that it would not be a critical determinant of outcomes after lung transplantation.
Methods
We retrospectively reviewed the United Network for Organ Sharing lung transplant database from 2000 to 2005 to allow sufficient time for bronchiolitis obliterans syndrome (BOS) development. Allograft recipients were stratified by geographic distance from their donors (local, regional, and national) and had yearly follow-up. The primary end points were the development of BOS and 1-year and 3-year mortality. Posttransplant outcomes were compared using a multivariable Cox proportional hazard model. Kaplan-Meier curves were compared by log-rank test.
Results
Of 6,055 allograft recipients, donors were local in 59%, regional in 19.3%, and national in 21.7%. BOS-free survival did not differ by geographic distance. Geographic distance did not independently predict BOS (hazard ratio, 1.03; 95% confidence interval, 0.96 to 1.10). Similarly, Kaplan-Meier survival curves were not significantly worse for recipients with national donors. Geographic distance did not independently predict 3-year mortality (hazard ratio, 0.95; 95% confidence interval, 0.89 to 1.01).
Conclusions
With appropriate donor selection, moderately long geographic distance (average ischemic time < 6 hours) between the donor and recipient is not associated with the development of BOS or increased death after lung transplantation. By placing less emphasis on distance, more donors could potentially be used to expand the donor pool.
Lung transplantation (LT) is the only treatment for many patients with end-stage lung disease. During the last 2 decades, significant changes have occurred in procurement strategies, allocation, surgical techniques, and perioperative management, making LT a successful option for many patients. More than 1,780 patients are actively waiting to receive an allograft, however, and an average of 128 patients will die for every 1000 patients on the waiting list.
The scarcity of lung donors, the increasing waiting time, and the increasing mortality rate of patients on the LT waiting list [1] have led many transplant centers to reevaluate their donor criteria. Over the years, centers have investigated the use of living donors, non–heart-beating donors [2], and the relaxation of donor criteria to increase the donor pool [3, 4]. Understanding the interplay of donor characteristics and procurement strategies on ischemia-reperfusion injury and bronchiolitis obliterans syndrome (BOS) is one important method for narrowing the gap between organ donation and transplantation.
Although the geographic distance between the donor and recipient is not part of the donor acceptance criteria [5], many transplant surgeons hesitate to accept donor lungs from a significant distance because distant harvest sites result in longer graft ischemic times. The influence of graft ischemic time on the development of ischemia-reperfusion injury and then the subsequent development of BOS and overall survival is debated.
The shortage in organ donation is a major limiting factor for patients with end-stage lung disease, and expanding the donor pool would be beneficial. The importance of geographic distance between the donor and recipient has been investigated in single-institutional studies, but not in a large multiple-institutional study [6]. We hypothesized that geographic distance between the donor and recipient is not a critical determinant of outcomes after LT.
Patients and Methods
This study was reviewed by our Institutional Review Board and was granted exemption from approval and patient consent.
Data Source
United Network for Organ Sharing (UNOS) provided transplant and follow-up information from the UNOS Standard Transplant Analysis and Research files for LTs, with all patient and center identifiers excluded.
The UNOS lung transplant and follow-up data set is a prospectively collected database of every organ donation and transplantation in the United States. Standardized data collection forms are used by each transplant center. Donor, preoperative, intraoperative, and postoperative variables are contained within the UNOS data set, and each recipient had yearly follow-up. LT recipients (LTRs) from 2000 to 2005 were included in the study to allow sufficient time for development of BOS.
Study Population
A total of 6,055 LTRs were identified, of whom 3,575 had a local donor, 1,166 had a regional donor, and 1,314 had a national donor. Geographic distance was defined by UNOS and the Organ Procurement and Transplantation Network. Local donation is served by the local organ procurement organization and is usually statewide. If no suitable local matches are available, then regional donation occurs, and the organ is offered to candidates within 500 miles, then 1,000 miles, and then 1,500 miles of the donor site. If there are no matches in the local or regional areas, then national donation occurs and the organ is offered to any candidate who is a potential match.
Variables Examined and Outcomes Measured
A retrospective review of all patients undergoing LT from 2000 to 2005 was performed. All variables were included in the univariate analysis. Factors included in the multivariate analysis were selected by clinical relevance in the literature and LT surgeon experience.
Relevant variables examined for each LTR included demographic factors (age, sex, race, etc), factors related to their pulmonary disease (diagnosis, oxygen requirement, etc), comorbidities, perioperative variables (transplant type, ischemic time, etc), and postoperative outcomes and complications (dialysis, airway dehiscence, pulmonary infection, acute rejection, etc). Ischemic time was measured as the total organ ischemia time, which includes cold ischemic time, warm ischemic time, and anastomotic time. The donor characteristics of each LTR were also examined.
The influence of geographic distance on the development of BOS after LT as well as mortality rates at 1 and 3 years were analyzed. BOS was defined as BOS potential (OP), grade 1, grade 2 or grade 3.
