Abstract
Previous studies with nonhuman species have shown that animals exposed to early adversity show differential DNA methylation relative to comparison animals. The current study examined differential methylation among 14 children raised since birth in institutional care and 14 comparison children raised by their biological parents. Blood samples were taken from children in middle childhood. Analysis of whole-genome methylation patterns was performed using the Infinium HumanMethylation27 BeadChip assay (Illumina), which contains 27,578 CpG sites, covering approximately 14,000 gene promoters. Group differences were registered, which were characterized primarily by greater methylation in the institutionalized group relative to the comparison group, with most of these differences in genes involved in the control of immune response and cellular signaling systems, including a number of crucial players important for neural communication and brain development and functioning. The findings suggest that patterns of differential methylation seen in nonhuman species with altered maternal care are also characteristic of children who experience early maternal separation.
It is widely recognized that early environment is crucially important for all aspects of human development, both for physical (Harkonmäki, et al., 2007; Thomas, Hyppönen, & Power, 2008) and psychological (Heim, Newport, Mletzko, Miller, & Nemeroff, 2008; Kishiyama, Boyce, Jimenez, Perry, & Knight, 2009) outcomes. Human and nonhuman studies (Champagne & Curley, 2005; Gunnar & Fisher, 2006) have contributed to the field’s growing understanding of the consequences of early neglect, both in terms of the physiological and neurological substrates affected most proximally, as well as downstream physical and mental health outcomes.
Research among nonhuman species has provided evidence of the pernicious effects of maternal deprivation on infant development. Behaviorally, maternal deprivation has been associated with marked deficits in the offspring’s play behavior (Suomi, Harlow, & Kimball, 1971), high levels of social aggression (Heinrichs & Koob, 2006), deficient cognitive functioning and learning (Enthoven, de Kloet, & Oitzl, 2008), impaired social behaviors (Sabatini et al., 2007), increased emotional reactivity to novelty (Gilad, Rabey, Eliyayev, & Gilad, 2000), and harmful and abusive parenting behaviors (Seay, Alexander, & Harlow, 1964).
Studies of adverse early experiences suggest similar effects among human children (e.g., Cicchetti, 2002; Cicchetti, Rogosch, Gunnar, & Toth, 2010; Dozier et al., 2006; Ladd et al., 2000). Epidemiological research has demonstrated that heart disease, diabetes, obesity, depression, substance abuse, and other health maladies might originate from the early stages of development (Harkonmäki et al., 2007; Kreppner et al., 2007). Similarly, physical and psychological maltreatment and related stress in early childhood has also been associated with subsequent challenged development and functioning that cascade throughout the life span (Bateson et al., 2004; Gottlieb, 1998; Schneirla, 1966).
Arguably, institutional care may represent one of the most extreme privations seen in human children. Consequently, institutional care has been associated with the most pervasive effects on children’s development, with developmental deficits seen across virtually every domain examined (O’Connor, Rutter, & The English and Romanian Adoptees Study Team, 2000). Such children are often delayed in physical growth and motor development as well as cognitive functioning and language development (O’Connor et al., 2000). Social behaviors are often odd and may be characterized by one of two extremes: some children are withdrawn and depressed in appearance, whereas others are indiscriminate in their attachment behaviors (O’Connor et al., 2000). Even high-quality institutional care has deleterious effects on young children’s development (Gunnar, Van Dulmen, & The International Adoption Project Team, 2007; Rutter et al., 2007). As a rule, such children often miss the opportunity to develop selective attachment relationships with caregivers in institutions. Institutional care seems to have specific adverse effects on children that other depriving conditions do not.
Yet, both human and nonhuman studies (Gunnar et al., 2007; Juffer & van IJzendoorn, 2005; Meaney et al., 1996; Nelson et al., 2007) suggest that there is rapid catch-up in physical and cognitive development following placement in enriched environments after even severe deprivation. Thus, adoptive placement in itself appears to represent a significant intervention with regard to physical and cognitive development catch-up, although problems persist among some children years after placement into adoptive homes (van IJzendoorn & Juffer, 2006). Researchers have investigated the associations between individual differences in early care and adult outcomes. Generally speaking, high-quality early care is associated with a range of positive outcomes, and low-quality early care with a host of negative outcomes. More specifically, these differences modulate offspring’s gene expression and the consequences of this modulation for physiology and behavior (Meaney, 2001a). Animal literature reveals associations of early care with the expression patterns of brain derived neurotrophic factor, GABA (Caldji, Francis, Sharma, Plotsky, & Meaney, 2000; Liu, Diorio, Day, Francis, & Meaney, 2000), oxytocin (Champagne, Diorio, Sharma, & Meaney, 2001), estrogen (Champagne, Weaver, Diorio, Sharma, & Meaney, 2003), and glucocorticoid receptors (Francis, Diorio, Liu, & Meaney, 1999), and other crucial players in the development, maturation, and functioning of the brain.
Many developmental psychologists are becoming interested in the “biological embedding” of early experience (Hertzman, 1999), alterations of which might result in shifting biological processes and influencing health and/or behavior over a lifespan. At the present time, it is known that one of the mechanisms determining changes in the functioning of the organism, that is, the physical and mental health of the individual, is induced by environmental changes of physical properties of the genome. One such property is DNA methylation, which together with histone deacetylation regulates gene expression (Razin, 1998), and consequently might affect the course of biological processes controlled by these genes. The study of genes’ functional activity that is not associated with changes in the primary structure of these genes’ DNA component is the main subject of epigenetics. The epigenome is thought to consist of chromatin and its modifications, and the methylation of cytosine rings found at the di-nucleotide sequence CG, as well as in microRNAs and other noncoding RNAs. More recently an additional modification of 5-methylcytosine 5-hydroxymethylcytosine was discovered in the brain but its role is still unknown (Kriaucionis & Heintz, 2009). In other words, the epigenome might be represented as the pattern of DNA methylation (or localization of methylated CpG sites across the genome) and the histone modifications of a particular genome.
The development of epigenetics and the accumulation of new knowledge on the functioning of genes in the contexts of specific environments allow us to get closer to understanding the environment-driven and/or environment-dependent aspects of the realization of genetic information. According to the conventional view, the epigenetic inheritance, being imposed on genetic heredity, is manifested at embryonic stages of development to create specific epigenome patterns in different cells and tissues that are a crucial part of normal organismal development and cellular differentiation. Today we know that environmental factors might cause epigenetic modifications of the genome (such as changes in the level of DNA methylation) and thus might affect gene expression during the whole lifespan. This has adaptive value, in terms of the organism’s plasticity in interacting with a dynamic environment, and at the same time might cause negative outcomes, increasing the risk of disorders and/or diseases (Gorman, Kent, Sullivan, & Coplan, 2000; McEwen, 2008a; Meaney, 2011; Meaney, Szyf, & Seckl, 2007; Zeisel, 2009). It has been shown that the epigenetic status might be changed not only by the action of diets, chemical substances, and other triggers (Cooney, Dave, & Wolff, 2002; Verhoeven, Jansen, vanDij, & Biere, 2010; Waterland, Lin, Smith, & Jirtle, 2006), but also through behavioral programming and early experiences (such as child maltreatment and parental stress), which have particular importance and power to influence a developing organism through the lifespan (Essex et al., in press; McGowan et al., 2008, 2009; Murgatroyd et al., 2009; Oberlander et al., 2008).
