Abstract
Neutrophils are pivotal effector cells of innate immunity. Their recruitment into peripheral tissues is indispensable for host defense. Given their destructive potential, neutrophil entry into tissue must be tightly regulated in vivo to avoid damage to the host. An array of chemically diverse chemoattractants is active on neutrophils and participates in recruitment. Neutrophil chemoattractants were thought redundant in the control of neutrophil recruitment into peripheral tissue, based on their often indistinguishable effects on neutrophils in vitro and their frequently overlapping patterns of expression at inflammatory sites in vivo. Recent data, however, suggest that neutrophil chemoattractants have unique functions in the recruitment of neutrophils into inflammatory sites in vivo dictated by their distinct patterns of temporal and spatial expression.
The ostensibly redundant role of chemoattractants in the recruitment of neutrophils
Neutrophils are essential effector cells of the innate immune response forming the first line of defense against bacterial and fungal pathogens. The engulfment of pathogens and the release of reactive oxygen species (ROS) and proteases contribute to the key role of neutrophils in host defense [1]. Accordingly, neutropenia is an alarming condition that renders patients susceptible to fulminant, life-threatening infections. However, neutrophils also contribute significantly to tissue damage in acute disease processes, such as acute lung injury and spinal cord injuries, as well as in chronic diseases processes, such as rheumatoid arthritis, chronic obstructive pulmonary disease (COPD), and asthma [2-12]. The destructive potential of neutrophils requires the tight control of their recruitment into tissue compartments. Neutrophils are responsive to a plethora of diverse chemoattractants (Table 1) that have similar functions in in vitro studies. In addition, a large number of different chemoattractants are usually present in vivo at sites of inflammation. Thus, overlapping signals were considered to induce neutrophil recruitment in a redundant fashion, to ensure that these crucial innate immune cells quickly and faithfully reached the site of infection. Recent discoveries, however, highlight non-redundant roles for chemoattractants in mouse models of sterile inflammation, suggesting that in vivo chemoattractants collaborate sequentially in temporal and spatial cascades in order to choreograph the recruitment of neutrophils[et2]. The absolute requirement for an individual chemoattractant at a specific step in the cascade might come about through unique temporal and/or spatial patterns of expression of the chemoattractant and the corresponding receptor on neutrophils, and through inherent differences in the biophysical properties of chemoattractants. Although the specific chemoattractants, and the order in which they act, likely differ depending on the specific pathological stimulus, the emerging concept of non-redundant cascades of chemoattractants offers the opportunity to tailor drugs meant to hit a chemoattractant or its receptor at a specific place and time in order to arrest the cascade and thus inhibit the pathogenic process. In this review, we will summarize the current knowledge on the journey of neutrophils from the bone marrow to the inflammatory site. We will demonstrate that on this journey there are three major decision points presiding over the neutrophil's migratory fate: exit from the bone marrow into the circulation, movement from the blood into the tissue, and eventually recruitment to the site of inflammation.
Table 1.
Major human and murine chemoattractants and their receptors expressed in neutrophils.
| Chemoattractant | Receptor | |||
|---|---|---|---|---|
| Chemokines | ||||
| Nomenclature | Receptors | |||
| Systemic | Human | Murine | Human neutrophils | Murine neutrophils |
| CXCL1 | GROα | KC | CXCR2 | CXCR2 |
| CXCL2 | GROβ | MIP-2 | CXCR2 | CXCR2 |
| CXCL3 | GROγ | n/a | CXCR2 | n/a |
| CXCL5 | ENA-78 | LIX | CXCR2 | CXCR2 |
| CXCL6 | GCP-2 | n/a | CXCR1/CXCR2 | n/a |
| CXCL7 | NAP-2 | NAP-2 | CXCR1/CXCR2 | CXCR2 |
| CXCL8 | IL-8 | n/a | CXCR1/CXCR2 | CXCR2 |
| CCL3 | MlP-lα | MIP-1α | n/a | CCR1 |
| CCL5 | RANTES | RANTES | n/a | CCR1 |
| CCL6 | (MPIF-1) | C10 | n/a | CCR1 |
| CCL7 | MCP-3 | MARC | n/a | CCR1 |
| CCL9 | (HCC-2) | MIP-1γ | n/a | CCR1 |
| CXCL12 | SDF-1α | SDF-1α | CXCR4 | CXCR4 |
| Peptides/Cytokines | |
|---|---|
| C5a | C5aR |
| C3a | C3aR |
| Formylated peptides (e.g. fMLF) | FPR1 |
| Pro-Gly-Pro (PGP) | CXCR2 |
| LL37 | FPR2 |
| MIF | CXCR2 |
| Eicosanoids | |
| Leukotriene B4 (LTB4) | BLT1 |
| Platelet activating factor (PAF) | PAFR |
Notes: The human and mouse chemokine system is not completely orthologous. For example, IL-8 and GCP-2 are not found in mice; MIP-1γ is not found in humans; CCR1 is not an important receptor on human neutrophils; and CXCR1 is not an important receptor on murine neutrophils. “n/a” stands for non-applicable. HCC-2 and MPIF-1 have also been given the systematic names CCL15 and CCL23, respectively [78].