Statistical Analysis
Descriptive analyses were performed between LTRs with local vs regional vs national donors to compare demographic data. A univariate analysis described donor and recipient variables by geographic distance. Categoric variables were compared using χ2 test or the Fisher exact test, and continuous variables were compared with the two-sided t test. Categoric data are reported as frequencies and percentages, and a statistical significance of p < 0.05 was used. A subanalysis included Kaplan-Meier curves of ischemic times of less than 4 hours, 4 to 6 hours, and more than 6 hours, which were analyzed for BOS-free survival and death. Kaplan-Meier curves of local vs regional vs national donation were analyzed for BOS-free survival, 1-year mortality, and 3-year mortality.
Multivariate Cox proportional hazard models were used to identify independent predictors of BOS development and 3-year mortality. The multivariate models were constructed using a priori variables derived from a literature review and clinical knowledge. The variables included donor old age, donor female sex, graft ischemic time, geographic distance, human leukocyte antigen mismatch, recipient diagnosis, single vs double LT, recipient old age, recipient female sex, and recipient peptic ulcer disease at LT. Hazard ratios (HR) are presented with 95% confidence intervals (CI). All statistical analysis was performed with SAS 9.1.3 software (SAS Institute, Cary, NC).
Results
Baseline Demographics
Lung transplantation was performed in 6,055 patients from 2000 to 2005. This cohort was divided by geographic distance between the donor and recipient, of which 3,575 (59%) had a local donor, 1,166 (19.3%) had a regional donor, and 1,314 (21.7%) had a national donor. The mean age of this cohort was 50.0 years old, with 2,961 female recipients (48.9%).
Donor Characteristics
Lung transplant recipients with local, regional, and national donors had similar donor characteristics. Donor age, sex, ethnicity, and preoperative comorbidities were essentially identical between the three geographic distances (Table 1). The rate of traumatic cause of death was also not significantly different between local, regional, and national donors.
Table 1.
Donor Characteristics as Described by Geographic Distance
| Variablesa | Local (n = 3,575) |
Regional (n = 1,166) |
National (n = 1,314) |
p Valueb |
|---|---|---|---|---|
| Old age (≥60 years) | 1045 (29.2) | 352 (30.2) | 404 (30.8) | 0.55 |
| Female sex | 1429 (40.0) | 451 (38.7) | 529 (40.3) | 0.68 |
| White ethnicity | 2506 (70.1) | 856 (73.1) | 929 (70.7) | 0.68 |
| Cytomegalovirus status (positive) | 2008 (57.4) | 681 (58.4) | 768 (58.5) | 0.69 |
| Creatinine (> 75th percentile) | 1017 (28.5) | 3.12 (26.8) | 358 (27.3) | 0.46 |
| Hypertension | 529 (15.1) | 169 (14.5) | 202 (15.4) | 0.88 |
| Diabetes | 111 (3.2) | 43 (3.7) | 59 (4.5) | 0.18 |
| History of | ||||
| Myocardial infarction | 64 (2.1) | 20 (2.1) | 20 (1.8) | 0.82 |
| Tobacco use | 880 (25.0) | 259 (22.0) | 306 (23.3) | 0.27 |
| Intravenous drug use | 39 (1.5) | 9 (1.0) | 8 (0.9) | 0.32 |
| Malignancy | 56 (1.5) | 18 (1.5) | 20 (1.6) | 0.71 |
| Traumatic cause of death | 1862 (54.2) | 606 (56.2) | 666 (52.0) | 0.32 |
| Active pulmonary infection | 741 (21.1) | 235 (20.2) | 219 (16.7) | 0.003b |
Values are shown as number (%).
Significance is set at p < 0.05.
Recipient Characteristics
Lung transplant recipients with donors from different geographic distances varied on several characteristics. There were small differences in recipient older age, ABO match, and previous malignancy (Table 2). Larger differences were seen in single vs double transplant, and graft ischemic time. There was an even distribution between single and double transplants in recipients with local and regional donors (Table 2). However in LTRs with national donors, there were significantly more double than single transplants (60.8% vs 39.2%, p < 0.0001). Not surprisingly, the further the donor geographic distance from the LTR, the longer the graft ischemic time (in minutes): 252 ± 96 local vs 292 ± 90 regional vs 342 ± 90 national (p < 0.0001).
Table 2.