Thus, recent discoveries in the field of epigenetics might be of particular interest to developmentalists in general and psychologists in particular for the purposes of understanding how environmental influences “get under the skin” and interact with, or even embed themselves in, the genome (Hertzman & Boyce, 2010). Since the release of the human genome sequence in 2001, researchers have generated an impressive amount of data connecting environmental adversity and human health through epigenetic mediation. The hypothesized mechanism assumes that cellular signaling pathways activated in response to these negative environmental conditions trigger long-term patterns of genome expression, and that these patterns, in turn, influence behavior and health. As stated above, the epigenome is a candidate system for the mediation of the genome’s response to environmental signals, whether external or internal to the organism, modulating the interactions between environmental and genetic factors, and health outcomes. There are a number of studies connecting alterations of the epigenome to physical health (Szyf, 2009), but the role of epigenetic factors in mental health has only begun to be considered (McGowan et al., 2009; Sfoggia, Pacheco, & Grassi-Oliveira, 2008).
The study presented here is one of the first attempts to investigate how such factors as the complete deprivation of parental attention and care, and residence at institutions from birth, might impact the epigenome of children. Thus, this research represents an attempt to identify the main biological pathways that might be affected by a negative behavioral environment. Broadly, we hypothesize that early adversity directly and indirectly affects the long-term expression pattern of critical genes involved in such early processes as immune regulation and function, stress reactivity, and the formation of social bonding, affiliation, and attachment through epigenetic reprogramming. This broad hypothesis makes several assumptions that are tested here. Specifically, first we assume that early social environment alters epigenetic states in humans systemically in several tissues, and that these are measurable in peripheral lymphocytes, specifically through DNA methylation signatures; as epigenetic states are tissue specific, we anticipate that some of the changes in DNA methylation will be unique to different tissues, whereas others will be common to several tissues. Second, different epigenetic states are related to altered gene expression in important pathways that, in turn, affect physiology and behavior later in life.
To verify, at least preliminarily, these hypotheses, we carried out a study of two groups of children, one—a group placed in institutional care at birth, and the other—a group of typically developing children being raised by their biological parents.
Methods
Participants
Participants for this study were recruited from a northwest region of the Russian Federation where the population is predominantly ethnically homogeneous and of Slavic origin. We elected to work in Russia for a number of reasons. First, the Russian Federation only recently implemented the practice of foster families; previously, most orphaned children were placed in state-run institutions (Schwirtz, 2008). Second, an extensive amount of societal turmoil that lasted through the early 1990s has substantially differentiated the society, such that a large subpopulation of those of very low socioeconomic status has emerged (Alvazian & Kolenikov, 2000). Third, as the result of the sheer size of the population of the Russian Federation, we know that there are many institutionalized children. According to the report from the Federal Social Program “Children of Russia” (http://www.usynovite.ru/f/experience/byulleten/bill.doc) and journalistic data (BBCRussia.com, May 28, 2008), the current estimate of children deprived of parental care is 731,000. Most of them are adopted or placed into foster families; approximately 180,000 children are living in children’s institutions, where they are being raised, cared for both medically and psychologically, and educated until the age of 18.
The study presented is based on a sample that included 28 children (9 girls, 19 boys) ranging in age from 7 to 10 years. The children represented two groups, a group of institutionalized children and a group of typically developing children being raised by their biological parents (Table 1). Institutionalized children (n = 14, mean age = 8.14, SD = 0.77, 35.71% girls) were recruited based on their records of being placed into institutional care at birth. This recruitment decision allowed us to control for the confounding factor of early experiences, whether positive (e.g., early interactions with biological parents) or negative (e.g., child neglect and abuse). Although, to our knowledge, no specific statistics are available on the characteristics of the families or mothers who choose to leave their children, either in the Russian Federation as a whole or in the particular geographic region where the study took place, sociological, judicial (Sapogov, 2010), and journalistic (http://www.youtube.com/watch?v=tl9WIb8odpw) evidence suggest that the dominant reason to leave a child immediately after birth is economic.
Table 1.
Participants in the study: Institutionalized versus comparison children
| N | Institutionalized Children
|
Comparison Children
|
||
|---|---|---|---|---|
| Age (years) | Gender | Age (years) | Gender | |
| 1 | 7 | F | 7 | F |
| 2 | 7 | F | 8 | F |
| 3 | 9 | F | 10 | F |
| 4 | 9 | F | 10 | F |
| 5 | 9 | F | 7 | M |
| 6 | 7 | M | 7 | M |
| 7 | 8 | M | 7 | M |
| 8 | 8 | M | 8 | M |
| 9 | 8 | M | 8 | M |
| 10 | 8 | M | 8 | M |
| 11 | 8 | M | 8 | M |
| 12 | 8 | M | 9 | M |
| 13 | 9 | M | 10 | M |
| 14 | 9 | M | 10 | M |
| Mean, F | 8.20 ± 1.09 | 8.74 ± 1.50 | ||
| Mean, M | 8.11 ± 0.60 | 8.20 ± 1.14 | ||
| Mean | 8.14 ± 0.77 | 35.71% F | 8.35 ± 1.21 | 28.57% F |
Orphan children were recruited through the regional social-service office of a large industrial center. The conditions in Russian specialized institutions for children in the care of the State are variable (The St. Petersburg–USA Orphanage Research Team, 2008), but the Russian government is striving to provide homogeneously good care to children who are raised outside of family life. The orphanages in the region where the study unfolded were well equipped, had an adequate ratio of children to adults (regulated by the state), had good physical plant facilities, and demonstrated adequate administrative leadership.
Comparison children (n = 14, mean age = 8.35, SD = 1.21, 28.57% girls) were recruited from biological families whose socioeconomic status was assumed to match those families who had made a decision to leave their children to alternative care. Specifically, we recruited families with an income level of no more than $350/month, which is substantially below that which is considered average across the Russian Federation ($724/month; according to the data from the Russia Federal State Statistics Service, http://www.gks.ru). Only families with no evidence of marital dysfunction, records of child abuse, or indications of any substance abuse were included in the comparison sample.
The exclusion criteria in both samples were the presence of known severe and/or chronic health conditions, HIV/ AIDS, diagnosed developmental disorders, dysmorphology, and any ethnicity other than Slavic. For all participants, care-givers’ consent (the Russian State for institutionalized children and parents for children from biological families) was obtained first, and the child assent second. There were no statistically significant differences in age and gender ratios between the institutionalized and comparison groups.
Genomic DNA preparation and DNA methylation analysis
A total of 10 ml of whole blood was collected from each assenting child from an arm vein via phlebotomy. Genomic DNA was isolated from blood samples using FlexiGene DNA kits, according to the manufacturer’s instructions (Qiagen, Chatsworth, CA). Sample yield and purity were assessed using NanoDrop spectrophotometer, and DNA QC was assessed by visualization in 2% agarose gel. For each individual, 1 μg of the genomic DNA was analyzed.
Bisulfite treatment (or the conversion of unmethylated cytosine to uracil), whole genome amplification, labeling, hybridization to the Infinium HumanMethylation27 BeadChip array, and scanning were performed at the Yale Center for Genomic Analysis (http://medicine.yale.edu/keck/ycga/index.aspx). The technical personnel at the Center were unaware of the replication experiment and of the group (institutionalized vs. comparison) status of the DNA specimens. To ensure consistency in methylation level measurements, two technical replicates were included (i.e., two samples were analyzed twice).
DNA methylation measurement
Quantitative DNA methylation measurements of purified genomic DNA were performed with the Infinium HumanMethylation27 BeadChip array (Illumina, San Diego, CA). The Infinium HumanMethylation27 BeadChip assay allows for the simultaneous measurement of the DNA methylation status within 27,578 CpG sites, covering more than 13,500 promoters of well-annotated genes and about 110 microRNA loci. The number of CpG sites per gene ranges from one site in 2541 genes, to two sites in 11,711 genes, to three or more sites in 195 genes. Thus, the BeadChip assay represents more than half of all human well-annotated protein-coding genes (~25,000; International Human Genome Sequencing Consortium, 2004).