Decision point I: trafficking from the bone marrow into the peripheral blood
Neutrophils are the most abundant immune cell type. It is estimated that each day 5 × 1010 – 10 × 1010 new neutrophils are formed in the bone marrow [13]. A neutrophil spends the majority of its life in the bone marrow: under physiological conditions, less than 2% of neutrophils are found in the bloodstream [14]. In the latter location neutrophils have a short half life (~ 6-8 hrs in human and ~ 11 hrs in mice) [13,15]. Neutrophil homeostasis in peripheral blood is tightly regulated primarily as a consequence of: 1) the proliferation/differentiation rate of neutrophil precursors in the bone marrow; 2) the egress of mature neutrophils from the bone marrow into the periphery; and 3) neutrophil clearance by the reticuloendothelial phagocytic system in the spleen, liver and bone marrow [15].
Neutrophil release form the bone marrow is a rapid way to increase the number of circulating neutrophls available for recruitment into tissue in response to infection or inflammation. Recently, neutrophil egress from the bone marrow into the peripheral blood has been found to be antagonistically regulated by the chemokine receptors CXCR2 and CXCR4 [14,16-18], which are both expressed on neutrophils. While CXCR4 retains neutrophils in the bone marrow, CXCR2 facilitates their egress. The CXCR4 ligand SDF-1 (CXCL12) and the CXCR2 ligands KC (CXCL1) and MIP-2 (CXCL2) are both constitutively expressed by endothelial cells and osteoblasts in the bone marrow. For SDF-1, however, osteoblasts are the major source, while for the CXCR2 ligands, endothelial cells are the major cellular source in the bone marrow. In this permanent tug-of-war between SDF-1 and CXCR2 ligands, the former usually dominates to retain most neutrophils in the bone marrow (Fig. 1). The dominance of SDF-1 over CXCR2 ligands in this regulatory network is revealed in the absence of CXCR4, where CXCR2 becomes dispensable for the mobilization of neutrophils from the bone marrow [18]. Mechanistically, heterologous desensitization and internalization of CXCR4 by CXCR2 ligands also contribute to neutrophil mobilization [16]. Therefore, the mobilization of neutrophils from the bone marrow by CXCR2 ligands can be described as inhibition of CXCR4-mediated retention. Furthermore, neutrophils in the bone marrow appear to lower their surface expression of CXCR4 in the course of maturation [16], which may be a mechanism to ensure the mobilization of functionally mature neutrophils while retaining less mature ones. Recently, it has been suggested that SDF-1 augments the binding of the α4 integrin VLA-4 on neutrophils to VCAM-1 on bone marrow endothelial and stromal cells this way keeping neutrophils in the bone marrow [19]. Combined inhibition of CXCR4 and VLA-4 potentiated the mobilization of neutrophils from the bone marrow, and notably, similar to CXCR4, VLA-4 expression on neutrophils decreases during maturation. The pivotal role of CXCR4 signaling in the retention of neutrophils in the bone marrow and its regulation by internalization was revealed in a human disease associated with a group of autosomal dominant mutations of CXCR4, called WHIMs syndrome, and characterized by warts, hypogammaglobulimenia, infections, and myelokathexis [22,23]. Mutations in the carboxy terminal tail of CXCR4 result in the expression of truncated forms of CXCR4, which are impaired in their desensitization and internalization, and therefore cause an enhanced signaling of CXCR4 [20,21]. Phenotypically, this leads to myelokathexis, a condition characterized by retention of mature neutrophils within the bone marrow and peripheral neutropenia.