Recipient Characteristics Described by Geographic Distance
| Variablesa | Local (n = 3,575) |
Regional (n = 1,166) |
National (n = 1,314) |
p Valueb |
|---|---|---|---|---|
| Old age (≥60 years) | 1090 (30.5) | 415 (35.6) | 398 (30.1) | 0.003b |
| Female sex | 1753 (49.0) | 557 (47.7) | 651 (49.5) | 0.66 |
| White ethnicity | 3135 (87.7) | 1026 (88.0) | 1180 (89.8) | 0.59 |
| ABO match | 0.01b | |||
| 1 | 3236 (90.5) | 1073 (92.0) | 1224 (93.2) | |
| 2 | 338 (9.5) | 93 (8.0) | 87 (6.6) | |
| 3 | 1 (0.03) | 0 (0) | 3 (0.2) | |
| Cytomegalovirus (IgG positive) | 1865 (58.5) | 649 (60.1) | 674 (57.0) | 0.56 |
| Creatinine (> 75th percentile) | 1159 (32.4) | 339 (29.1) | 440 (33.5) | 0.05 |
| Cerebrovascular disease | 18 (0.5) | 7 (0.6) | 12 (0.9) | 0.37 |
| Diabetes | 371 (10.4) | 126 (10.8) | 144 (11.0) | 0.64 |
| Peripheral vascular disease | 34 (1.0) | 12 (1.1) | 13 (1.0) | 0.91 |
| History of malignancy | 124 (3.5) | 47 (4.0) | 27 (2.1) | <0.0001b |
| Diagnosis | 0.005b | |||
| COPD | 1423 (39.8) | 456 (39.1) | 503 (38.3) | |
| Pulmonary fibrosis | 807 (22.6) | 295 (25.3) | 273 (19.9) | |
| Cystic fibrosis | 534 (14.9) | 149 (12.8) | 245 (18.7) | |
| Sarcoidosis | 97 (2.7) | 30 (2.6) | 48 (3.7) | |
| α-1 antitrypsin deficiency | 238 (6.7) | 71 (6.1) | 76 (5.8) | |
| Pulmonary hypertension | 126 (3.5) | 33 (2.8) | 35 (2.6) | |
| Bronchiectasis | 71 (1.99) | 27 (2.32) | 24 (1.8) | |
| Other | 279 (7.8) | 105 (9.0) | 110 (8.4) | |
| Transplant type | ||||
| Single | 1876 (52.5) | 595 (51.0) | 515 (39.2) | <0.0001b |
| Double | 1699 (47.5) | 571 (49) | 799 (60.8) | |