The technique is based on measuring differential methylation using the two-color fluorescent hybridization of target fragments with specific DNA probes contained in the array. Each CpG site is represented by two oligonucleotide probes with sequences targeting methylated DNA and unmethylated DNA, respectively. The methylation status of each CpG site was measured as the ratio of signal from methylated probe to the sum of both methylated and unmethylated signals (β value), using the IlluminaGenomeStudio software package. Beta values range from 0 (completely unmethylated) to 1 ( fully methylated) and provide a quantitative readout of relative DNA methylation for each CpG site.
Raw scanned data were normalized; average beta values were recalculated using background intensity, measured by negative background probes present on the array, using GenomeStudio software (Illumina). All CpG sites that had a detection p value of >.001 were removed to ensure that only high-confidence probes would be included in the subsequent analysis.
Differential methylation analysis
The Illumina methylation data were processed and analyzed using the Methylation Module v1.8 of the GenomeStudio software (Illumina). For interindividual comparison of whole-genome methylation profiles the clustering analysis based on Pearson correlations (r) was used. The results of the analysis are provided in the form of a dendrogram. The distances were defined as 1 − |r|.
The comparison of methylation patterns between groups was based on the difference in mean beta value (Avgβ) of each CpG site, or Delta Avgβ (Δβ). To identify differentially methylated CpG sites in the target and comparison groups we applied significance criteria based on the Illumina Custom model. This error model operates under the assumption that the methylation value (beta value) is normally distributed among biological replicates and estimates variation of β as a function of β, a method developed by Illumina based on estimates for repeated measures of loci with known methylation fractions. The methylation difference score (DiffScore) for a probe provided by Genome Studio Software takes into account background noise and sample variability (Chudin et al., 2006). Targets showing significant (DiffScore >|20|, corresponding to p < .01) intergroup differences in methylation level were considered to be differentially methylated CpG sites. To account for multiple testing, the procedure of permutations of repeated measures to estimate the false discovery rate was used, which is integrated into the Genome-Studio software. In all cases, we ran 1000 permutations and included false discovery rates up to 20%.
Functional annotation of differentially methylated genes
To identify common biological processes and pathways, molecular functions, and cellular components for genes that showed differential methylation in the target and comparison groups of children, we applied the Database for Annotation, Visualization and Integrated Discovery (DAVID) bioinformatics software (available through http://david.abcc.ncifcrf.gov; Huang, Sherman, & Lempicki, 2009). For this analysis the default (medium stringency) setting of the DAVID analysis tool was used, which compares the enrichment of gene ontology (GO) with the list of differentially methylated genes using Fisher’s exact test. The p values of the DAVID tool and the Benjamini corrections of the scores from the tool were used as inclusion criteria in the trimming of the clusters to overrepresented term lists.
Results
We carried out global methylation profiling of the genomes of 14 institutionalized children and 14 typically developing children being raised by their biological parents using the Infinium HumanMethylation27 BeadChip array. The number of detected Illumina probes (detection p < .01) was high for all DNA samples and varied between 99.39% and 99.99%. The comparison of methylation profiles of the two technical replicates showed good reproducibility of methylation level measurements (r2 = .9946 and .9949, respectively; Figure S.1, see offsite materials at http://journals.cambridge.org/dpp). Only 14 and 15 of 27,578 Illumina probes have subsequently shown statistically significant ( p < .01) differences in methylation level measurements in the pairwise comparison between technical replicates (Table S.1, see online data at http://journals.cambridge.org/dpp). This indicates that the expected error in detecting the methylation level of CpG sites does not exceed 0.05% of the total number of 27,578 probes contained in the Infinium27 array.
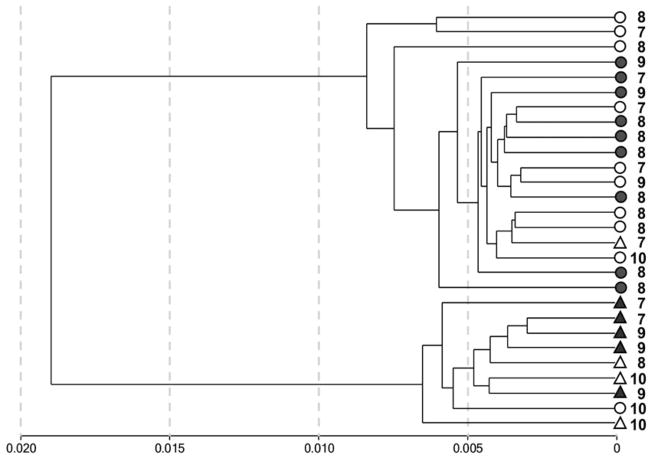
All CpG sites that had a detection p value of >.001 (316 targets) were removed to include only high-confidence probes in the subsequent analysis. This left a total of 27,262 CpG sites to be analyzed to detect methylation profiles in the genomes of the studied children. Hierarchical cluster analysis of individual methylation patterns was carried out; the results are represented in a dendrogram (see Figure 1). The dendrogram shows two large branches that are monomorphic, combining only individuals from one gender. Thus, hierarchical clustering indicates that gender is the main factor in the differentiation of methylation profiles in the genomes of the children who participated in this research. Nevertheless, with rare exceptions, individuals from the same group (i.e., the group of institutionalized children or the group of children living with their biological families) were clustered together in the closest clusters of the first order within each large gender cluster (Figure 1). This finding was confirmed by the results of hierarchical clustering performed using data on methylation levels of CpG sites located only in autosomes, excluding those located on the sex chromosomes (Figure S.2; see at http://journals.cambridge.org/dpp). Thus, these patterns suggest the presence of slight but consistent differences in gene methylation profiles between the two groups of children. Such differences were further validated in the analysis of the intergroup comparison of gene methylation profiles.
Figure 1.
The clustering analysis of 28 individuals from the groups of institutionalized children (gray figures) and comparison children (white figures) groups on the basis of pairwise correlations of Illumina Infinium27 whole genome methylation profiles. There were 27,262 CpG sites with detection p < .01 featured in this analysis. The gender is shown as circle (male) or triangle (female), the digit shows age of the child.
Taking into account previous findings, and to prevent interindividual variability by gender, CpG sites on the sex chromosomes were excluded from the differential methylation analysis. This left a total of 26,214 targets to be analyzed to detect differentially methylated sites and genes. The comparison was based on the average beta value of each CpG site. The difference in methylation level was estimated as the difference between the average beta values (DeltaAvβ) in the institutionalized children and comparison children groups. Only targets showing significant ( p < .01) intergroup differences in methylation level were considered to be differentially methylated CpG sites. Using the selected threshold, 914 of the 26,214 CpG sites were found to be differentially methylated in the institutionalized group relative to the comparison group (see Table 2). The results of the intergroup differential methylation analysis as well as the list of 914 Illumina probes with individual AgBeta values are provided in Tables S.2 and S.3 (http://journals.cambridge.org/dpp).
Table 2.
Number of CpG sites and genes differentially methylated (p < .01) in institutionalized children versus comparison children
| Differentially Methylated CpG Sites
|
|||
|---|---|---|---|
| Increased Methylation | Decreased Methylation | Total | |
| No. of CpG sites (of genes) | 815 (744) | 99 (94) | 914 (838) |
| Differences in methylation level, FoldChange (average) | 1.03–2.36 (1.09) | 1.04–2.04 (1.33) | 1.03–2.36 (1.22) |
Based on the set of differentially methylated sites, it was established that the main intergroup difference is the increase of methylation in the genomes of institutionalized children compared with those of children living with their biological families. This observation is graphically depicted in the box plot in Figure 2. Most of the sites (815 of 914, or 89.17%) were characterized by increased methylation in the genomes of institutionalized children relative to the comparison children (see Table 2 and Figure 3). These intergroup differences in methylation measurements among the 914 CpG sites are small, the differences in foldchanges ranged from 1.03 to 2.36 with the average value of 1.22 (see Table 2), but at same time they are very stable across the set of CpG sites, as shown in Figure 4.