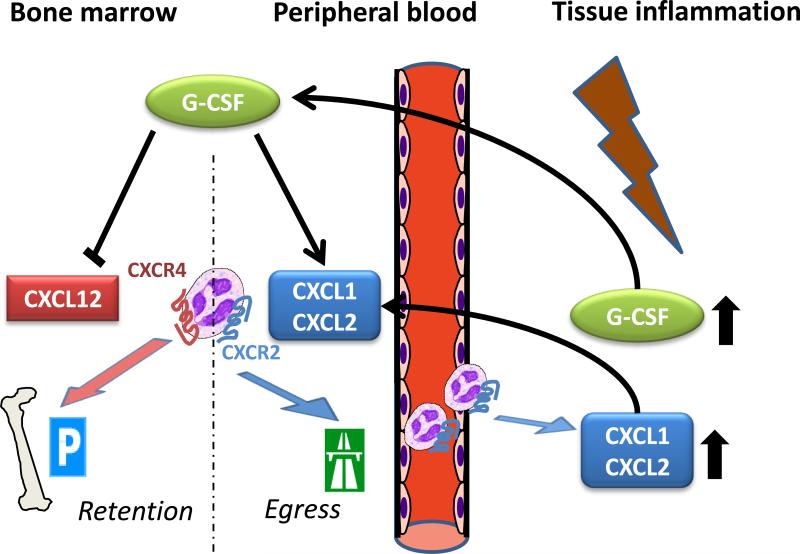
Figure 1. Regulation of neutrophil egress from the bone marrow by CXCR4 and CXCR2 chemokine ligands.
The CXCR4 ligand SDF-1 (CXCL12) functions to retain neutrophils in the bone marrow, while the CXCR2 ligands KC (CXCL1) and MIP-2 (CXCL2) promote neutrophil egress. GCSF mobilizes neutrophils from the bone marrow by increasing the ratio of CXCR4 to CXCR2 ligands in the bone marrow. Neutrophil egress is influenced by the local production of G-CSF and KC within the bone marrow as well as the release of these mediators from inflamed peripheral tissues.
In acute inflammation mobilization of neutrophils from the bone marrow is orchestrated by the hematopoietic cytokine G-CSF. G-CSF mobilizes neutrophils indirectly shifting the balance between SDF-1 and CXCR2 ligands in the bone marrow. G-CSF does this by reducing the absolute number of osteoblasts, the major cellular source of SDF-1, while simultaneously increasing KC and MIP-2 and decreasing SDF-1 expression in endothelial cells of the bone marrow [14,24]. In addition to mediators produced and released within the bone marrow, inflammatory mediators released in peripheral tissues may also make their way to the bone marrow and modulate neutrophil egress. In thioglycollate-induced peritonitis, for instance, neutrophil blood counts were elevated by 4.5-fold two hours after intraperitoneal injection of thioglycollate. The elevation was decreased by 84% and 72% when MIP-2 and KC, or G-CSF, respectively, were neutralized prior to the administration of thioglycollate by intraperitoneal antibody injection [24]. Neutralizing only one chemokine diminished the elevation by approximately 50%. Intraperitoneal injection of MIP-2 or G-CSF mimicked the effect of acute peritonitis on the peripheral neutrophil blood count. Therefore, local peripheral release of chemokines in inflammation may directly affect neutrophil mobilization from the bone marrow. Unlike MIP-2, however, intraperitoneal injection of G-CSF did not induce neutrophil recruitment into the peritoneum. Consistent with this, intraperitoneal injection of MIP-2- and KC-neutralizing antibodies also attenuated the influx of neutrophils into the peritoneum after thioglycollate administration, while neutralizing of G-CSF had no effect on the influx [24]. Thus, chemokines can act locally to induce neutrophil recruitment into peripheral tissue and at distance to induce neutrophil mobilization from the bone marrow (Fig. 1).
Decision point II: entering peripheral tissues or not
Neutrophils in the peripheral blood can be rapidly recruited into peripheral tissues in the event of pathogenic invasion or sterile tissue damage. The disturbance of tissue homeostasis is recognized either by professional tissue-resident sentinel cells, such as macrophages and mast cells, or by stromal cells [25,26]. A panel of diverse stimuli, especially pathogen-associated molecular pattern (PAMPs) and damage-associated molecular pattern (DAMPs) molecules, activates these sentinel cells to release pro-inflammatory mediators (e.g., IL-1β and TNF), and neutrophil-active chemoattractants (e.g., chemokines and lipid mediators) [25,26]. These mediators initiate the recruitment of neutrophils into the tissue by diverse actions.
