Introduction
This document is complementary to an Expert Review Document on Optical Coherence Tomography (OCT) for the study of coronary arteries and atherosclerosis.1 The goal of this companion manuscript is to provide a practical guide framework for the appropriate use and reporting of the novel frequency domain (FD) OCT imaging to guide interventional procedures, with a particular interest on the comparison with intravascular ultrasound (IVUS).1–4
Technique for optical coherence tomography imaging
In the OCT Expert Review Document on Atherosclerosis, a comprehensive description of the physical principles for OCT imaging and time domain (TD) catheters (St Jude Medical, Westford, MA, USA) was provided.1
The main advantage of FD-OCT is that the technology enables rapid imaging of the coronary artery, using a non-occlusive acquisition modality. The FD-OCT catheter (DragonflyTM; St Jude Medical) employs a single-mode optical fibre, enclosed in a hollow metal torque wire that rotates at a speed of 100 r.p.s. It is compatible with a conventional 0.014″ angioplasty guide wire, inserted into a short monorail lumen at the tip. The frequency domain optical coherence tomography lateral resolution is improved in comparison with TD-OCT, while the axial resolution did not change. These features, together with reduced motion artefacts and an increased maximum field of view up to 11 mm, have significantly improved both the quality and ease of use of OCT in the catheterization laboratory.3,4 However, the imaging depth of the FD-OCT is still limited to 0.5–2.0 mm.5
Methodological issues: image acquisition, safety, and effectiveness
The main obstacle to the adoption of TD-OCT imaging in clinical practice is that OCT cannot image through a blood field, and therefore requires clearing or flushing of blood from the lumen.1 The 6 Fr compatible DragonflyTM FD-OCT catheter is so far the only one in the market, as two other systems from Volcano and Terumo, which have function similar to the DragonflyTM, are not yet available. The DragonflyTM catheter is first advanced over a regular guide wire, distal to the region of interest. A dedicated marker, located 10 mm distal to the OCT lens, enables the pull-back starting point selection.
The acquisition of an OCT image sequence requires a bolus of crystalloid solution (usually contrast) injected through the guiding catheter. The acquisition speed can be set up in a range between 5 and 40 mm/s, based on the used OCT system. Most expert users advocate the use of automated contrast injection to optimize the image quality.
The previous experience with TD and FD-OCT technology shows OCT acquisition to be safe,1,4,6 effective,1,4,6–8 and highly reproducible for the assessment of the luminal areas and length.9–11 A fair correlation between OCT and IVUS quantitative measurements of the lumen areas was reported,9–11 despite comparative studies showing that IVUS tends to slightly overestimate lumen areas, while stent and neointimal areas are slightly higher on OCT.9–11 Frequency domain optical coherence tomography image quality depends on an accurate acquisition technique and proper guiding catheter engagement is needed to optimize directional contrast flushing.
Procedural definitions
Metallic stent struts are highly reflective structures that lead to specular reflection with the excessive signal usually resulting in ‘blooming’ of the strut surface. Optical coherence tomography shows only the reflection of the luminal surface of the stent strut and is unable to provide a direct measurement of the strut thickness.8,12
Two features indicate a strut: a highly reflective spot and/or an associated shadow behind the strut. The presence of only one of these two defining features is sufficient for strut identification.
For bioresorbable stents, the assessment of the whole thickness is possible, since light can usually penetrate non-metallic materials. They can be followed by serial images; the degradation process, characterized by the presence of empty spaces replacing stent struts, was documented for the first generation design,13 but was not visible with the second-generation model, which maintains its mechanical properties.14 Based on preliminary unpublished data, the bioabsorbable magnesium stent has different properties; it is visible after implantation and tends to disappear at the follow-up due to magnesium degradation.
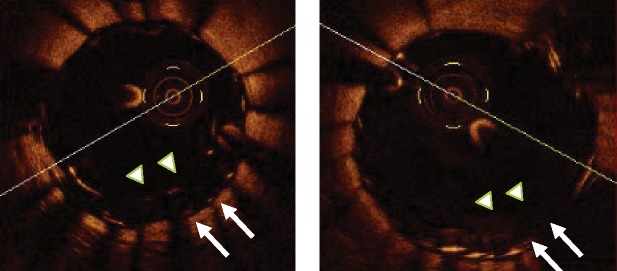
Stent overlap occurs in the cross sections in which two superimposed layers of stent struts are visible (Figure 1). Incomplete stent apposition or malapposition occurs if there is a separation of a stent strut from the vessel wall. Malapposition can be addressed at a cross-section level and expressed as an area, or can be evaluated at the strut level analysis.15 In the latter case, it is defined as a measured distance greater than the strut thickness for bare metal stents or greater than the sum of the thickness of the strut plus polymer for drug eluting stents (DES)8,15 (Figure 2). Acute malapposition is diagnosed immediately after stent deployment (Figure 1),14 late malapposition at the follow-up, while late acquired malapposition requires the comparison of the post-intervention and follow-up images. Intra-stent tissue protrusion is defined as tissue prolapsed between stent struts extending inside a circular arc connecting adjacent struts (Figure 3).16 A thrombus is identified as an intra-luminal mass, with no direct continuity with the surface of the vessel wall or as a highly backscattered luminal protrusion in continuity with the vessel wall and resulting in signal-free shadowing.17 However, the discrimination between a tissue prolapse and intra-stent thrombosis is not simple and sufficiently validated. A dissection flap is a linear rim of tissue with a clear separation from the vessel wall, plaque, or the stent struts.18 Dissections are also frequent at the stent edges (Figure 4) and are defined by their longitudinal extension (mm), circumferential extension (degrees or quadrants), and width.
Figure 1.
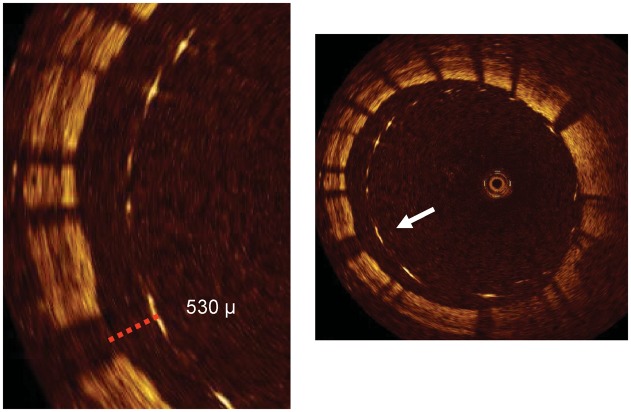
Left panel: Stent overlapping in the left anterior descending artery. Despite an optimal angiographic result, optical coherence tomography shows a malapposition of the proximal edge of the distal stent, with a 430 µ distance from the inner (arrow heads) to the outer struts (arrows). Right panel: the optical coherence tomography image of the same cross section obtained after additional high pressure inflation with a non-compliant oversized balloon shows a correction of the malapposition.