Figure 2.
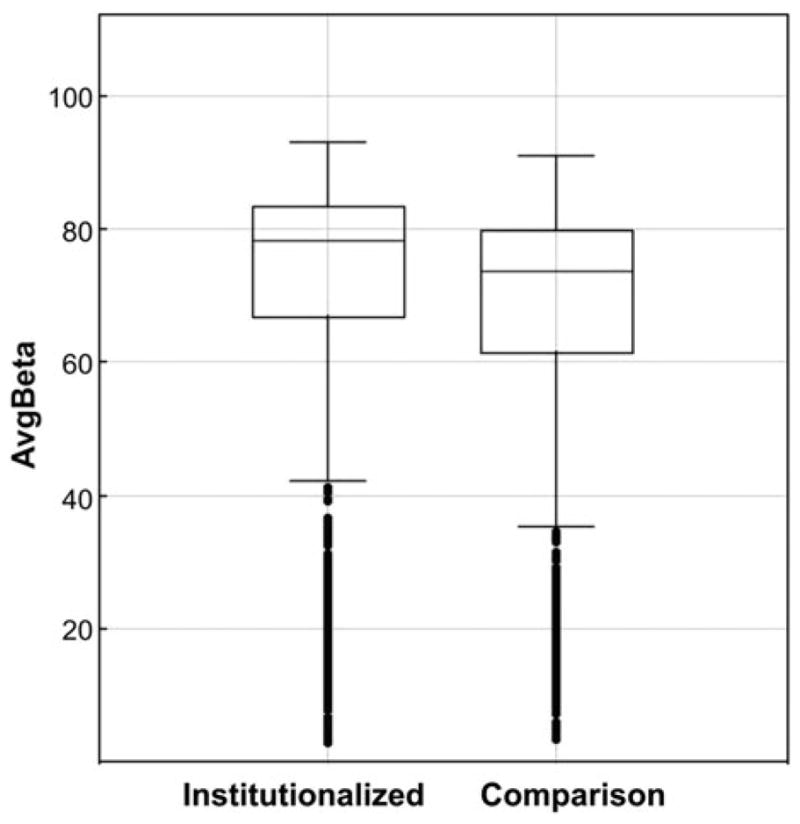
A box plot of data on the methylation levels (AvgBeta) of 914 CpG sites that have shown differential methylation in genomes of children from the institutionalized and comparison groups.
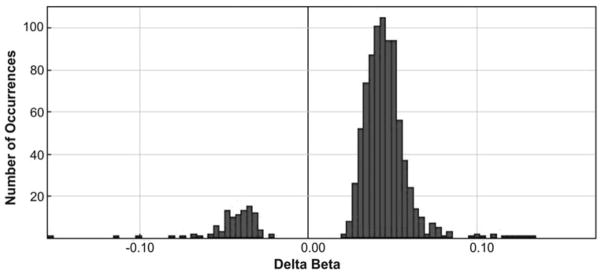
Figure 3.
The distributions of DeltaAvβ values for 914 CpG sites differentially methylated in the group of institutionalized children compared to the group of children living with their biological parents (min DeltaAvβ = −0.155, max = 0.170, mean = 0.036, SD = 0.032). These distributions show a significant predominance of positive values of DeltaAvβ. This means the predominance of sites that are characterized by higher levels of methylation in the genomes of children from target group, relative to the comparison group.
Figure 4.
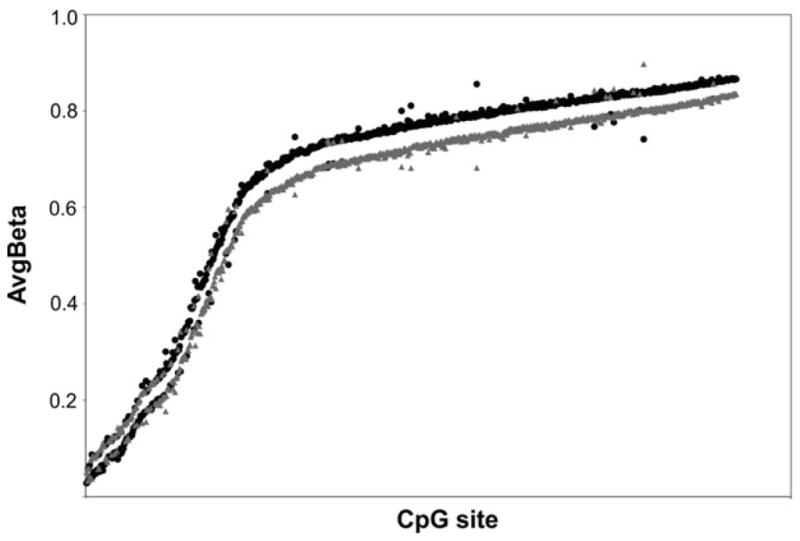
A comparison of the levels of methylation (AvgBeta) for 914 CpG sites differentially methylated in genomes of institutionalized children (black) and children living with their biological families (gray). It is important to recognize that the differences between the two groups’ means are small, but stable.
The intergroup analysis described above is based on the comparison of each group’s average methylation level for each site and the difference between these averages. To validate the power of the set of differentially methylated sites in an intergroup differentiation of individuals we performed a cluster analysis of the individual methylation patterns based on those 914 sites. The results of the hierarchical clustering are represented in a dendrogram (Figure 5). The dendrogram shows a clear separation of the target and comparison groups of children. Thus, Branches II and III contain individuals from the group of children raised by their biological families, and Branch I predominantly consists of individuals from the group of institutionalized children (Figure 5). It is necessary to note that age, which ranged from 7 to 10 years, and gender, whose effect was partly eliminated by removing from the analysis sites localized on the sex chromosomes, seemed to make no significant contribution to the clustering of individuals. Children of similar age did not show a tendency to cluster into separate branches on the dendrogram. In addition, no stable clustering of individuals of the same gender into single clusters (see Figure 5) has been observed. Taken together, the results of hierarchical clustering indicate that the sites detected as differentially methylated between target and comparison groups are sufficiently reliable to reflect interindividual differences in the methylation profiles of the genomes of the children from these groups.
Figure 5.
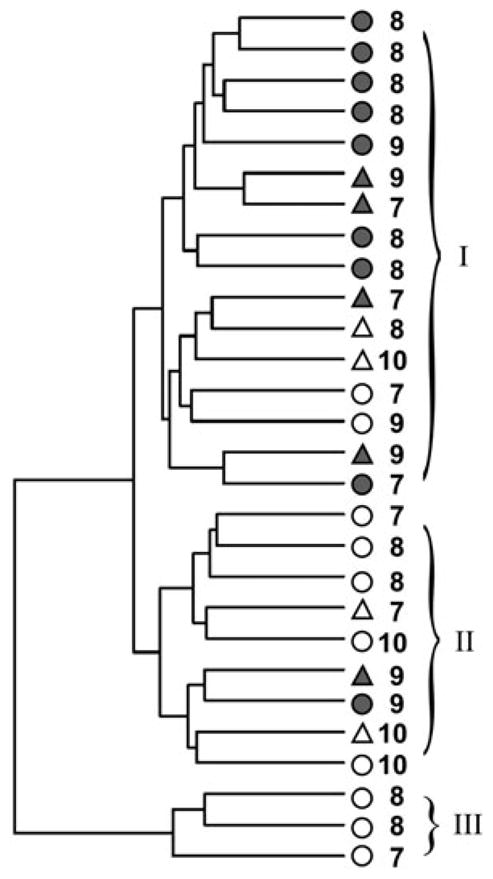
The clustering analysis of 28 individuals from the groups of institutionalized children (gray figures) and comparison children (white figures) using pairwise correlations of methylation profiles of 914 CpG sites differentially methylated in the target group of children compared with the comparison group. The gender is shown as a circle (male) or triangle (female), and the digit shows the age of the child.