One initial action of utmost importance is the activation of adhesion molecules on the endothelium neighboring the injury or inflammation in order for neutrophils to exit the vasculature. It has recently been suggested that neutrophils, rather than taking the shortest linear route through tissues, approach the inflammatory site as closely as possible within the blood vessels. The blood vessels, therefore, apparently function as fast-track transfer routes for neutrophils [27]. Approaching the injured site, neutrophils face a second major migratory decision point –namely, whether or not they should leave the peripheral blood and transmigrate into peripheral tissues. This migration mainly occurs at the postcapillary venules.
Having approached the endothelium, the neutrophils engage in a sequence of physical interactions with endothelial cells, referred to as the leukocyte adhesion cascade. The leukocyte adhesion cascade was originally thought to consist of three distinct steps: rolling, activation, and arrest, followed by diapedesis (transmigration through the endothelium). In recent years, it has become evident that more steps can be distinguished. Now slow rolling, adhesion strengthening, and intraluminal crawling are regarded as additional steps, each requiring different molecular mechanisms [28,29]. While the adhesion cascade is reversible and can be stopped at any point, transmigration is irreversible. Therefore, “Decision point II” consists of several independent decisions, which are integrated to make the final decision to leave the blood and enter the tissue.
The first step, rolling, is mediated predominantly by selectins, while the β2-integrins LFA-1 (αLβ2-integrin) and MAC-1 (αMβ2-integrin) mediate adhesion and the subsequent intraluminal crawling of the neutrophil to an optimal anatomical site for transmigration, respectively [30]. Thus, each integrin exerts fundamentally different functions, although both bind to endothelial ICAM-1.
When moving over the endothelium, neutrophils are activated by chemokines, which are bound to the endothelium via glycoaminoglycans [31]. Activation of the neutrophil by chemokines enhances the affinity of their β2-integrins for their binding partner and subsequently induces arrest. Activated and arrested, the neutrophils start transmigrating. Neutrophils can transmigrate via the paracellular or the transcellular route, i.e., either through junctions between endothelial cells or through an endothelial cell. The former is thought to be the predominant route. Paracellular transmigration is mediated by integrins, junction adhesion molecules, and the adhesion molecules platelet/endothelial cell adhesion molecule 1 (PECAM-1), CD99, and the endothelial cell-selective adhesion molecule (ESAM) [32,33].
The contribution of chemokines, particularly CXCR2 ligands, to the transmigration of neutrophils is not limited to the direct activation of neutrophils on the endothelial cell surface. Thus, in a model of acute lung injury, activation of CXCR2 was not only required on neutrophils, but also on endothelial cells [34]. This finding extends the complexity of the role of chemokines in mediating transmigration of neutrophils. For further details about the mechanisms of neutrophil transmigration other reviews are suggested [28,29].
Having traversed the endothelial cell layer, neutrophils must penetrate the perivascular basement membrane. The detailed mechanism of the penetration of the basement membrane is still elusive. Homophilic interactions of PECAM-1, however, appear to be crucial as well as a PECAM-1-dependent α6β1 integrin up-regulation on transmigrated neutrophils, enabling the interaction of neutrophils with laminin in the basement membrane [35]. Proteases, expressed on the cell surface of neutrophils, may facilitate the penetration of the basement membrane. Neutrophils are capable of expressing a number of different proteases, but the importance of neutrophil-derived proteases for transmigration is contentious. MMP9 has been reported to be essential for the activity of Mac-1 in transendothelial migration of neutrophils in vitro [36]. Neutrophil proteases may also subtly alter the basement membrane or the subendothelial ECM and thus facilitate the recruitment of subsequent neutrophils [36-38].
Intriguingly, the structural composition of the vascular basement membrane differs in specific anatomical sites. Particularly in the early phases of inflammatory reactions, these differences can impact the ability of neutrophils to enter the inflammatory site. For example, a decreased density of the components collagen IV, laminin-10, and nidogen-2 facilitates the transmigration of neutrophils and favors transmigration [37]. Transmigration may also alter the phenotype of neutrophils and prime them for their functions in peripheral tissues. As a result, transmigrated neutrophils exhibit differences in their general protein and surface receptor expression [39-41].