| Ischemic time, hours | <0.0001b | |||
| <4 | 1930 (54) | 493 (42.3) | 280 (21.3) | |
| 4–6 | 1261 (35.3) | 491 (42.1) | 618 (47.0) | |
| >6 | 384 (10.7) | 182 (15.6) | 416 (31.7) | |
| Ischemic time (min) | 252 ± 96 | 292 ± 90 | 342 ± 90 | <0.0001b |
| Postoperative outcomes | ||||
| Airway dehiscence | 45 (1.3) | 14 (1.2) | 13 (1.0) | 0.0005b |
| Dialysis | 202 (5.7) | 73 (6.3) | 66 (5.0) | <0.0001b |
| Stroke | 63 (1.8) | 19 (1.6) | 25 (1.9) | 0.0002b |
| Infection | 1444 (40.4) | 504 (43.2) | 536 (40.8) | 0.02b |
| Antiviral treatment | 2908 (81.4) | 946 (81.1) | 1091 (83.0) | 0.2 |
Values are reported as number (%).
Significance set at p < 0.05.
COPD = chronic obstructive pulmonary disease; IgG = immunoglobulin G.
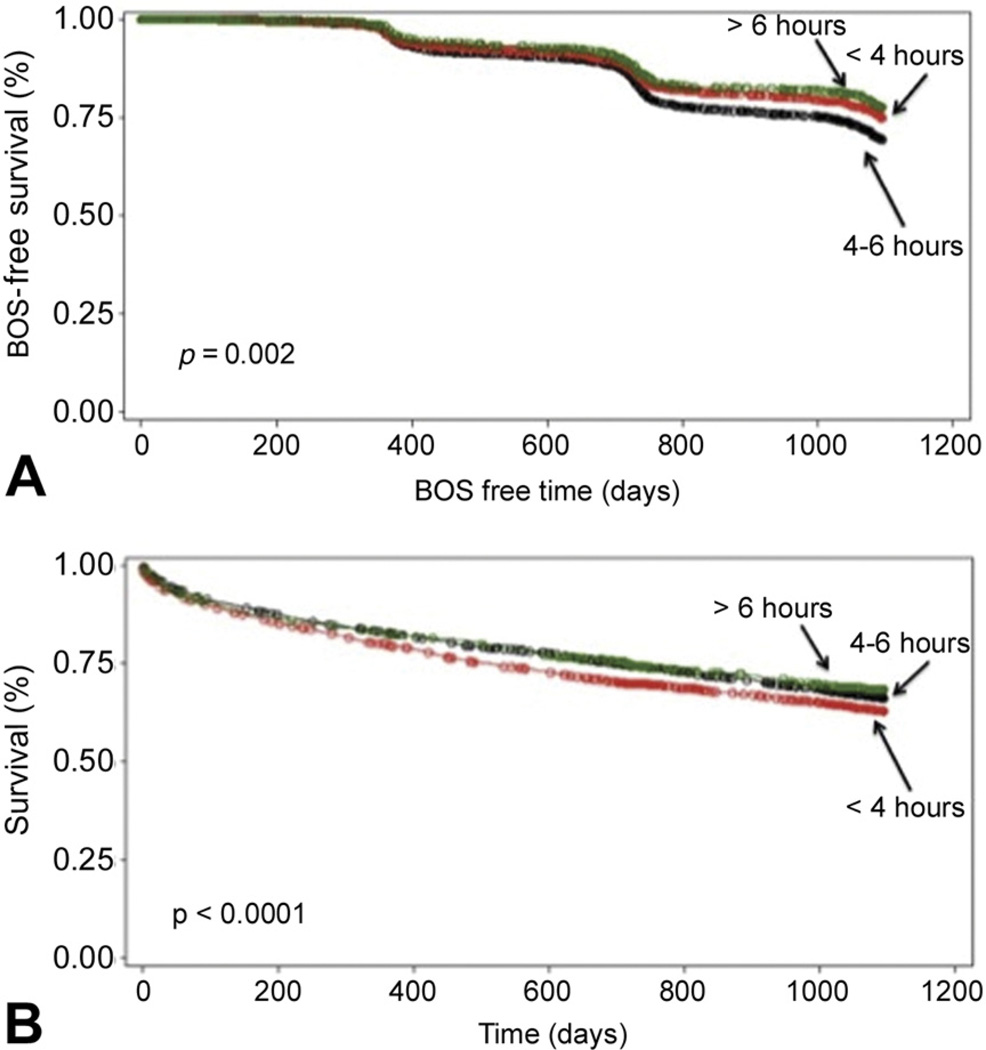
A subanalysis to further categorize ischemic times of less than 4 hours, 4 to 6 hours, and exceeding 6 hours demonstrated that a significantly greater number grafts in the national pool had ischemic times exceeding 6 hours compared with regional or local grafts (p < 0.001). Also, most local grafts had ischemic times of less than 4 hours (Table 2). Kaplan-Meier survival curves (p = 0.002) demonstrated no improvement in survival with ischemic times of less than 4 hours, and this was similarly found with Kaplan-Meier curves of BOS-free survival (p < 0.0001; Fig 1).
Fig 1.
(A) Kaplan-Meier bronchiolitis obliterans syndrome (BOS)-free survival curve for ischemic times. (B) Kaplan-Meier survival curve for ischemic times.
Outcomes by Geographic Distance