The 914 sites detected as differentially methylated between the groups of children are located in the promoters of 838 genes; 744 of them (88.78%) are characterized by a gain in methylation, and only 94 (11.22%) show less methylation in the group of institutionalized children (see Table 2). To identify the common biological processes and molecular functions in which those genes are involved, we performed functional annotation analyses separately for genes which have shown, consequently, upmethylation and downmethylation in the group of institutionalized children, using DAVID bioinformatics software (Huang et al., 2009). The results of the analysis are presented in Tables S.4 and S.5 (http://journals.cambridge.org/dpp).
It is important to note that no significant enrichment of the GO terms with the list of downmethylated genes in the genomes of institutionalized children was found (see Table S.4 at http://journals.cambridge.org/dpp). At the same time, statistically significant enrichment was found in some of the GO terms with the list of genes upmethylated in the genomes of the institutionalized children. The top list of the annotation clusters (for those whose Benjamini corrections of the scores <0.05) are represented in Table 3. An analysis of the functions of these clusters revealed that the genes with increased methylation in the genomes of the institutionalized children are involved predominantly in the control of cellular signaling systems (Table 3, Cluster 1) and the immune response (Clusters 2 and 3). These functional groups of genes are overrepresented in the list of genes upmethylated in the target group of children at least 1.5–2.0 times more (Table 3).
Table 3.
Functional annotation and clusters of genes that gain methylation in the genomes of institutionalized relative to comparison children
| Annotation Cluster | Term | Count | % | p | Fold Enrichment | Benjamini | FDR |
|---|---|---|---|---|---|---|---|
| Cluster 1 | Disulfide bond | 181 | 28.59 | 7.65E-19 | 1.90 | 4.26E-16 | 1.12E-15 |
| EScore: 13.96 | Secreted | 124 | 19.59 | 4.78E-18 | 2.25 | 1.33E-15 | 6.99E-15 |
| Disulfide bond | 170 | 26.86 | 5.35E-16 | 1.84 | 1.34E-12 | 9.77E-13 | |
| Signal | 185 | 29.23 | 2.65E-15 | 1.74 | 4.95E-13 | 3.90E-12 | |
| Signal peptide | 185 | 29.23 | 5.08E-15 | 1.73 | 6.17E-12 | 8.97E-12 | |
| Glycoprotein | 223 | 35.23 | 4.26E-14 | 1.58 | 5.92E-12 | 6.22E-11 | |
| GO:0005576~extracellular region | 139 | 21.96 | 4.53E-14 | 1.86 | 1.56E-11 | 6.18E-11 | |
| GO:0044421~extracellular region part | 82 | 12.95 | 1.29E-12 | 2.30 | 2.21E-10 | 1.75E-09 | |
| GO:0005615~extracellular space | 65 | 10.27 | 5.41E-12 | 2.55 | 6.20E-10 | 7.38E-09 | |
| Glycosylation site: N-linked (GlcNAc…) | 205 | 32.39 | 7.48E-11 | 1.51 | 4.52E-08 | 1.32E-07 | |
| Cluster 2 | GO:0006952~defense response | 50 | 7.90 | 1.08E-07 | 2.27 | 2.47E-04 | 1.88E-04 |
| EScore: 3.85 | GO:0006954~inflammatory response | 23 | 3.63 | 3.13E-03 | 1.98 | 8.35E-03 | 1.34E-03 |
| GO:0009611~response to wounding | 31 | 4.90 | 8.45E-03 | 1.63 | 8.85E-03 | 1.18E-02 | |
| Cluster 3 | IPR012351:Four-helical cytokine, core | 12 | 1.90 | 2.71E-07 | 7.71 | 2.64E-04 | 4.27E-04 |
| EScore: 3.78 | IPR000471:Interferon alpha/beta/delta | 8 | 1.26 | 7.03E-07 | 13.90 | 3.42E-04 | 1.11E-03 |
| SM00076:IFabd | 8 | 1.26 | 7.33E-07 | 13.74 | 1.60E-04 | 9.32E-04 | |
| PIRSF001934:interferon alpha | 8 | 1.26 | 1.30E-06 | 12.25 | 4.05E-04 | 1.74E-03 | |
| Cytokine | 21 | 3.32 | 1.99E-06 | 3.55 | 1.58E-04 | 2.91E-03 | |
| GO:0005125~cytokine activity | 23 | 3.63 | 2.18E-06 | 3.26 | 1.58E-03 | 3.30E-03 | |
| Antiviral | 7 | 1.11 | 2.81E-06 | 15.31 | 1.96E-04 | 4.11E-03 | |
| hsa05320:Autoimmune thyroid disease | 12 | 1.90 | 6.40E-06 | 5.54 | 4.35E-04 | 7.51E-03 | |
| IPR015589:Interferon alpha | 6 | 0.95 | 4.45E-05 | 13.63 | 1.43E-02 | 7.00E-02 | |
| hsa04060:Cytokine-cytokine receptor interaction | 26 | 4.11 | 9.19E-05 | 2.34 | 3.12E-03 | 1.08E-01 | |
| hsa04612:Antigen processing and presentation | 12 | 1.90 | 6.58E-04 | 3.40 | 1.48E-02 | 7.70E-01 | |
| Antiviral defense | 9 | 1.42 | 8.73E-04 | 4.45 | 3.19E-02 | 1.27E+00 | |
| hsa04622:RIG-I-like receptor signaling pathway | 10 | 1.58 | 2.77E-03 | 3.32 | 3.10E-02 | 3.21E+00 | |
| hsa04620:Toll-like receptor signaling pathway | 12 | 1.90 | 3.33E-03 | 2.80 | 3.43E-02 | 3.84E+00 | |
| hsa04630:Jak-STAT signaling pathway | 15 | 2.37 | 5.56E-03 | 2.28 | 4.93E-02 | 6.34E+00 |
Moreover, we performed a functional annotation of the 744 genes, detected as upmethylated in the group of institutionalized children, in terms of their expression in different tissues. DAVID indicated that these upmethylated genes are expressed in a number of different tissues, such as whole blood, salivary gland, skin, tongue, liver, muscle, lung, spinal cord, and brain (see Table S.6 at http://journals.cambridge.org/dpp). Among the genes characterized by an increase of methylation level in the genomes of institutionalized children, many were found to play a critical role in the development and function of the brain (i.e., genes involved in the regulation of ion channels; genes coding membrane transport proteins, such as the solute carrier group of proteins, SLC, and transcription factors, such as zinc-finger proteins; neuro-transmitters and receptors [see http://journals.cambridge.org/dpp; Tables S.4 and S.5]). To illustrate, some of those genes are listed on Table 4, such as genes involved in the control of the dopaminergic system (TERF2IP), serotonin biosynthesis and serotonin receptor activity (TPH, HTR1D, and HTR1F), glucocorticoid and steroid biosynthesis and their receptor activity (NRIP1, PPARGC1B, and UGT), genes coding the arginine vasopressin receptor, glutamate, cadherin, and cholinergic receptors, and other genes, which individually and collectively, are important for neural communication, memory formation, learning and retention, and have been implicated in the pathogenesis of a number of neurodegenerative diseases.
Table 4.