Decision point III: finding the cue into the inflammatory site
It was recently shown that diverse chemoattractants can act in a sequential cascade in order to recruit neutrophils into an inflammatory site. In this case, immune complex-induced arthritis (K/BxN serum transfer-induced arthritis) is driven by a lipid – cytokine – chemokine cascade, precisely by LTB4 – IL-1β – CCR1/CXCR2 ligands [42]. LTB4 and its high-affinity receptor BLT1 are absolutely required for the induction of immune complex-induced arthritis. In this model, release of LTB4 by neutrophils, and expression of BLT1 specifically on neutrophils is sufficient to induce arthritis [43,44]. Adoptive transfer of wild-type neutrophils into BLT1-deficient (Ltb4r1-/-) mice restored arthritis in these mice. Interestingly, analysis of the synovial infiltrate showed that a few days after adoptive transfer, the bulk of neutrophils in the synovial fluid were Ltb4r1-/-, suggesting the BLT1 is only needed on a small proportion of all neutrophils for the induction of arthritis and that Ltb4r1+/+ neutrophils facilitate the entry of Ltb4r1-/- neutrophils into the joint. Following up on these observations, it was found that Ltb4r1+/+ neutrophils were required to express IL-1β for the induction of arthritis. IL-1β induced the release of CCR1 and CXCR2 ligands. Although both CCR1 and CXCR2 ligands recruit neutrophils into the joint, they do so non-redundantly due to differences in the timing of their expression and their cellular source. CCR1 ligands are primarily synthesized in the earlier phase of arthritis by synovial tissue, whereas CXCR2 ligands are required for the later phase and are released by the neutrophils themselves. The molecular basis for the sequential expression of CCR1 and CXCR2 ligands during induction of arthritis is not completely understood. The sequential cascade must be run through entirely (Fig. 2) and inhibiting any point in the cascade attenuates the development of full-blown arthritis. Of note, the cascade described thus far is likely incomplete. The complement receptor C5aR, another important neutrophil chemoattractant receptor, is also required for arthritis development in this model and it remains unknown if it is also required for neutrophil recruitment, and if so, where it fits into the cascade of other neutrophil chemoattractants. Furthermore, it is unclear which chemoattractant recruits the very first neutrophils into the joint. Adding further complexity, MIF, a cytokine capable of recruiting neutrophils by binding to and activating CXCR2 as a non-cognate ligand [45,46], has recently been implicated in neutrophil recruitment in immune complex-induced arthritis with arthritis almost completely abrogated in Mif-/- mice [47]. The protective effect of MIF deficiency was more pronounced than that of CXCR2 deficiency, suggesting that MIF might contribute to the pathogenesis of arthritis in this model by mechanisms other than CXCR2 activation.
Figure 2. Recruitment of neutrophils into the joint in immune complex-induced arthritis is mediated by a temporal cascade of chemoattractants.
(1) At early time points, LTB4 is required to recruit and activate a small number of neutrophils. (2) These recruited neutrophils release IL-1β in the joint, which induces the release of predominantly CCR1 ligands at first and later CXCR2 ligands from cells in the joint. (3) CCR1 ligands are required to recruit the next wave of neutrophils into the joint, and this recruitment of neutrophils is broadly amplified in the last step of the cascade when (4) CXCR2 ligands released form neutrophils themselves potently recruit large numbers of neutrophils into the joint.
LTB4 – BLT1 is not only crucial for neutrophil recruitment in models of autoimmune diseases, but it is also important for the inflammatory processes in the aftermath of traumatic injuries. In experimental spinal cord injuries, for instance, BLT1 on neutrophils was required for their recruitment into spinal cord tissue [48]. Absence of BLT1 or its inhibition ameliorated the inflammatory process. Neutrophils that had infiltrated the spinal cord tissue had elevated levels of IL-6, IL-1β, TNF, KC, MIP-2, and MCP-1 compared to blood neutrophils, suggesting that they amplify inflammation and the recruitment of more neutrophils. Together, these findings imply a sequence of events similar to those described in immune complex-induced arthritis.
Lipid mediators such as LTB4 are rapidly produced and have a short half-life, thus it is not surprising that they frequently act at the beginning of neutrophil recruitment cascades and at the local site of inflammation. In contrast, chemokines are well poised to act later in cascades and at longer distances. The production of chemokines is slower than that lipid chemoattractants. Chemokines are often transcriptionally regulated and their release is often also subject to post-transcriptional regulation. However, the ability of chemokines to bind glycosaminoglycans, which increases their retention in the tissue and protects them from proteolysis likely prolonging their half-life [31,49,50], enables chemokines to act later in chemoattractant cascades and at farther distances than lipid mediators [51-53].