Figure 2.
An optical coherence tomography cross section of a stent immediately following implantation in the right coronary artery. The stent struts are clearly visible. There are struts that are apposed to the vessel wall. However, what is clearly evident is a group of stent struts (between 4 and 12 o'clock) which are grossly malapposed to the vessel wall. Optical coherence tomography cannot penetrate through metallic structures and hence only shows the endoluminal aspect of the stent strut. To be classified as malapposed, the strut must not be in contact with the vessel wall with the distance between the endoluminal aspect and the vessel wall greater than the known thickness of the stent strut itself, including the polymer thickness (arrow in the right panel and dotted line in the left panel with a magnified view).
Figure 3.
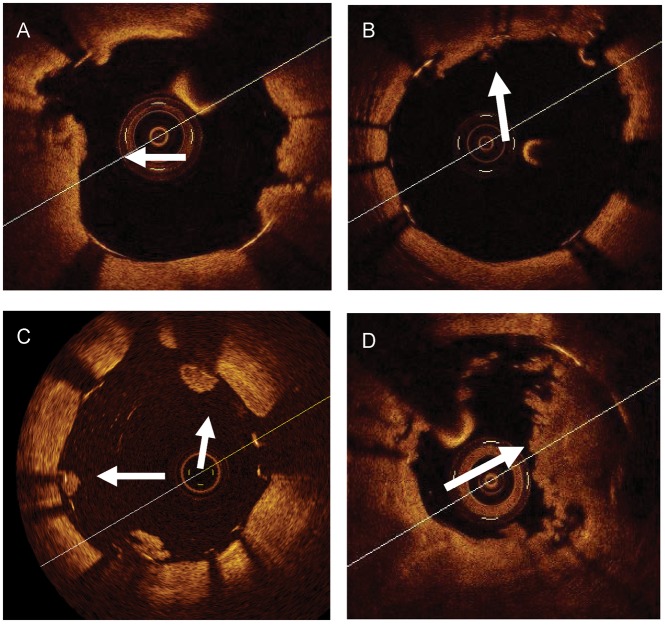
Examples of intra-stent thrombotic formations and a plaque prolapse. (A and B) A tissue protrusion. Arrows show tissue prolapsed between stent struts and extending inside a circular arc, connecting adjacent struts. (C) Intra-luminal globular protrusions at stent strut locations with no direct continuation from the surface of the vessel wall (arrows). (D) A marked intra-stent thrombosis observed in a drug eluting stents at a late follow-up. Arrows show an intra-stent mass with an irregular inner border. Images in (A) and (D) were obtained with frequency domain optical coherence tomography, those in (B) and (C) with time domain optical coherence tomography.
Figure 4.
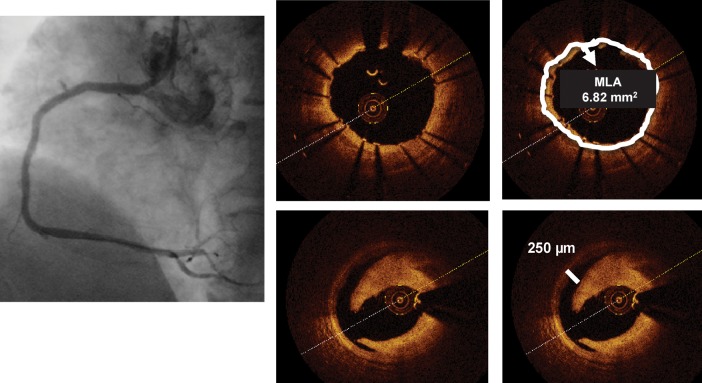
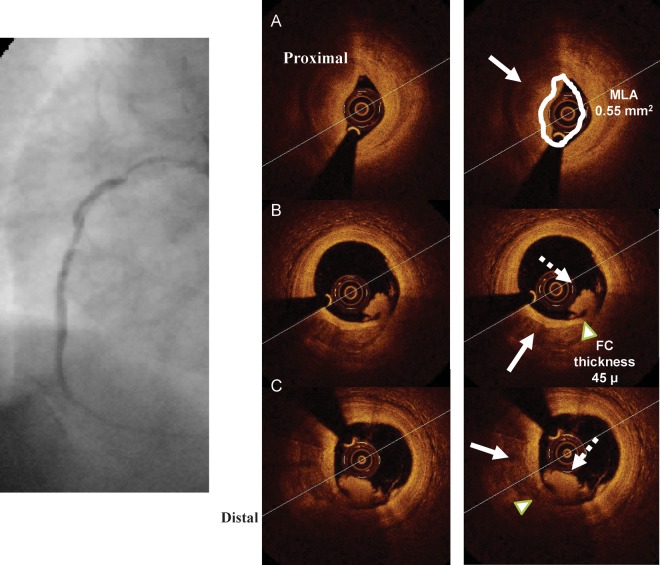
Left panel: Cypher stent deployed in the mid-right coronary artery with an optimal final angiographic result. Optical coherence tomography (right inferior panels) shows a moderate dissection distal to the stent ending, missed by angiography. The distance between the rim of the dissection and the vessel wall is 250 µm (line), slightly above the resolution of intravascular ultrasound, while the circumferential extension of the plaque dissection is 90°. The right upper panels are obtained at the stent minimum lumen area and show a well-apposed stent with an MLA of 6.82 mm2.
Clinical applications of optical coherence tomography and comparison with intravascular ultrasound
Impact of plaque composition on coronary intervention
Calcification
Unlike angiography, IVUS identifies calcium with high accuracy,19 but is unable to measure its thickness.1 Infrared light penetrates calcium better, but calcific components with a thickness >1–1.3 mm or a deep intra-plaque location can prove impossible to penetrate.