Small differences were noted in acute postoperative outcomes, including dehiscence, stroke, dialysis, and infection (Table 2). However, because of a very large population, small differences between LTRs who received donor lungs from different geographic distances were statistically significant.
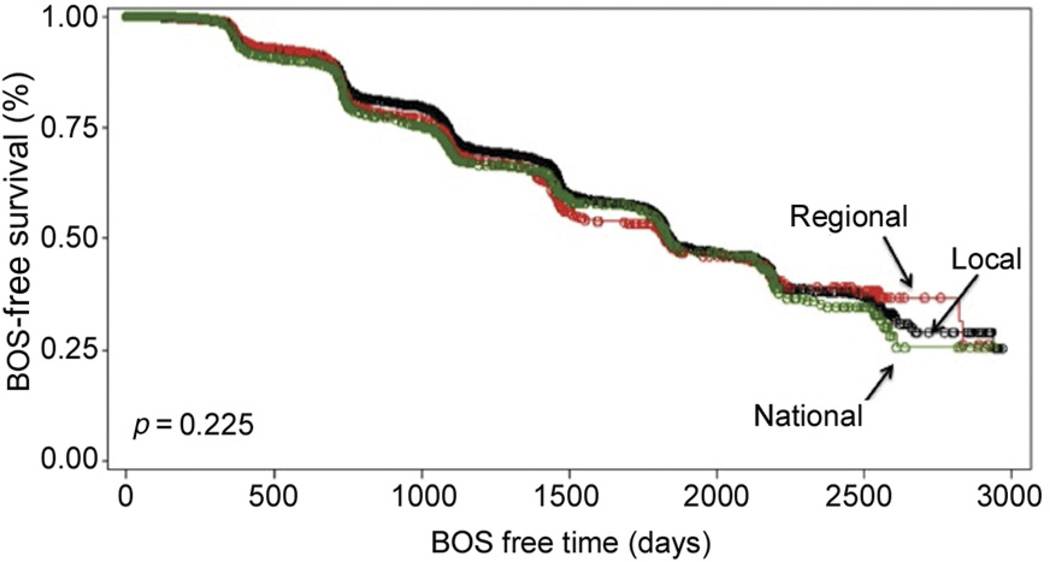
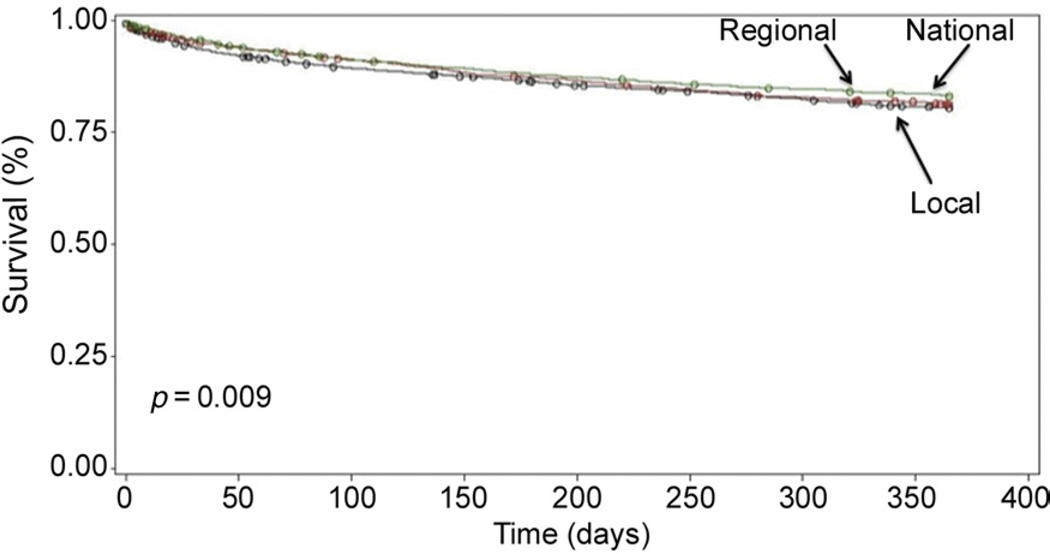
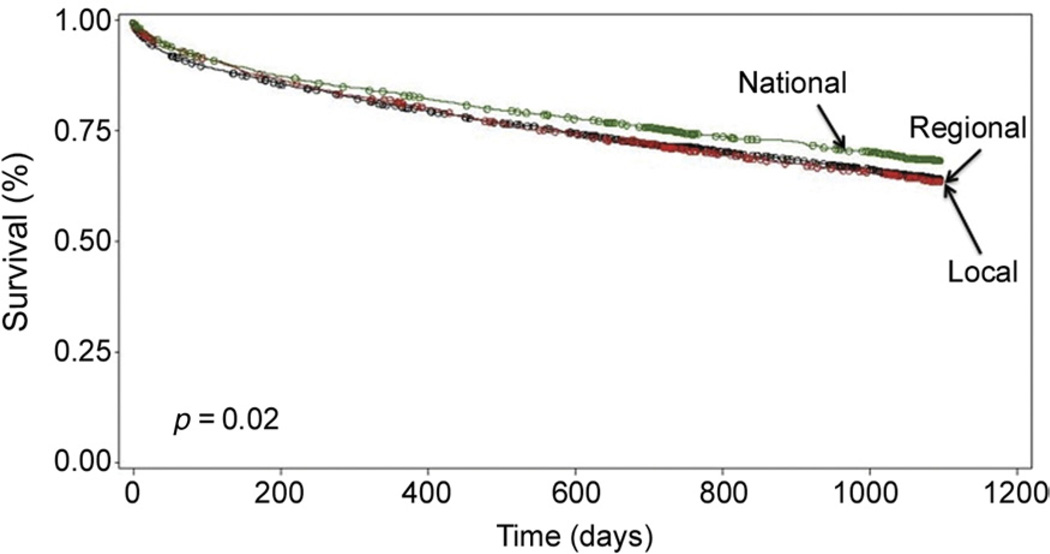
There was no significant difference in development of BOS in LTRs who received a lung from a local vs regional vs national donor (Table 3). Kaplan-Meier curves of BOS-free survival demonstrated no significant difference in BOS between LTRs by geographic distance of their donor (p = 0.23; Fig 2). At 1 year, there was no significant difference in death among the geographic distances (Table 3). Kaplan-Meier survival curves at 1 year were also not different (p = 0.09; Fig 3). At 3 years, however, the mortality rate was significantly lower in LTRs who received lungs from national donors vs regional or local donors (Table 3). Kaplan-Meier survival curves for 3-year mortality demonstrated improved survival for LTRs who received allografts from national donors (p = 0.02; Fig 4).
Table 3.
Outcomes Based on Geographic Distance
| Variablesa | Local (n = 3,575) |
Regional (n = 1,166) |
National (n = 1,314) |
p Valueb |
|---|---|---|---|---|
| BOS | 1115 (31.2) | 364 (31.2) | 429 (32.7) | 0.60 |
| 1-year mortality | 694 (19.4)c | 216 (18.5) | 219 (16.7) | 0.09 |
| 3-year mortality | 1234 (34.5)c | 405 (34.7) | 399 (30.4) | 0.02b |
Values are reported as number (%).