List of genes that gain methylation in the genomes of institutionalized relative to comparison children and which products are known to be involved in the development and functioning of the brain
| Gene Symbol | Chromosome | Gene Name | Annotationa |
|---|---|---|---|
| AVPR1A | 12 | Arginine vasopressin receptor 1A | Protein kinase C binding; vasopressin receptor activity; signaling pathway; social behaviors |
| CELSR1 | 22 | Cadherin, EGF LAG seven-pass G-type receptor 1 | Calcium ion binding; structural molecule activity; cell adhesion; neuropeptide signaling pathway; CNS development |
| CHRNB3 | 8 | Cholinergic receptor, nicotinic, beta 3 | Neurotransmitter receptor activity; extracellular ligand-gated ion channel activity; synaptic transmission; cholinergic |
| DDR2 | 1 | Discoidin domain receptor tyrosine kinase 2 | ATP binding; receptor activity, transferase activity; cell adhesion; signal transduction; regulation of cell growth, differentiation, and metabolism |
| ENO2 | 12 | Enolase 2 (gamma, neuronal) | Lyase activity; magnesium ion binding; phosphopyruvate hydratase activity; glycolysis; neurotrophic and neuroprotective properties |
| GABRA5 | 15 | Gamma-aminobutyric acid A receptor, alpha 5 | GABA-A receptor activity; transporter activity; extracellular ligand-gated ion channel activity; chloride transport; signal transduction |
| GRM5 | 11 | Glutamate receptor, metabotropic 5 | Metabotropic glutamate; GABA-B-like receptor activity; signal transduction; synaptic transmission |
| HSD3B2 | 1 | Hydroxyl-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 2 | Isomerase activity; oxidoreductase activity; glucocorticoid biosynthetic process; steroid biosynthesis |
| HTR1D | 1 | 5-Hydroxytryptamine (serotonin) receptor 1D | Rhodopsin-like receptor activity; serotonin receptor activity; signal transduction; synaptic transmission; G-protein signaling; involved in anxiety, depression, and other neuropsychiatric disorders |
| HTR1F | 3 | 5-Hydroxytryptamine (serotonin) receptor 1F | Rhodopsin-like receptor activity; serotonin receptor activity; signal transduction; synaptic transmission |
| IGSF11 | 3 | Immunoglobulin superfamily, member 11 | Brain- and testis-specific immunoglobulin superfamily protein; receptor activity; signal transduction; stimulator of cell growth |
| LONRF2 | 2 | LON peptidase N-terminal domain and ring finger 2 | Zinc ion binding; ATP-dependent peptidase activity; ubiquitin-protein ligase activity; neurohypophyseal hormone activity |
| MRGPRX2 | 11 | MAS-related GPR, member X2 | Neuropeptide binding; rhodopsin-like receptor activity; signal transduction; sensory perception of pain; sleep |
| NLN | 5 | Neurolysin (metallopeptidase M3 family) | Zinc ion binding; hydrolase activity; metalloendopeptidase activity; termination of neurotensinergic signal in CNS and in gastrointestinal tract |
| NRIP1 | 21 | Nuclear receptor interacting protein 1 | Regulation of transcription; protein binding; androgen receptor binding; estrogen receptor binding; glucocorticoid receptor binding |
| OPN5 | 6 | Opsin 5 | Rhodopsin-like receptor activity; phototransduction; visual perception; sensory perception; signal transduction |
| PCP4 | 21 | Purkinje cell protein 4 | Brain-specific polypeptide PEP19; CNS development |
| PMCH | 12 | Pro-melanin-concentrating hormone | Melanin-concentrating hormone activity; spermatogenesis; feeding behavior; cell differentiation; synaptic transmission; signal transduction |
| PMCHL2 | 5 | Pro-melanin-concentrating hormone | Melanin-concentrating hormone activity; synaptic transmission |
| PPARGC1B | 5 | Peroxisome proliferator-activated receptor gamma, coactivator 1 | DNA and RNA binding; receptor activity; regulation of transcription–transcriptional activity of estrogen receptor alpha and glucocorticoid receptor; chromatin modification; cell glucose homeostasis; signal transduction |
| SCRG1 | 4 | Stimulator of chondrogenesis 1 | Neurogenesis |
| TERF2IP | 16 | Telomeric repeat binding factor 2, interacting protein | Dopamine receptor interacting protein 5; DNA binding; protein binding |
| TPH1 | 11 | Tryptophan hydroxylase 1 | Iron ion binding; amino acid binding; monooxygenase activity; tryptophan 5-monooxygenase activity; serotonin biosynthesis from tryptophan |
| TUBA3 | 12 | Tubulin, alpha 1a | GTP binding; GTPase activity; microtubule-based movement; expressed predominantly in morphologically differentiated neurologic cell |
| UGT2B11 | 4 | UDP glucuronosyltransferase 2 family, polypeptide B11 | Glucuronosyltransferase activity; estrogen metabolism; xenobiotic metabolism |
| UGT2B4 | 4 | UDP glucuronosyltransferase 2 family, polypeptide B4 | Glucuronosyltransferase activity; estrogen metabolism; xenobiotic metabolism |
| UGT8 | 4 | UDP glycosyltransferase 8 | Glycosphingolipid biosynthesis; CNS development; peripheral nervous system development |
| WNT8B | 10 | Wingless-type MMTV integration site family, member 8B | Signal transducer activity; frizzled-2 signaling pathway; nervous system development |
The public database GeneCards (http://www.genecards.org; Rebhan et al., 1997) was used for the annotation of the genes in terms of GO functions and processes.
Discussion
A number of studies have shown that the epigenetic status of an organism is susceptible to change through behavioral programming. The psychological stresses and early experiences that occur during the early stages of an organism’s development, that is, in childhood, have a particular importance and power in developmental change. It is known that occurrences, such as birth by Caesarean section, which changes the timing and preliminary stages of delivery (Schlinzig, Johansson, Gunnar, Ekström, & Norman, 2009), parental abuse (McGowan et al., 2008, 2009), and parental stress during the early stages of life, in infancy, and preschool years (Essex et al., in press) might result in epigenetic changes in children’s genomes.
Acquired under the influence of behavioral programming, epigenetic changes are detected in various cells and tissues, such as the central nervous system (Franklin et al., 2010; McGowan, Sasaki, D’Alessio, et al., 2009; McGowan, et al., 2008; Murgatroyd, et al., 2009), liver (Bateson et al., 2004; Meaney et al., 2007), epithelial cells (Essex et al., in press), and peripheral blood lymphocytes (Schlinzig et al., 2009). It has been shown that these changes in genome properties might be stable and have long-term effects; although emerging in childhood, they may also be detected in later developmental stages up to adulthood. In addition, these changes have great potential for heritability in subsequent generations (Francis et al., 1999; Franklin et al., 2010; McGowan et al., 2009; Meaney, 2001b; Verhoeven et al., 2010).
One important behavioral factor that might affect the epigenetic status of an individual is maternal care. It has been shown (mostly on the animal model) that this factor determines the behavior and the hormonal status of the offspring; particularly, the presence and level of maternal care are highly responsible for the epigenetic regulation of genes involved in the control of the hypothalamic–pituitary–adrenal system (Caldji et al., 2000; Champagne et al., 2003; Franklin et al., 2010; Liu et al., 2000; Meaney & Szyf, 2005; Murgatroyd et al., 2009; Oberlander et al., 2008; Weaver et al., 2004).
The most extreme manifestation of negative early experience is a complete deprivation of parental attention and care. Our research is one of the first attempts to investigate the peculiar properties of the epigenetic status of the genomes of children who have been placed in an orphanage shortly after birth and thus completely deprived of parental care. The main goal of the study was to identify the systematic differences in the methylation status of the genomes of children placed in institutional care at birth in comparison with children reared by their biological parents, to detect the main biological pathways that might be affected by the lack of parental care and stay in institutional care.