In the presence of an important circumferential calcification direct stenting should be avoided and the strategy can span from a careful pre-dilatation to test expansion, to cutting balloons for very short calcific rings or to pre-treatment with a rotational atherectomy.19,20
As OCT accurately measures the radial and longitudinal extension of a lipid pool, its use may be encouraged to also predict the risk of embolization of plaque components;1 this is in line with the previous experience with VH-IVUS.21,22
Unlike angiography or IVUS, OCT holds promise in identifying thrombi, measuring their dimensions and guiding their removal23 (Figures 5 and 6).
Figure 5.
The figure shows optical coherence tomography potentialities to detect components of ruptured plaques. Left panel shows a suboccluded right coronary artery in a patient with an inferior myocardial infarction. Optical coherence tomography cross sections (B) and (C) were obtained at the site of a plaque rupture and show a plaque with mixed composition (calcium + lipid) (arrow). At the shoulder of the plaque, at the site of minimum thickness of the fibrous cap (arrow head), an intra-luminal thrombotic formation is shown (dotted arrow). (A) A severe reduction in the lumen area due to a marked concentric thrombotic formation. As the penetration of optical coherence tomography through a thrombus is limited, vessel media cannot be studied (arrow).
Figure 6.
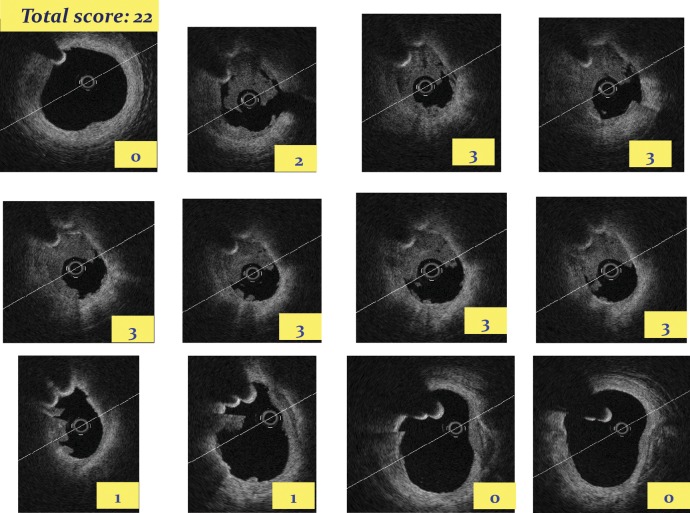
The application of a score based on the semi-quantitative assessment of a thrombus (number of the involved quadrants on the cross-sectional optical coherence tomography images) and the longitudinal extension of the thrombus itself. By applying this semi-quantitative grading, in each cross section, a thrombus is classified as absent or subtending 1, 2, 3, or 4 quadrants. The score is then calculated as the sum of each cross-section score.
Assessment of ambiguous angiographic lesions and deferral of interventions
In angiographically hazy lesions, OCT often detects ruptured plaques with a thrombus attached to the site of rupture of the fibrous caps over a partially emptied lipid pool.1,6 Under these circumstances, the decision for the need to proceed with treatment can stem more from these morphological observations than from the absolute measurement of the lumen area.
A technical drawback of both TD-OCT and FD-OCT in this application is that a plaque located at the very ostium of the left or right coronary arteries cannot be accurately imaged.1 As for IVUS, preliminary data indicate that OCT can change the operator's intention to treat, avoiding unnecessary interventional procedures or modifying the strategy in some cases.6,24,25
As FD-OCT probes have a slightly thinner profile than IVUS probes and pull-back imaging can be done at very high speeds (normally 20 mm/s), a significant fraction of severely diseased target lesions can be imaged without causing luminal obstruction, with symptomatic ischaemia being less likely. However, in the presence of subocclusive lesions the OCT probe can cause a luminal obstruction and it can be more convenient to perform OCT after gentle pre-dilatation.
Identification of vulnerable plaques
A number of IVUS studies attempted to characterize the appearance of vulnerable plaques. Recently, the PROSPECT trial, based on a signal radiofrequency analysis of the IVUS backscatter, showed for the first time that IVUS can identify plaques with a risk of events at 3 years.26 OCT, due to its high accuracy in the detection of superficial plaque components, can directly measure the fibrous cap thickness and can possibly detect plaques with a risk of a rupture with greater accuracy. The utility of a combined approach based on the use of OCT and VH-IVUS has been recently proposed to better characterize deep lesion components.27,28 Possibly, in the near future, a real-time application of OCT algorithms to characterize plaque components and identify local signs of inflammation will facilitate OCT detection of plaque vulnerability.29,30
Advantages and pitfalls of optical coherence tomography for guidance of coronary interventions
Optical coherence tomography easily enables the comparison of the minimal stent area with the reference area, which is the most often used IVUS criterion for optimal stent expansion. The demonstration of the usefulness of an IVUS-guided approach of a bare metal stent expansion to reduce restenosis is more by meta-analyses31 than by single randomized studies.32,33 There is some evidence that IVUS guidance can improve the clinical outcome in the presence of left main disease34 and tackles the occurrence of thrombosis.35,36 The ability of OCT to address luminal areas and identify underexpansion, malapposition, uneven stent strut distribution, or small intra-stent thrombotic formations makes the technique a very attractive tool for the prevention of thrombosis. However, future studies are required to determine whether OCT is as useful or more useful than IVUS in this regard.
Acute malapposition can contribute to a stent thrombosis by disturbing the normal laminar blood flow along the vessel wall, by promoting the deposition of platelets and fibrin37 and reducing re-endothelialization and neointima formation.38 However, to date, IVUS data suggest that acute and late-stent malapposition do not increase the risk of major adverse cardiac events.39,40 Nevertheless, both studies detected a surprisingly small number of malapposed stents (<7.2%) using the IVUS-derived criterion of at least one malapposed strut. Optical coherence tomography studies following stent implantation have demonstrated a much greater proportion of malapposed struts, even after optimal high-pressure post-dilatation, with this phenomenon being particularly evident in regions of stent overlap,41 after deployment of DES or long stents, and in type C lesions.15
In patients with acute coronary syndromes, the occurrence of an in-stent tissue protrusion, due to the presence of residual thrombus, is a common finding on OCT1,8,42 that may elevate the risk for an acute and subacute stent thrombosis.