In Bovine serum albumin (BSA) antigen-induced arthritis, another complex cascade of chemoattractants was identified that orchestrates neutrophil recruitment. In this model, neutrophil entry into the joint was initiated by IL-23, which then induced IL-17A production within the joint. This induced TNF, KC and LIX, which subsequently induced the release of LTB4 and further recruitment of neutrophils into the joint [54,55]. IL-23 regulates this cascade through a positive feedback loop, which began with IL-23 inducing COX-2-dependent production of PGE2, which then reinforced the release of IL-23 [56]. Accordingly, neutralization of IL-23 inhibited the recruitment of neutrophils into the joint after intraarticular injection of BSA in immunized mice, while intraarticular injection of recombinant IL-23 alone induced the recruitment of neutrophils to the joint. The cellular sources of these mediators in this complex cascade were not identified.
A spatial cascade of chemoattractants has also recently been described in mouse models of focal liver and skin necrosis [27]. Here, it was found that multiple distinct zones of chemoattractants existed around foci of necrosis (Fig. 3). The cascade started with the release of damage-associated molecular patterns (DAMPs) and ATP by necrotic cells. This induced the release of IL-1β, which in turn generated an inflammatory microenvironment around the area of necrosis. This was due in part to the increased expression of adhesion molecules on endothelial cells, thereby promoting neutrophil adherence to the vascular wall. Although usually neutrophil adherence to the endothelium is mainly mediated by LFA-1 – ICAM1 interactions, in this particular model neutrophil adhesion was primarily mediated by Mac1 – ICAM1 interactions. In addition, an intravascular gradient of the CXCR2 ligands MIP-2 and KC towards the necrotic site was formed and guided neutrophils to the lesion. This gradient abruptly ended in a zone approximately 150 μm around the lesion. From this point, the recruitment of neutrophils became independent of CXCR2 and dependent on FPR1 activated by endogenous formyl-peptide signals released from necrotic cells. FPR1 signaling precisely directs neutrophils into the site of necrosis. This exemplifies the coordinated recruitment of neutrophils by a spatial cascade. In spatial cascades like this one, neutrophils must prioritize between diverse chemotactic signals. Neutrophils are able to distinguish between “end-target” (e.g., C5a, C3a, and N-formly-peptides) and “intermediate target” (e.g., chemokines and LTB4) chemoattractants [57]. On the molecular level, the activity of the PI(3)K and p38 MAPK pathways is pivotal for the prioritization between opposing signals from end-target and intermediate target chemoattractants. Thus, end-target chemoattractants, such as N-formlypeptides, activate both p38 MAPK and PI(3)K, while intermediate chemoattractants, such as MIP-2, only activate PI(3)K [57-60]. Accordingly, in neutrophil recruitment to sites of focal necrosis, the MIP-2 signal is hierarchically overridden by formyl-peptide signals as neutrophils leave the zone of maximal CXCL2 concentration to enter the zone dominated by FPR1 signals [27].
Figure 3. Recruitment of neutrophils into a sterile necrotic site is mediated by a spatial cascade of chemoattractants.
(1) Necrotic tissues release DAMPs and ATP, which induce the release of IL-1β from resident tissue macrophages. (2) Secreted IL-1β induces the intravascular expression of ICAM-1, resulting in the adhesion of neutrophils to the endothelium surrounding the necrotic focus. Zones of chemoattractants are formed at different distances from the necrotic focus that sequential guide neutrophil migration into the lesion. (3) Farthest from the necrotic focus, a gradient of CXCR2 ligands is induced in the vasculature that guides neutrophils to the vicinity of the necrotic focus. (4) This chemokine gradient falls off close to the necrotic site, and neutrophils are then guided into the necrotic site by a gradient of FPRL1 ligands released from dying cells.
Spatially- and temporally-restricted expression of chemokines might also result in non-redundancy between chemokines that activate the same receptor. Five ELR+ CXC chemokines bind to murine CXCR2 and have similar effects in vitro. In vivo, however, these ligands may exert distinct roles. Thus, intratracheal instillation of TNF alone, or in combination with IL-17A, induced the CXCR2 ligands KC, MIP-2, and LIX (CXCL5). While the expression of KC and MIP-2 peaked at 4 hrs, only LIX expression remained high for at least 24 hrs [61]. Evaluation of the neutrophil recruitment into the lung showed that while LIX did not play a significant role at early time points, it was indispensable for sustaining neutrophil infiltration. In vitro, alveolar type II cells were identified as a major source for LIX after stimulation with TNF and IL-17A. They released LIX in a polarized fashion on the basolateral or the apical membrane, dependent on the direction of stimulation. This suggests that alveolar type II cells may also establish a locally directed chemokine gradient in vivo.