In brief, the results of this small-scale investigation can be grouped into four observations. First, although the cluster analysis did not show a complete separation between the two studied groups based on the whole-genome analysis, it did indicate the presence of a nontrivial amount of within-group clustering. Second, a further investigation of this clustering revealed that approximately 6% of the investigated genes (specifically, 838 of the 14,000 contained by the Infinium HumanMethylation27 BeadChip) showed small but statistically significant ( p <.01) intergroup differences in levels of methylation. Third, the absolute majority (~89%) of these differences are due to an increase in the levels of methylation in the genomes of the children from the group of institutionalized children. Fourth, among the genes that showed gains in methylation in the genomes of the institutionalized children, most are involved in the control of immune response and cellular signaling systems.
These findings are consistent with the growing body of research connecting early experiences with subsequent developmental outcomes (Champagne & Curley, 2005). Early experiences largely exist as sensory stimulation (Grubb & Thompson, 2004). The developing organism is tuned to respond to this stimulation at a variety of levels from switching specific genes on and off to developing highly specialized neuronal pathways. Early adverse experiences can, in turn, jeopardize these fundamental processes that lay the foundation for many subsequent outcomes (McEwen, 2008b). In addition, both short- and long-range signaling systems are central to receiving and processing sensory stimulation and responding to stress (McEwen, 2008a).
It was also found that genes that were detected as hypermethylated in the genomes of institutionalized children are known to be expressed in the cells of at least nine different tissues and organs, for instance blood, salivary glands, skin, tongue, liver, muscles, lungs, spinal cord, and brain. Specifically focusing on genes that play a critical role in the development and function of the brain, we found that among those hypermethylated in the group of institutional children there are a number of genes involved in the biosynthesis of hormones and neurotransmitters, and in the control of their receptor activity, including members of the hypothalamic–pituitary–adrenal system. These findings are consistent with data from the animal literature that reveal associations of early care with the expression patterns of a number of receptors and other crucial players in the development, maturation, and functioning of the brain (Caldji et al., 2000; Champagne et al., 2001, 2003; Francis et al., 1999; Liu et al., 2000), as well as with data from human studies showing the important role of early experiences in the neuroendocrine system development and functioning (Cicchetti, 2002; Cicchetti et al., 2010; Ladd et al., 2000; McEwen, 2008b; McGowan et al., 2008, 2009).
Taking into account the small differences in the methylation levels of those genes found to be hypermethylated in the group of institutional children relative to the comparison group (the intergroup difference varied from a 1.03- to 2.36-fold change with an average of 1.09), as well as the complexity of the relationship between gene methylation and expression, we are far from speculating on the critical changes in the expression of these genes, as well as on the long-term outcomes of the epigenetic differences observed. For that, additional investigations, including a longitudinal study, are necessary. In addition, we cannot definitively associate the epigenetic modifications that we detected in the genomes of institutional children with the lack of parental care only; especially, considering (a) the environmental differences between orphanage and family, and (b) insufficient information on the physical and behavioral statuses of the institutional children’s mothers during the prenatal period, which are serious limitations of our study. Yet, although further investigations are needed to confirm the observed group differences and further explore them with regard to specific behavioral and psychological phenotypes, the groups are quite systematically distinct in terms of their patterns of methylation. In general, even considering the limitations of the study and though preliminary in nature and generated in the relative void of comparable research, these findings look promising. In addition, the results obtained are logical, expected, and consistent with the growing literature on the association of early experiences and epigenetic regulation of gene activity. Of particular interest is the presence of hypermethylation that has been previously associated with adverse developmental impacts (Essex et al., in press).
The strength of the work presented here is in assembling a well-defined sample of children placed into institutional care at birth and matching this sample with a sample of typically developing children being raised by their biological families, whose socioeconomic status is thought to be comparable to that of the parents who placed their children in institutional care. Nonetheless, it is critical to stress the tentative nature of these results. To our knowledge, this study makes one of the first steps toward understanding the changes in the methylation profiles of the whole genome based on exposure to the highly adverse circumstance of being placed into institutional care at birth. Although small-scale and exploratory, the research has revealed definitive indications of the presence of small, yet numerous, differences in the patterns of methylation in the two studied groups. As such, this work opens the gate for further validation and examination of this finding, so that the field can understand both the extent and the mechanics of the impact of the early loss of parental care on subsequent human development as captured by the epigenome.
Supplementary Material
Acknowledgments
This work was supported by funding from the Foundation for Child Development, NIH (MH81756 and MH84135), and Edna Bennett Pierce. We thank the young participants who provided samples of their blood for the study, as well as the parents, caregivers, and medical staff of orphanages for their understanding, support, and participation in collecting material for this research. We thank Dr. Dean Palejev for his help with data analysis and Ms. Mei Tan for her editorial assistance.
Footnotes
Supplementary materials and methods
The Supplementary Material referred to in this article can be found online at http://journals.cambridge.org/dpp
References
- Alvazian SA, Kolenikov SO. Final Report. Moscow, Russia: Economics Education and Research Consortium Russia; 2000. Poverty and expenditure differentiation of Russian population. Retrieved from www.komkon.org/~tacik/science/Aivazian-Kolenikov-FinalEng-2.pdf. [Google Scholar]
- Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Curley JP. How social experiences influence the brain. Current Opinion in Neurobiology. 2005;15:704–709. doi: 10.1016/j.conb.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Sharma S, Meaney MJ. Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology. 2003;144:4720–4724. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- Chudin E, Kruglyak S, Baker SC, Oeser S, Barker D, McDaniel TK. A model of technical variation of microarray signals. Journal of Computational Biology. 2006;13:996–1003. doi: 10.1089/cmb.2006.13.996. [DOI] [PubMed] [Google Scholar]
- Cicchetti D. The impact of social experience on neurobiological systems: Illustration from a constructivist view of child maltreatment. Cognitive Development. 2002;17:1407–1428. [Google Scholar]
- Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The differential impacts of early abuse on internalizing problems and diurnal cortisol activity in school-aged children. Child Development. 2010;25:252–269. doi: 10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. Journal of Nutrition. 2002;132:2393–2400. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall-McClough KC, et al. Foster children’s diurnal production of cortisol: An exploratory study. Child Maltreatment. 2006;11:189–197. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- Enthoven L, de Kloet ER, Oitzl MS. Effects of maternal deprivation of CD1 mice on performance in the water maze and swim stress. Behavioural Brain Research. 2008;187:195–199. doi: 10.1016/j.bbr.2007.08.037. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Boyce WT, Hertzman C, Lam L, Armstrong JM, Neumann SMA, et al. Epigenetic vestiges of early developmental adversity: Childhood stress exposure and DNA methylation in adolescence. Child Development. doi: 10.1111/j.1467-8624.2011.01641.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Russig H, Weiss IC, Gräff J, Linder N, Michalon A, et al. Epigenetic transmission of the impact of early stress across generations. Biological Psychiatry. 2010;68:408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Gilad VH, Rabey JM, Eliyayev Y, Gilad GM. Different effects of acute neonatal stressors and long-term postnatal handling on stress-induced changes in behavior and in ornithine decarboxylase activity of adult rats. Brain Research. Developmental Brain Research. 2000;120:255–259. doi: 10.1016/s0165-3806(00)00012-2. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuro-anatomical hypothesis of panic disorder revised. American Journal of Psychiatry. 2000;157:493–505. doi: 10.1176/appi.ajp.157.4.493. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Normally occurring environmental and behavioral influences on gene activity: From central dogma to probabilistic epigenesis. Psychological Reviews. 1998;105:792–892. doi: 10.1037/0033-295x.105.4.792-802. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Thompson ID. The influence of early experience on the development of sensory systems. Current Opinion in Neurobiology. 2004;14:503–512. doi: 10.1016/j.conb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Fisher PA. Early experience, stress, and prevention network. Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Development and Psychopathology. 2006;18:651–677. [PubMed] [Google Scholar]