The need of serial OCT acquisitions to guide the selection of balloons and stents and to correct underexpansion means repeated contrast injections which may significantly increase the total amount of procedural contrast. The main drawback of OCT is its inability, unlike IVUS, to outline the vessel architecture, and measure the external elastic membrane, and the longitudinal extent of plaque burden in lesions with thicknesses exceeding 1.0–1.5 mm (Figure 7). This drawback may have some clinical implications, as the accepted criterion for the identification of reference segments by IVUS is a plaque burden <40%.33 Therefore, the pre-intervention use of OCT to select the appropriate stent size and length in diffusely diseased vessels is questionable.
Figure 7.
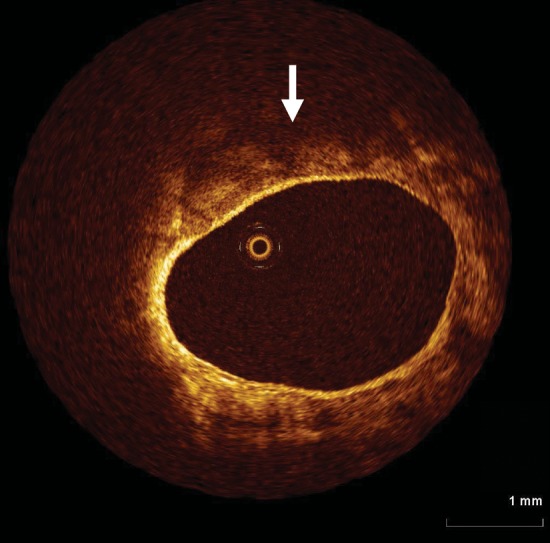
Optical coherence tomography is very well versed at detecting microstructures in close proximity to the vessel wall. Its limited tissue penetration (<1.5 mm), however, means that the extent of plaque is unable to be determined. In this example, diffuse, poorly reflective tissue is seen consistent with a lipid rich plaque (arrow), however, its extent beyond 1 mm from the vessel wall is limited.
Optical coherence tomography has an easier application for the treatment of late in-stent restenosis because the strong reflective power of the stent struts allows their detection through thick layers of hyperplasia, allowing optimal sizing of high-pressure balloons to correct underexpansion and facilitating the use of cutting balloons.15
Stent follow-up
Delayed healing and poor endothelialization are common findings in the pathological specimens of vessels treated with DES,43 and recent post-mortem studies demonstrated that a late-stent thrombosis was strongly associated with the ratio of uncovered/total stent struts.44
Several experimental and clinical studies have demonstrated the high correlation between OCT and histological measurements of tissue coverage of stent struts,45,46 although OCT is unable to identify the endothelium.1 In a subanalysis of the ODESSA trial, 8% of the stented segments with no detectable neointima by IVUS were found to have neointimal coverage by OCT.41
Follow-up OCT data revealed that most of the DES, including those with a biodegradable polymer, were covered with thin neointima, but few revealed complete coverage.47–51 Incomplete stent apposition without neointimal hyperplasia was significantly associated with the presence of an OCT-detected thrombus at the follow-up, and may constitute a potent substrate for a late-stent thrombosis;52 however, so far a subclinical thrombus has not been related to the risk of major adverse cardiac events.53
Furthermore, incomplete stent apposition and the absence of OCT tissue coverage are more frequently found in the setting of acute coronary syndromes, particularly after DES deployment (Figure 8).54 It is difficult to offer any recommendation at this stage for the use of OCT for the late follow-up of individual patients but anecdotal cases of OCT application to rule out the need for the prolongation of a dual antiplatelet treatment in patients requiring undeferrable surgery have been reported. The main application is, at present, the comparison of different stent platforms, assuming that a more uniform strut coverage could improve the late outcome.
Figure 8.
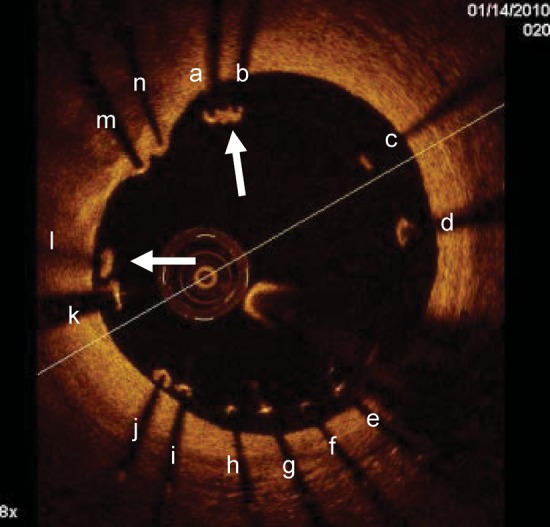
Malapposed struts tend not to heal with tissue coverage unlike well-apposed ones. Optical coherence tomography images obtained 1 month after Taxus stent deployment in the right coronary artery. Optical coherence tomography showed malapposition of the drug eluting stents with many struts not adhering at the vessel wall. The vast majority of malapposed struts (from a to l) were uncovered while only two of them (arrows) had some tissue coverage. On the other hand the two well-apposed struts (m, n) were covered.
Standardized methodology for core laboratory optical coherence tomography image analysis
Optical coherence tomography is being increasingly used as a surrogate method for evaluating stents.15 The ability to provide serial images and analyse a large number of OCT cross section and stent struts represent an advantage over standard histopathology. Core laboratory OCT image analysis should, however, be performed according to a standardized methodology and after a deep understanding of the method.15 Many OCT readers suggest to address images not only at a strut level, but including also cross-sectional and in-stent level analyses, to further study an in-stent coverage uniformity and individual propensity towards incomplete stent healing. Furthermore, if available, automated tools should be applied to reduce possible intra- and inter-observer' variability.55
Ideally, for each strut information on malapposition, coverage, presence of thrombotic formations, and tissue prolapse should be given. Different classifications have been adopted so far by OCT readers and data on intra- and inter-observer variability for the applied methods are not available. As a rationale solution, the following classification can be adopted to define stent struts throughout the pull-back image sequence: embedded covered struts: covered by tissue with at least 50% of the strut boundary below the level of the luminal surface; protruding covered struts: covered by tissue and with the strut boundary located above the level of the luminal surface; uncovered apposed struts: for those not covered by tissue but abutting the vessel wall; uncovered and malapposed struts: for those not covered by tissue and not abutting the vessel wall.1,15,41
Some OCT readers explored different patterns of stent coverage56,57 describing a homogeneous vs. heterogeneous pattern of intimal thickening.56 An intra-stent thrombus is typically shown as an in-stent globular appearance and can be reasonably identified. In the presence of stent malapposition, the lumen area should be divided into an in-stent lumen area and an extra-stent lumen area. Stents overlapping segments and bifurcation lesions with major side branches should be noted.