LIX also plays a role in other inflammatory models. In a model of chronic peritonitis induced by injection of 2,6,10,14-tetramethylpentadecane (pristane), inhibition of LIX lowers the influx of neutrophils into the peritoneum. Although MIP-2 is strongly induced by pristane in this model, its neutralization had no effect on neutrophil recruitment [62]. LIX and MIP-2 are both thought to exclusively bind to CXCR2 and, therefore, they are believed to play redundant roles if both chemokines are strongly expressed at the same time and place. This finding, however, suggests that in vivo each might fulfill a unique role.
Self-perpetuation through neutrophils recruiting neutrophils
Neutrophils have been regarded as purely phagocytotic effector cells, lacking the noteworthy capability to synthesize proteins once they are terminally differentiated. However, it has become evident that neutrophils in peripheral tissues are more active transcriptionally and translationally than their counterparts in the blood, and that they contribute to the orchestration of inflammatory reactions by releasing chemokines, cytokines and lipid mediators [28,63,64]. Although the quantity of protein that a single neutrophil can synthesize is usually surpassed by other immune cells such as macrophages, neutrophils can nevertheless contribute to overall biological mediator production due to their sheer number [42,65-68].
Many mediators released by neutrophils themselves are neutrophil-chemoattractants. Therefore, neutrophils may recruit other neutrophils. In immune complex-induced arthritis, the recruitment of neutrophils by neutrophils into the joint is apparently crucial for the development of arthritis. Here, neutrophils in the joint released LTB4, MIP-1α (CCL3), MIP-2, and IL-1β [42]. While the first three most likely recruit neutrophils into the joint directly, the latter induces the release of neutrophil-chemoattractant mediators from other cell types in the joint (Fig. 2). Thus, in response to IL-1β, fibroblast-like synoviocytes produce KC and LIX, endothelial cells produced KC, and macrophages produced MIP-1γ (CCL9).
Moreover, neutrophils may also alter the activity of neutrophil-chemoattractants through the activity of their proteases. In some instances, proteases may inactivate chemokines and cytokines while in other instances proteases may activate chemokines or convert a chemokine agonist into an antagonist [69,70]. Proteolysis by the protease MMP-2, for instance, enhances the potency of LIX, whereas MMP-2 deactivates SDF-1 and converts CX3CL1 and CCL7 into antagonists. By cleaving ECM components, proteases can also generate newly derived peptides with neutrophil-attractant activity [71]. MMP-8, MMP9, and prolyl endopeptidase, for instance, in concert cleave collagen to proline-glycine-proline (PGP), a peptide activating CXCR1 and CXCR2 on neutrophils [72]. PGP may particularly contribute to neutrophil recruitment when chemokine levels are already declining. Surprisingly, PGP is apparently degraded by leukotriene A4 hydrolase, an enzyme required for the generation of LTB4 [73]. This enzyme, therefore, exerts opposing effects on the recruitment of neutrophils. As the net effect on neutrophil recruitment is likely to depend on the stage of the inflammatory process, it is conceivable that this behavior expresses the specialization of the enzyme to regulate acute neutrophilic inflammatory processes with a fast recruitment of neutrophils and then its quick termination. Intriguingly, neutrophils not only promote the recruitment of other neutrophils to the inflammatory site, but also choreograph the natural progression of an acute inflammatory reaction from a predominantly neutrophilic infiltrate to a monocytic one, as reviewed elsewhere [74], and finally, they also contribute to the resolution of inflammation as reviewed elsewhere [75,76].