Significance set at p < 0.05;
Significant (p < 0.05) local vs national.
BOS = bronchiolitis obliterans syndrome.
Fig 2.
Kaplan-Meier bronchiolitis obliterans syndrome (BOS)-free survival curve for geographic distance.
Fig 3.
Kaplan-Meier 1-year survival by geographic distance.
Fig 4.
Kaplan-Meier 3-year survival by geographic distance.
Independent Predictors
In the multivariate model, geographic distance between the recipient and donor was not an independent predictor of BOS (HR, 1.02; 95% CI, 0.96 to 1.10; p = 0.40) and neither was graft ischemic time (HR, 0.89; 95% CI, 0.77 to 1.04; p = 0.14). Similarly, neither geographic distance (HR, 0.95; 95% CI, 0.89 to 1.01; p = 0.10) nor graft ischemic time (HR, 0.91; 95% CI, 0.80 to 1.04; p = 0.18) was an independent predictor of 3-year mortality.
Comment
Several strategies have been undertaken in attempts to expand the donor pool for LT. Despite these attempts, there continues to be a considerable shortage, and the mortality rate for patients on the LT waiting lists continues to rise [1]. Given the pressure to expand the donor pool, accepting donor lungs from long geographic distances may be one simple and effective way to use more donors and perform more LTs.
Many transplant centers hesitate accepting donor lungs from distant harvest sites because the long geographic distances contribute to longer graft ischemic times; however, few studies have examined the influence of recipient and donor geographic distance on LT outcomes. Given the very limited literature, we decided to review the literature on graft ischemia. The influence of graft ischemic time on postoperative outcomes after LT is debated. Many of the current studies report conflicting information, which is frequently due to small sample sizes and single-institution studies.
In this study 59% of LTRs received lungs from local donors, 19% received lungs from regional donors, and 23% received lungs from national donors. The longer geographic distance between the donor and recipient was associated with longer ischemic times (Table 2). However, longer ischemic times (> 6 hours) were not associated with worse outcomes after LT (Fig 1) and neither was a long geographic distance (Fig 2 to Fig 4). Geographic distance between the donor and recipient was not an independent predictor of BOS (Table 4) or death (Table 5).
Table 4.
Multivariate Cox Proportional Hazards Model for Predictors of Bronchiolitis Obliterans Syndrome
| Variable | HR (95% CI) | p Valuea |
|---|---|---|
| Geographic distance | 1.03 (0.96–1.10) | 0.40 |
| Graft ischemic time | 0.89 (0.77–1.04) | 0.14 |
| Donor sex (female) | 0.85 (0.75–1.01) | 0.08 |
| Donor old age (≥60 years) | 1.02 (0.90–1.16) | 0.78 |
| Diagnosis | 0.97 (0.94–1.01) | 0.14 |
| Transplant type (double vs single) | 0.94 (0.83–1.07) | 0.34 |
| Recipient sex (female) | 1.07 (0.95–1.20) | 0.26 |
| Recipient old age | 0.95 (0.81–1.04) | 0.18 |
| HLA mismatch | 1.02 (0.98–1.0) | 0.47 |
| Peptic ulcer disease | 1.0 (0.80–1.23) | 0.97 |
Significance p < 0.05.
CI = confidence interval; HLA = human leukocyte antigen; HR = hazard ratio.
Table 5.
Multivariate Cox Proportional Hazards Model for Predictors of 3-Year Mortality
| Variable | HR (95% CI) | p Valuea |
|---|---|---|
| Geographic distance | 0.95 (0.89–1.01) | 0.10 |
| Graft ischemic time | 0.91 (0.80–1.04) | 0.18 |
| Donor sex (female) | 1.01 (0.90–1.13) | 0.84 |
| Donor old age (≥60 years) | 1.13 (1.02–1.26) | 0.03a |
| Diagnosis | 1.0 (0.99–1.01) | 0.10 |
| Transplant type (double vs single) | 0.97 (0.87–1.08) | 0.58 |
| Recipient sex (female) | 0.95 (0.85–1.06) | 0.39 |
| Recipient old age | 1.2 (1.08–1.34) | 0.001a |
| HLA mismatch | 1.03 (0.98–1.07) | 0.24 |
Significance set at p < 0.05.
CI = confidence interval; HLA = human leukocyte antigen; HR = hazard ratio.
Kaplan-Meier survival curves and BOS-free survival were significantly different between graft ischemic times, with longer graft ischemic times conferring improved outcomes. These findings support our conclusions that donor geographic distance should not influence a center’s criteria for allograft selection for transplant. Improved outcomes with longer graft ischemic times was an unexpected finding and can likely be explained by donor selection criteria of each transplant center. To account for such selection bias, donor and recipient variables were included in the multivariate analysis for independent predictors of death and BOS.