Conclusions
Optical coherence tomography enables an accurate vessel lumen and stent assessment, providing a high image quality with fast pull-back speeds, procedural safety, and patient tolerability. Optical coherence tomography offers unique insights into stenting procedures that otherwise would be missed using conventional angiography or IVUS. Whether such anatomical details will lead to superior or complementary information with respect to IVUS in guiding coronary intervention, particularly in the era of DES, remains to be defined.
Funding
Funding to pay the Open Access publication charges for this article was provided by CLI Foundation.
Conflict of interest: F.P. has received consultant fees from St Jude Medical. G.G. has received consultant fees from Boston Scientific, St Jude Medical, Volcano, and Cordis; and research grants from Boston Scientific, Medtronic Vascular, Abbott, and LightLab Imaging. G.S.M. has received consultant fees from Volcano and St Jude Medical; and grant support from Volcano and Boston Scientific. M.C. has received consultant fees from Abbott, St Jude Medical, LightLab Imaging, and Biosense Webster; and is a scientific advisor for Scitech. G.J.T. has received consultant fees from Samsung and Merck; royalties from Terumo and MIT; and has received sponsored research from Terumo. I.K.J. has received consultant fees and research grant from St Jude Medical. H.G.B. has received consultant fees and honoraria from St Jude Medical. D.D. has received consultant fees and research grants from Abbott, Adamed, AstraZeneca, Biotronik, Balton, Bayer, BBraun, BioMatrix, Boston Scientific, Boehringer Ing, Bristol Myers Squibb, Cordis, Cook, Eli-Lilly, EuroCor, Glaxo, Invatec, Medtronic, Medicines Company, Merck, Nycomed, Orbus-Neich, Pfizer, Possis, Promed, sanofi-aventis, Siemens, Solvay, Stentys, Terumo, and Tyco; and is an advisory board member. P.M. has received consultant fees from St Jude Medical and Terumo. T.A. has received consultant fees and carried out educational activity for St Jude Medical. C.D.M. has received speaker fees from St Jude Medical.
References
- 1.Prati F, Regar E, Mintz GS, Arbustini A, Di Mario C, Jang IK, Akasaka T, Costa M, Guagliumi G, Grube E, Ozaki Y, Pinto F, Serruys PWJ for the Expert's OCT Review Document. Expert review document on methodology and clinical applications of OCT. Physical principles, methodology of image acquisition and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31:401–415. doi: 10.1093/eurheartj/ehp433. [DOI] [PubMed] [Google Scholar]
- 2.Jang IK, Bouma BE, Kang DH, Park SJ, Park SW, Seung KB, Choi KB, Shishkov M, Schlendorf K, Pomerantsev E, Houser SL, Aretz HT, Tearney GJ. Visualization of coronary atherosclerotic plaques in patients using Optical Coherence Tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002;39:604–609. doi: 10.1016/s0735-1097(01)01799-5. [DOI] [PubMed] [Google Scholar]
- 3.Tearney GJ, Waxman S, Shishkov M, Vakoc BJ, Suter MJ, Freilich MI, Desjardins AE, Oh WY, Bartlett LA, Rosenberg M, Bouma BE. Three-dimensional coronary artery microscopy by intracoronary Optical Frequency Domain Imaging. J Am Coll Cardiol Img. 2008;1:752–761. doi: 10.1016/j.jcmg.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takarada S, Imanishi T, Liu Y, Ikejima H, Tsujioka H, Kuroi A, Ishibashi K, Komukai K, Tanimoto T, Ino Y, Kitabata H, Kubo T, Nakamura N, Hirata K, Tanaka A, Mizukoshi M, Akasaka T. Advantage of next-generation frequency-domain optical coherence tomography compared with conventional time-domain system in the assessment of coronary lesion. Catheter Cardiovasc Interv. 2010;75:202–206. doi: 10.1002/ccd.22273. [DOI] [PubMed] [Google Scholar]
- 5.Jang IK, Tearney GJ, MacNeill B, Takano M, Moselewski F, Iftima N, Shishkov M, Houser S, Aretz HT, Halpern EF, Bouma BE. In vivo characterization of coronary atherosclerotic plaque by use of Optical Coherence Tomography. Circulation. 2005;111:1551–1555. doi: 10.1161/01.CIR.0000159354.43778.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imola F, Mallus MT, Ramazzotti V, Manzoli A, Pappalardo A, Di Giorgio A, Albertucci M, Prati F. Safety and feasibility of frequency domain Optical Coherence Tomography to guide decision making in percutaneous coronary intervention. EuroInterv. 2010;6:575–581. doi: 10.4244/EIJV6I5A97. [DOI] [PubMed] [Google Scholar]
- 7.Barlis P, Gonzalo N, Di Mario C, Prati F, Buellesfeld L, Rieber J, Dalby MC, Ferrante G, Cera M, Grube E, Serruys PW, Regar E. A multicentre evaluation of the safety of intracoronary optical coherence tomography. EuroInterv. 2009;5:90–95. doi: 10.4244/eijv5i1a14. [DOI] [PubMed] [Google Scholar]
- 8.Tanigawa J, Barlis P, Di Mario C. Intravascular optical coherence tomography: optimization of image acquisition and quantitative assessment of stent strut apposition. EuroInterv. 2007;3:128–136. [PubMed] [Google Scholar]
- 9.Gutiérrez-Chico JL, Serruys PW, Girasis C, Garg S, Onuma Y, Brugaletta S, Garcia-Garcia H, van ES GA, Regar E. Int J Cardiovasc Imaging. Quantitative multi-modality imaging analysis of a fully bioresorbable stent; a head-to head comparison between QCA, IVUS and OCT. 2011 Feb 26 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capodanno D, Prati F, Pawlowsky T, Cera M, Albertucci M, Tamburino C. Comparison of optical coherence tomography and intravascular ultrasound for the assessment of in-stent tissue coverage after stent implantation. EuroInterv. 2009;5:538–543. doi: 10.4244/eijv5i5a88. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalo N, Serruys PW, García-García HM, van Soest G, Okamura T, Ligthart J, Knaapen M, Verheye S, Bruining N, Regar E. Quantitative ex vivo and in vivo comparison of lumen dimensions measured by optical coherence tomography and intravascular ultrasound in human coronary arteries. Rev Esp Cardiol. 2009;62:615–624. doi: 10.1016/s1885-5857(09)72225-x. [DOI] [PubMed] [Google Scholar]