- Gunnar MR, Van Dulmen MHM The International Adoption Project Team. Behavior problems in post-institutionalized internationally adopted children. Development and Psychopathology. 2007;19:129–148. doi: 10.1017/S0954579407070071. [DOI] [PubMed] [Google Scholar]
- Harkonmäki K, Korkeila K, Vahtera J, Kivimäki M, Suominen S, Sillanmäki L, et al. Childhood adversities as a predictor of disability retirement. Journal of Epidemiology and Community Health. 2007;61:479–484. doi: 10.1136/jech.2006.052670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF. Application of experimental stressors in laboratory rodents. Current Protocols in Neuroscience. 2006;Chap 8(Unit 8.4) doi: 10.1002/0471142301.ns0804s34. [DOI] [PubMed] [Google Scholar]
- Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Annals of New York Academy of Sciences. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- Hertzman C, Boyce T. How experience gets under the skin to create gradients in developmental health. Annual Review of Public Health. 2010;31:329–347. doi: 10.1146/annurev.publhealth.012809.103538. [DOI] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Research. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juffer F, van IJzendoorn MH. Behavior problems and mental health referrals of international adoptees: A meta-analysis. Journal of the American Medical Association. 2005;293:2501–2515. doi: 10.1001/jama.293.20.2501. [DOI] [PubMed] [Google Scholar]
- Kishiyama MM, Boyce WT, Jimenez AM, Perry LM, Knight RT. Socioeconomic disparities affect prefrontal function in children. Journal of Cognitive Neuroscience. 2009;21:1106–1115. doi: 10.1162/jocn.2009.21101. [DOI] [PubMed] [Google Scholar]
- Kreppner JM, Rutter M, Beckett C, Castle J, Colvert E, Groothues C, et al. Normality and impairment following profound early institutional deprivation: A longitudinal follow-up into early adolescence. Developmental Psychology. 2007;43:931–946. doi: 10.1037/0012-1649.43.4.93. [DOI] [PubMed] [Google Scholar]
- Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–930. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Progress in Brain Research. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nature Neuroscience. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008a;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Understanding the potency of stressful early life experiences on brain and body function. Metabolism: Clinical & Experimental. 2008b;57:S11–S15. doi: 10.1016/j.metabol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, Huang TCT, Unterberger A, Suderman M, Ernst C, et al. Promoter-wide hypermethylation of the ribosomal RNA gene promoter in the suicide brain. PLoS ONE. 2008;3:e2085. doi: 10.1371/journal.pone.0002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001a;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001b;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene environment interactions. Child Development. 2011;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlanta P, Caldji C, et al. Early environmental regulation of forebrain glucocorticoid receptor gene expression: Implications for adrenocortical responses to stress. Developmental Neuroscience. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Maternal effects as a model for environmentally-dependent chromatin plasticity. Trends in Neuroscience. 2005;28:456–463. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic–pituitary–adrenal function and health. Trends in Molecular Medicine. 2007;13:269–277. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmühl Y, Fischer D, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nature Neuroscience. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Nelson CA, III, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Rutter M The English and Romanian Adoptees Study Team. Attachment disorder behaviour following early severe deprivation: Extension and longitudinal follow-up. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:703–712. doi: 10.1097/00004583-200006000-00008. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Virsi S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Razin A. CpG methylation, chromatin structure and gene silencing—A three-way connection. EMBO Journal. 1998;17:4905–4908. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Colvert E, Kreppner J, Beckett C, Castle J, Groothues C, et al. Early adolescent outcomes for institutionally-deprived and non-deprived adoptees. I: Disinhibited attachment. Journal of Child Psychology and Psychiatry. 2007;48:17–30. doi: 10.1111/j.1469-7610.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- Sabatini MJ, Ebert P, Lewis DA, Levitt P, Cameron JL, Mirnics K. Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. Journal of Neuroscience. 2007;27:3295–3304. doi: 10.1523/JNEUROSCI.4765-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapogov MV. Пpaвocoзнaниe и пpaвoвaя coциaлизaция нecoвepшeннoлeтниx ocyждeнныx из Чиcлa дeтeй-cиpoт и дeтeй, ocтaвшиxcя бeз пoпeЧeния poдитeлeй. Pskov, Russia: ANO Logos; 2010. [Google Scholar]
- Schlinzig T, Johansson S, Gunnar A, Ekström TJ, Norman M. Epigenetic modulation at birth—Altered DNA-methylation in white blood cells after Caesarean section. Acta Pædiatrica. 2009;98:1096–1099. doi: 10.1111/j.1651-2227.2009.01371.x. [DOI] [PubMed] [Google Scholar]
- Schneirla TC. Behavioral development and comparative psychology. Quarterly Review of Biology. 1966;41:283–302. doi: 10.1086/405056. [DOI] [PubMed] [Google Scholar]
- Schwirtz M. An experiment in orphan care in Russia. The New York Times. 2008 Oct 1; Retrieved from http://www.nytimes.com/2008/10/01/world/europe/01iht-russia.4.16620179.html.
- Seay B, Alexander BK, Harlow HF. Maternal behavior of socially deprived Rhesus monkeys. Journal of Abnormal Psychology. 1964;69:345–354. doi: 10.1037/h0040539. [DOI] [PubMed] [Google Scholar]
- Sfoggia A, Pacheco MA, Grassi-Oliveira R. History of childhood abuse and neglect and suicidal behavior at hospital admission. Crisis: Journal of Crisis Intervention & Suicide. 2008;29:154–158. doi: 10.1027/0227-5910.29.3.154. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Harlow HF, Kimball SD. Behavioral effects of prolonged partial social isolation in the rhesus monkey. Psycholological Reports. 1971;29:1171–1177. doi: 10.2466/pr0.1971.29.3f.1171. [DOI] [PubMed] [Google Scholar]
- Szyf M. Epigenetics, DNA methylation, and chromatin modifying drugs. Annual Review of Pharmacology & Toxicology. 2009;49:243–263. doi: 10.1146/annurev-pharmtox-061008-103102. [DOI] [PubMed] [Google Scholar]
- The St. Petersburg–USA Orphanage Research Team. The effects of early social–emotional and relationship experience on the development of young orphanage chidren. Monographs of the Society for Research in Child Development. 2008;73:1–297. doi: 10.1111/j.1540-5834.2008.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Hyppönen E, Power C. Obesity and type 2 diabetes risk in midadult life: The role of childhood adversity. Pediatrics. 2008;121:e1240–e1249. doi: 10.1542/peds.2007-2403. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn MH, Juffer F. The Emanuel Miller Memorial Lecture 2006: Adoption as intervention. Meta-analytic evidence for massive catch-up and plasticity in physical, socio-emotional, and cognitive development. Journal of Child Psychology and Psychiatry. 2006;47:1228–1245. doi: 10.1111/j.1469-7610.2006.01675.x. [DOI] [PubMed] [Google Scholar]
- Verhoeven KJF, Jansen JJ, vanDij PJ, Biere A. Stress-induced DNA methylation changes and their heritability in asexual dandelions. New Phytologist. 2010;185:1108–1118. doi: 10.1111/j.1469-8137.2009.03121.x. [DOI] [PubMed] [Google Scholar]
- Waterland RA, Lin JR, Smith CA, Jirtle RL. Post-weaning diet affects genomic imprinting at the insulin-like growth factor 2 (Igf2) locus. Human Molecular Genetics. 2006;15:705–716. doi: 10.1093/hmg/ddi484. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. Epigenetic mechanisms for nutrition determinants of later health outcomes. American Journal of Clinical Nutrition. 2009;89:1488S–1493S. doi: 10.3945/ajcn.2009.27113B. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.