Patterson and colleagues [6] are one of the few groups that has investigated the influence of geographic distance between the donor and recipient, local vs distant. They found no significant difference in survival and concluded that survival after LT is unaffected by long-distance harvest [6]. In comparison, no other series has reported higher rates of mortality or increased BOS with longer geographic distances between LTRs and their donors.
Bronchiolitis obliterans syndrome occurs in up to 50% of LTRs and is a major cause of morbidity and late death after LT [7]. In their series, Fiser and colleagues [8] reported no significant difference in BOS onset or progression when comparing ischemic times of less than 4 hours, 4 to 6 hours and more than 6 hours. Several other groups have also demonstrated that prolonged ischemic time had no influence on the development of BOS [9–12]. Similar to previous findings, our study found no significant difference in the development of BOS between LTRs who received donor lungs from local vs regional vs national donors (Table 3). The Kaplan-Meier curve for BOS-free survival was not significantly different after adjustment for geographic distance. Most importantly, geographic distance and ischemic time were not independent predictors for BOS development.
The influence of prolonged graft ischemic time on survival after LT has always been controversial. In their series, Thabut and colleagues [13] reported that graft ischemic time was associated with long-term mortality, and they found a steep increase the relative risk of death when graft ischemic time was more than 6 hours. Other groups have also found an association between prolonged ischemic time and decreased survival [11]. However, several groups have demonstrated no relationship between graft ischemic time and death after LT [8, 9, 14–17].
Geographic distance did not influence 1-year survival in our series (Fig 3), and 3-year survival was not worse with national donors (Fig 4). Mortality at 3 years was significantly different, with decreased mortality in LTRs with national donors compared with local donors (Table 3). This was an unexpected finding. There was a statistically significant difference in the number of double LTs in the national donor pool compared to the donor pool of single LTs. The improved 3-year mortality in LTRs from national donors may be explained by these recipients undergoing more double LTs than single LTs; however, this was controlled for in the multivariate analysis. Most importantly, geographic distance and graft ischemic time were not independent predictors of death at 3 years (Table 5).
The contradictory findings in previous studies may be explained by advances in LT over time, along with limitations of single-institutional studies. In the more recent era, significant changes have occurred in procurement strategies and in preservation fluids. Several studies have demonstrated superior preservation with low potassium dextran solutions, such as Perfadex (Vitrolife, Göteborg, Sweden) [14, 18, 19], and lower rates of moderate to severe ischemia–reperfusion injury after transplantation [18]. De Perrot and colleagues [12] also suggested that the significance of ischemic time is less important with such improvements.
This study has some limitations. It is inherently biased by its retrospective nature. The UNOS data set is collected by individual centers, and each center has an independent method of interpreting the variables measured by UNOS and patient outcomes. However, UNOS limited such confounders by the creation of standardized data collection forms. It is also likely that these confounders are equally distributed between all transplant centers.
We also recognize that other factors that were not collected by UNOS may influence outcomes after LT. The UNOS database represents a heterogeneous group and therefore it cannot be known whether higher-risk donors were not used for higher-risk recipients. This is an inherent limitation of a large multiple-institutional database; however, given the very large population in this database, it could be assumed the various donor-recipient matches would occur equally and randomly. Despite these limitations, the UNOS data set does allow us to study one of the largest populations of LTRs, which is often one of the major limitations of single-center studies [20].
It is important to identify that geographic distance may not always be a proxy for ischemic time, especially when looking at extremes of ischemic time. The average graft ischemic time significantly differs between local vs regional vs national donor pools; however, the average ischemic times are less than 6 hours for all groups and, therefore, the data set is biased. However, this study aims not to analyze the extremes of ischemic time but to demonstrate that long geographic distances with variable but reasonable ischemic times should not prevent a lung transplant from occurring given no difference in rates of BOS or mortality in this study.
In conclusion, with appropriate donor selection, moderately long geographic distance (average ischemic time < 6 hours) between the donor and recipient is not associated with the development of BOS or increased death after LT. By placing less emphasis on distance and ischemic time, more donors could potentially be used. This has the potential to increase lung transplantation and reduce waiting list mortality.
Acknowledgments
This work was supported in part by Health Resources and Services Administration contract 234-20050370011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Presented at Poster Session of the Forty-seventh Annual Meeting of The Society of Thoracic Surgeons, San Diego, CA, Jan 31-Feb 2, 2011.