- 12.Radu M, Jørgensen E, Kelbaek H, Helqvist S, Skovgaard L, Saunamaki K. Strut apposition after coronary stent implantation visualized with optical coherence tomography. EuroInterv. 2010;6:86–93. [PubMed] [Google Scholar]
- 13.Serruys PW, Ormiston JA, Onuma Y, Regar E, Gonzalo N, Garcia-Garcia HM, Nieman K, Bruining N, Dorange C, Miguel-Hébert K, Veldhof S, Webster M, Thuesen L, Dudek D. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet. 2009;373:897–910. doi: 10.1016/S0140-6736(09)60325-1. [DOI] [PubMed] [Google Scholar]
- 14.Serruys PW, Onuma Y, Ormiston JA, de Bruyne B, Regar E, Dudek D, Thuesen L, Smits PC, Chevalier B, McClean D, Koolen J, Windecker S, Whitbourn R, Meredith I, Dorange C, Veldhof S, Miquel-Hebert K, Rapoza R, García-García HM. Evaluation of the second generation of a bioresorbable everolimus drug eluting vascular scaffold for treatment of de novo coronary artery stenosis. Circulation. 2010;122:2301–2312. doi: 10.1161/CIRCULATIONAHA.110.970772. [DOI] [PubMed] [Google Scholar]
- 15.Bezerra HG, Costa MA, Guagliumi G, Rollins AM, Simon DI. Intracoronary optical coherence tomography: a comprehensive review clinical and research applications. J Am Coll Cardiol Intv. 2009;2:1035–1046. doi: 10.1016/j.jcin.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubo T, Imanishi T, Kitabata H, Kuroi A, Ueno S, Yamano T, Tanimoto T, Matsuo Y, Masho T, Takarada S, Tanaka A, Nakamura N, Mizukoshi N, Tomobuchi Y, Akasaka T. Comparison of vascular response after sirolimus-eluting stent implantation between patients with unstable and stable angina pectoris: a serial optical tomography study. J Am Coll Cardiol Img. 2008;1:475–484. doi: 10.1016/j.jcmg.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Kume T, Akasaka T, Kawamoto T, Ogasawara Y, Watanabe N, Toyota E, Neishi Y, Sukmawan R, Sadahira Y, Yoshida K. Assessment of coronary arterial thrombus by Optical Coherence Tomography. Am J Cardiol. 2006;97:1713–1717. doi: 10.1016/j.amjcard.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalo N, Serruys PW, Okamura T, Shen ZJ, Onuma Y, Garcia-Garcia HM, Sarno G, Schultz C, van Geuns RJ, Ligthart J, Regar E. Optical coherence tomography assessment of the acute effects of stent implantation on the vessel wall: a systematic quantitative approach. Heart. 2009;95:1913–1919. doi: 10.1136/hrt.2009.172072. [DOI] [PubMed] [Google Scholar]
- 19.Tenaglia AN, Buller CE, Kisslo KB, Phillips HR, Stack RS, Davidson CJ. Intracoronary ultrasound predictors of adverse outcomes after coronary artery interventions. J Am Coll Cardiol. 1992;20:1385–1390. doi: 10.1016/0735-1097(92)90252-i. [DOI] [PubMed] [Google Scholar]
- 20.Tanigawa J, Barlis P, Di Mario C. Heavily calcified coronary lesions preclude strut apposition despite high pressure balloon dilatation and rotational atherectomy: in vivo demonstration with optical coherence tomography. Circ J. 2008;72:157–160. doi: 10.1253/circj.72.157. [DOI] [PubMed] [Google Scholar]
- 21.Bae JH, Kwon TG, Hyun DW, Rihal CS, Lerman A. Acute coronary syndromes: predictors of slow flow during primary percutaneous coronary intervention: an intravascular ultrasound-virtual histology study. Heart. 2008;94:1559–1564. doi: 10.1136/hrt.2007.135822. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka A, Imanishi T, Kitabata H, Kubo T, Takarada S, Tanimoto T, Kuroi A, Tsujioka H, Ikejima H, Komukai K, Kataiwa H, Okouchi K, Kashiwaghi M, Ishibashi K, Matsumoto H, Takemoto K, Nakamura N, Hirata K, Mizukoshi M, Akasaka T. Lipid-rich plaque and myocardial perfusion after successful stenting in patients with non-ST-segment elevation acute coronary syndrome: an optical coherence tomography study. Eur Heart J. 2009;30:1348–1355. doi: 10.1093/eurheartj/ehp122. [DOI] [PubMed] [Google Scholar]
- 23.Prati F, Capodanno D, Pawlowski T, Ramazzotti V, Albertucci M, La Manna D, Di Salvo M, Gil RJ, Tamburino Cl. Local versus standard intracoronary infusion of abciximab in patients with acute coronary syndromes. J Am Coll Cardiol Intv. 2010;3:928–934. doi: 10.1016/j.jcin.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, Rosenfield K, Siegel RJ, Tuzcu EM, Yock PG. ACC Clinical Expert Consensus Document on standards for the acquisition, measurement and reporting of intravascular ultrasound studies: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents (Committee to Develop a Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies [IVUS] J Am Coll Cardiol. 2001;37:1478–1492. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 25.Abizaid AS, Mintz GS, Mehran R, Abizaid A, Lansky AJ, Pichard AD, Satler LF, Wu H, Pappas C, Kent KM, Leon MB. Long-term follow-up after percutaneous transluminal coronary angioplasty was not performed based on intravascular ultrasound findings: importance of lumen dimensions. Circulation. 1999;100:256–261. doi: 10.1161/01.cir.100.3.256. [DOI] [PubMed] [Google Scholar]