References
- 1.De Perrot M, Waddell TK, Shargall Y, et al. Impact of donors aged 60 years or more on outcome after lung transplantation: results of an 11-year single-center experience. J Thorac Cardiovasc Surg. 2007;133:525–531. doi: 10.1016/j.jtcvs.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 2.Steen S, Sjoberg T, Pierre L, et al. Transplantation of lungs from a non-heart-beating donor. Lancet. 2001;357:825–829. doi: 10.1016/S0140-6736(00)04195-7. [DOI] [PubMed] [Google Scholar]
- 3.Bhorade SM, Vigneswaran W, McCabe MA, Garrity ER. Liberalization of donor criteria may expand the donor pool without adverse consequence in lung transplantation. J Heart Lung Transplant. 2000;19:1199–1204. doi: 10.1016/s1053-2498(00)00215-1. [DOI] [PubMed] [Google Scholar]
- 4.Kawut SM, Reyentovich A, Wilt JS, et al. Outcomes of extended donor lung recipients after lung transplantation. Transplantation. 2005;79:310–316. doi: 10.1097/01.tp.0000149504.53710.ae. [DOI] [PubMed] [Google Scholar]
- 5.Yeung JC, Cypel M, Waddell TK, et al. Update on donor assessment, resuscitation, and acceptance criteria, including novel techniques—non-heart-beating donor lung retrieval and ex vivo donor lung perfusion. Thorac Surg Clin. 2009;19:261–274. doi: 10.1016/j.thorsurg.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Meyers BF, Lynch J, Trulock EP, et al. Lung transplantation: a decade of experience. Ann Surg. 1999;230:362–371. doi: 10.1097/00000658-199909000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verleden GM. Chronic allograft rejection (obliterative bronchiolitis) Semin Respir Crit Care Med. 2001;22:551–558. doi: 10.1055/s-2001-18427. [DOI] [PubMed] [Google Scholar]
- 8.Fiser SM, Kron IL, Long SM, et al. Influence of graft ischemic time on outcomes following lung transplantation. J Heart Lung Transplant. 2001;20:1291–1296. doi: 10.1016/s1053-2498(01)00355-2. [DOI] [PubMed] [Google Scholar]
- 9.Gammie JS, Stukus DR, Pham SM, et al. Effect of ischemic time on survival in clinical lung transplantation. Ann Thorac Surg. 1999;68:2015–2020. doi: 10.1016/s0003-4975(99)00903-0. [DOI] [PubMed] [Google Scholar]
- 10.Ueno T, Snell GI, Williams TJ, et al. Impact of graft ischemic time on outcomes after bilateral sequential single-lung transplantation. Ann Thorac Surg. 1999;67:1577–1582. doi: 10.1016/s0003-4975(99)00309-4. [DOI] [PubMed] [Google Scholar]
- 11.Snell GI, Rabinov M, Griffiths A, et al. Pulmonary allograft ischemic time: an important predictor of survival after lung transplantation. J Heart Lung Transplant. 1996;15:160–168. [PubMed] [Google Scholar]
- 12.De Perrot M, Bonser RS, Dark J, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part III: donor-related risk factors and markers. J Heart Lung Transplant. 2005;24:1460–1467. doi: 10.1016/j.healun.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Thabut G, Mal H, Cerrina J, et al. Graft ischemic time and outcome of lung transplantation: a multicenter analysis. Am J Respir Crit Care Med. 2005;171:786–791. doi: 10.1164/rccm.200409-1248OC. [DOI] [PubMed] [Google Scholar]
- 14.Kshettry VR, Kroshus TJ, Burdine J, et al. Does donor organ ischemia over four hours affect long-term survival after lung transplantation? J Heart Lung Transplant. 1996;15:169–174. [PubMed] [Google Scholar]
- 15.Meyer DM, Bennett LE, Novick RJ, Hosenpud JD. Effect of donor age and ischemic time on intermediate survival and morbidity after lung transplantation. Chest. 2000;118:1255–1262. doi: 10.1378/chest.118.5.1255. [DOI] [PubMed] [Google Scholar]
- 16.Winton TL, Miller JD, deHoyos A, et al. Graft function, airway healing, rejection, and survival in pulmonary transplantation are not affected by graft ischemia in excess of 5 hours. Transplant Proc. 1993;25:1649–1650. [PubMed] [Google Scholar]
- 17.Novick RJ, Bennett LE, Meyer DM, Hosenpud JD. Influence of graft ischemic time and donor age on survival after lung transplantation. J Heart Lung Transplant. 1999;18:425–431. doi: 10.1016/s1053-2498(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 18.Oto T, Griffiths AP, Rosenfeldt F, et al. Early outcomes comparing Perfadex, Euro-Collins, and Papworth solutions in lung transplantation. Ann Thorac Surg. 2006;82:1842–1848. doi: 10.1016/j.athoracsur.2006.05.088. [DOI] [PubMed] [Google Scholar]
- 19.Fischer S, Matte-Martyn A, De Perrot M, et al. Low-potassium dextran preservation solution improves lung function after human lung transplantation. J Thorac Cardiovasc Surg. 2001;121:594–596. doi: 10.1067/mtc.2001.109703. [DOI] [PubMed] [Google Scholar]
- 20.Hennessy SA, Hranjec T, Swenson BR, et al. Donor factors are associated with bronchiolitis obliterans syndrome after lung transplantation. Ann Thorac Surg. 2010;89:1555–1562. doi: 10.1016/j.athoracsur.2010.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]