- 26.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW for the PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 27.Manfrini O, Mont E, Leone O, Arbustini E, Eusebi V, Virmani R, Bugiardini R. Sources of error and interpretation of plaque morphology by optical coherence tomography. Am J Cardiol. 2007;99:1350. doi: 10.1016/j.amjcard.2006.01.097. [DOI] [PubMed] [Google Scholar]
- 28.Sawada T, Shite J, Garcia-Garcia HM, Shinke T, Watanabe S, Otake H, Matsumoto D, Tanino Y, Ogasawara D, Kawamori H, Kato H, Miyoshi N, Yokoyama M, Serruys PW, Hirata K. Feasibility of combined use of intravascular ultrasound radiofrequency data analysis and optical coherence tomography for detecting thin-cap fibroatheroma. Eur Heart J. 2008;29:1136–1146. doi: 10.1093/eurheartj/ehn132. [DOI] [PubMed] [Google Scholar]
- 29.Tearney GJ, Yabushita H, Houser SL, Aretz HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Halpern EF, Bouma BE. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003;107:113–119. doi: 10.1161/01.cir.0000044384.41037.43. [DOI] [PubMed] [Google Scholar]
- 30.Raffel OC, Tearney GJ, Gauthier DD, Halpern EF, Bouma BE, Jang IK. Relationship between a systemic inflammatory marker, plaque inflammation, and plaque characteristics determined by intravascular optical coherence tomography. Arterioscler Thromb Vasc Biol. 2007;27:1820–1827. doi: 10.1161/ATVBAHA.107.145987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mintz GS, Weissman NJ. Intravascular ultrasound in the drug-eluting stent era. J Am Coll Cardiol. 2006;48:421–429. doi: 10.1016/j.jacc.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 32.Gerber R, Colombo A. Does IVUS guidance of coronary interventions affect outcome? A prime example of the failure of randomized clinical trials. Cathet Cardiovasc Interv. 2008;71:646–654. doi: 10.1002/ccd.21489. [DOI] [PubMed] [Google Scholar]
- 33.Mudra H, Di Mario C, de Jaegere P, Figulla HR, Macaya C, Zahn R, Wennerblom B, Rutsch W, Voudris V, Regar E, Henneke KH, Schächinger V, Zeiher A for the OPTICUS (OPTimization with ICUS to reduce stent restenosis) Study Investigators. Randomized comparison of coronary stent implantation under ultrasound or angiographic guidance to reduce stent restenosis (OPTICUS study) Circulation. 2001;104:1343–1349. doi: 10.1161/hc3701.096064. [DOI] [PubMed] [Google Scholar]
- 34.Park SJ, Kim YH, Park DW, Lee SW, Kim WJ, Suh J, Yun SC, Lee CW, Hong MK, Lee JH, Park SW MAIN-COMPARE Investigators. Impact of intravascular ultrasound guidance on long-term mortality in stenting for unprotected left main coronary artery stenosis. Circ Cardiovasc Interv. 2009;3:167–177. doi: 10.1161/CIRCINTERVENTIONS.108.799494. [DOI] [PubMed] [Google Scholar]
- 35.Roy P, Steinberg DH, Sushinsky SJ, Okabe T, Pinto Slottow TL, Kaneshige K, Xue Z, Satler EF, Kent KM, Suddath WO, Pichard AD, Weissman NJ, Lindsay J, Waksman R. The potential clinical utility of intravascular ultrasound guidance in patients undergoing percutaneous coronary intervention with drug-eluting stents. Eur Heart J. 2008;29:1851–1857. doi: 10.1093/eurheartj/ehn249. [DOI] [PubMed] [Google Scholar]
- 36.Choi SY, Witzenbichler B, Maehara A, Lansky AJ, Guagliumi G, Brodie B, Kellett MA, Jr, Dressler O, Parise H, Mehran R, Dangas GD, Mintz GS, Stone GW. Intravascular ultrasound findings of early stent thrombosis after primary percutaneous intervention in acute myocardial infarction: a Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) substudy. Circ cardiovasc Interv. 2011;4:39–47. doi: 10.1161/CIRCINTERVENTIONS.110.959791. [DOI] [PubMed] [Google Scholar]
- 37.Alfonso F, Suárez A, Pérez-Vizcayno MJ, Moreno R, Escaned J, Bañuelos C, Jiménez P, Bernardo E, Angiolillo DJ, Hernández R, Macaya C. Intravascular ultrasound findings during episodes of drug-eluting stent thrombosis. J Am Coll Cardiol. 2007;50:2095–2097. doi: 10.1016/j.jacc.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Prati F, Stazi F, Dutary J, La Manna A, Di Giorgio A, Pawlosky T, Gonzalo N, Di Salvo ME, Imola F, Tamburino C, Albertucci M, Alfonso F. Detection of very early stent healing after primary angioplasty: an optical coherence tomographic observational study of chromium cobaltum and first generation drug eluting stents. The DETECTIVE study. Heart. 2011;97:1841–1846. doi: 10.1136/heartjnl-2011-300782. [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann R, Morice MC, Moses JW, Fitzgerald PJ, Mauri L, Breithardt G, Schofer J, Serruys PW, Stoll HP, Leon MB. Impact of late incomplete stent apposition after sirolimus-eluting stent implantation on 4-year clinical events: intravascular ultrasound analysis from the multicentre, randomised, RAVEL, E-SIRIUS and SIRIUS trials. Heart. 2008;94:322–328. doi: 10.1136/hrt.2007.120154. [DOI] [PubMed] [Google Scholar]
- 40.Steinberg DH, Mintz GS, Mandinov L, Yu A, Ellis SG, Grube E, Dawkins KD, Ormiston J, Turco MA, Stone GW, Weissman NJ. Long-term impact of routinely detected early and late incomplete stent apposition: an integrated intravascular ultrasound analysis of the TAXUS IV, V, and VI and TAXUS ATLAS workhorse, long lesion, and direct stent studies. J Am Coll Cardiol Intv. 2010;5:486–494. doi: 10.1016/j.jcin.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Guagliumi G, Musumeci G, Sirbu V, Bezerra HG, Suzuki N, Fiocca L, Matiashvili A, Lortkipanidze N, Trivisonno A, Valsecchi O, Biondi-Zoccai G, Costa MA, et al. on behalf of the ODESSA Trial Investigators. Optical coherence tomography assessment of in vivo vascular response after implantation of overlapping bare-metal and drug-eluting stents. J Am Coll Cardiol Intv. 2010;3:531–539. doi: 10.1016/j.jcin.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Guagliumi G, Costa MA, Sirbu V, Musumeci G, Bezerra HG, Suzuki N, Matiashvili A, Lortkipanidze N, Mihalcsik L, Trivisonno A, Valsecchi O, Mintz GS, Dressler O, Parise H, Maehara A, Cristea E, Lansky AJ, Mehran R, Stone GW. Strut coverage and late malapposition with paclitaxel-eluting stents compared with bare metal stents in acute myocardial infarction: optical coherence tomography substudy of the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) Trial. Circulation. 2011;123:274–281. doi: 10.1161/CIRCULATIONAHA.110.963181. [DOI] [PubMed] [Google Scholar]
- 43.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 44.Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435–2441. doi: 10.1161/CIRCULATIONAHA.107.693739. [DOI] [PubMed] [Google Scholar]
- 45.Prati F, Zimarino M, Stabile E, Pizzicannella G, Fouad T, Rabozzi R, Filippini A, Pizzicannella J, Cera M, De Caterina R. Does optical coherence tomography identify arterial healing after stenting? An in-vivo comparison with histology on a rabbit carotid model. Heart. 2008;94:217–221. doi: 10.1136/hrt.2006.112482. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki Y, Ikeno F, Koizumi T, Tio F, Yeung AC, Yock PG, Fitzgerald PJ, Fearon WF. In vivo comparison between optical coherence tomography and intravascular ultrasound for detecting small degrees of in-stent neointima after stent implantation. J Am Coll Cardiol Intv. 2008;1:168–173. doi: 10.1016/j.jcin.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 47.Takano M, Inami S, Jang IK, Yamamoto M, Murakami D, Seimiya K, Ohba T, Mizuno K. Evaluation by optical coherence tomography of neointimal coverage of sirolimus-eluting stent three months after implantation. Am J Cardiol. 2007;99:1033–1038. doi: 10.1016/j.amjcard.2006.11.068. [DOI] [PubMed] [Google Scholar]
- 48.Matsumoto D, Shite J, Shinke T, Otake H, Tanino Y, Ogasawara D, Sawada T, Paredes OL, Hirata K, Yokoyama M. Neointimal coverage of sirolimus-eluting stents at 6-month follow-up: evaluated by optical coherence tomography. Eur Heart J. 2007;28:961–967. doi: 10.1093/eurheartj/ehl413. [DOI] [PubMed] [Google Scholar]
- 49.Guagliumi G, Sirbu V, Bezerra H, Biondi-Zoccai G, Fiocca L, Musumeci G, Matiashvili A, Lortkipanidze N, Tahara S, Valsecchi O, Costa M. Strut coverage and vessel wall response to zotarolimus eluting and bare metal stents implanted in patients with ST segment elevation myocardial infarction: the OCTAMI (Optical coherence tomography in acute myocardial infarction) study. J Am Coll Cardiol Intv. 2010;3:680–687. doi: 10.1016/j.jcin.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Kim JS, Jang IK, Kim JS, Kim TH, Takano M, Kume T, Hur NW, Ko Y-G, Choi D, Hong M-K, Jang Y. Optical coherence tomography evaluation of zotarolimus eluting stents at 9 month follow-up: comparison with sirolimus eluting stents. Heart. 2009;95:1907–1912. doi: 10.1136/hrt.2009.167759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barlis P, Regar E, Serruys PW. An optical coherence tomography study of a biodegradable vs. durable polymer-coated limus-eluting stent: a LEADERS trial sub-study. Eur Heart J. 2010;31:165–176. doi: 10.1093/eurheartj/ehp480. [DOI] [PubMed] [Google Scholar]
- 52.Ozaki Y, Okumura M, Ismail TF, Naruse H, Hattori K, Kan S, Ishikawa M, Kawai T, Takagi Y, Ishii J, Prati F, Serruys W. The fate of incomplete stent apposition with drug-eluting stents: an optical coherence tomography-based natural history study. Eur Heart J. 2010;31:1470–1476. doi: 10.1093/eurheartj/ehq066. [DOI] [PubMed] [Google Scholar]
- 53.Otake H, Shite J, Ako J, Shinke T, Tanino Y, Ogasawara D, Sawada T, Miyoshi N, Kato H, Koo BK, Honda Y, Fitzgerald PJ, Hirata K. Local determinants of thrombus formation following sirolimus-eluting stent implantation assessed by optical coherence tomography. J Am Coll Cardiol Intv. 2009;2:459–466. doi: 10.1016/j.jcin.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 54.Gonzalo N, Barlis P, Serruys PW, Garcia-Garcia HM, Onuma Y, Ligthart J, Regar E. Incomplete stent apposition and delayed tissue coverage are more frequent in drug-eluting stents implanted during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction than in drug eluting stents implanted for stable/unstable angina. J Am Coll Cardiol Intv. 2009;2:445–452. doi: 10.1016/j.jcin.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 55.Sihan K, Botha C, Post F, de Winter S, Gonzalo N, Regar E, Serruys PJ, Hamers R, Bruining N. Fully automatic three-dimensional quantitative analysis of intracoronary optical coherence tomography: method and validation. Catheter Cardiovasc Interv. 2009;74:1058–1065. doi: 10.1002/ccd.22125. [DOI] [PubMed] [Google Scholar]
- 56.Alfonso F. Treatment of drug-eluting stent restenosis the new pilgrimage: quo vadis? J Am Coll Cardiol. 2010;55:2717–2720. doi: 10.1016/j.jacc.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 57.Templin C, Meyer M, Müller MF, Djonov V, Hlushchuk R, Dimova I, Flueckiger S, Kronen P, Sidler M, Klein K, Nicholls F, Ghadri JR, Weber K, Paunovic D, Corti R, Hoerstrup SP, Lüscher TF, Landmesser U. Coronary optical frequency domain imaging (OFDI) for in vivo evaluation of stent healing: comparison with light and electron microscopy. Eur Heart J. 2010;31:1792–1801. doi: 10.1093/eurheartj/ehq168. [DOI] [PMC free article] [PubMed] [Google Scholar]