Conclusions and future directions
In recent years, temporal and spatial cascades of chemically diverse chemoattractants have been identified that coordinate the recruitment of neutrophils in mouse models of acute local inflammation. These findings suggest chemoattractants have unique roles in vivo. Temporal and spatial cascades of appear to be necessary to guide the complex migratory path of neutrophils from the bone marrow into the blood, from the blood into the tissue, and then once in the tissue guide neutrophils to the exact location of the inflammatory focus. The involement of multipe chemoattractants, acting at different points in the migratory path, provides a mechanism to tightly and precisely control the recruitment of neutrophils into tissues. These cascades also provide multiple points for interventing therapeutically to attenuate neutrophil recrutiment into tissues. If a specific chemoattractant or receptor is not available at the appropriate time and place, the cascade comes to a halt and the neutrophil inflammatory reaction collapses. Animal models suggest that the cascade is more vulnerable to inhibition in its early stages as in later stages of inflammation the recruitment of neutrophils becomes reinforced by multiple overlapping pathways. Therefore, future research must aim to define chemoattractant cascades that drive specific inflammatory reactions and seek to identify the early acting mediators that initiate the process. We believe that targeting these early points in specific chemoattractant pathways will lead to new effective therapies.
Acknowledgement
This work was supported by grants of the Deutsche Forschungsgemeinschaft (Sa1960/1-1 to C.D.S.) and of the National Institutes of Health (R01-AI050892 to A.D.L. and K08-AR054094 to N.D.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Segal AW. How neutrophils kill microbes. Annu. Rev. Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal NR, et al. Moderate oxygen augments lipopolysaccharide-induced lung injury in mice. Am. J. Physiol Lung Cell Mol. Physiol. 2010;298:L371–L381. doi: 10.1152/ajplung.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards SW, Hallett MB. Seeing the wood for the trees: the forgotten role of neutrophils in rheumatoid arthritis. Immunol. Today. 1997;18:320–324. doi: 10.1016/s0167-5699(97)01087-6. [DOI] [PubMed] [Google Scholar]
- 4.Genovese T, et al. TNF-alpha blockage in a mouse model of SCI: evidence for improved outcome. Shock. 2008;29:32–41. doi: 10.1097/shk.0b013e318059053a. [DOI] [PubMed] [Google Scholar]
- 5.Monteseirin J. Neutrophils and asthma. J. Investig. Allergol. Clin. Immunol. 2009;19:340–354. [PubMed] [Google Scholar]
- 6.Taoka Y, et al. Activated protein C reduces the severity of compression-induced spinal cord injury in rats by inhibiting activation of leukocytes. J. Neurosci. 1998;18:1393–1398. doi: 10.1523/JNEUROSCI.18-04-01393.1998. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Eyles JL, et al. A key role for G-CSF-induced neutrophil production and trafficking during inflammatory arthritis. Blood. 2008;112:5193–5201. doi: 10.1182/blood-2008-02-139535. [DOI] [PubMed] [Google Scholar]
- 8.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J. Immunol. 2001;167:1601–1608. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- 9.Nabe T, et al. Important role of neutrophils in the late asthmatic response in mice. Life Sci. 2011 doi: 10.1016/j.lfs.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogg JC, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 11.Drost EM, et al. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax. 2005;60:293–300. doi: 10.1136/thx.2004.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papi A, et al. Pathophysiology of exacerbations of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2006;3:245–251. doi: 10.1513/pats.200512-125SF. [DOI] [PubMed] [Google Scholar]
- 13.Summers C, et al. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semerad CL, et al. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17:413–423. doi: 10.1016/s1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- 15.Rankin SM. The bone marrow: a site of neutrophil clearance. J. Leukoc. Biol. 2010;88:241–251. doi: 10.1189/jlb.0210112. [DOI] [PubMed] [Google Scholar]
- 16.Suratt BT, et al. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood. 2004;104:565–571. doi: 10.1182/blood-2003-10-3638. [DOI] [PubMed] [Google Scholar]
- 17.Wengner AM, et al. The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation. Blood. 2008;111:42–49. doi: 10.1182/blood-2007-07-099648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eash KJ, et al. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J. Clin. Invest. 2010;120:2423–2431. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petty JM, et al. Crosstalk between CXCR4/stromal derived factor-1 and VLA-4/VCAM-1 pathways regulates neutrophil retention in the bone marrow. J. Immunol. 2009;182:604–612. doi: 10.4049/jimmunol.182.1.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawai T, et al. WHIM syndrome myelokathexis reproduced in the NOD/SCID mouse xenotransplant model engrafted with healthy human stem cells transduced with C-terminus-truncated CXCR4. Blood. 2007;109:78–84. doi: 10.1182/blood-2006-05-025296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balabanian K, et al. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005;105:2449–2457. doi: 10.1182/blood-2004-06-2289. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez PA, et al. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat. Genet. 2003;34:70–74. doi: 10.1038/ng1149. [DOI] [PubMed] [Google Scholar]
- 23.Gorlin RJ, et al. WHIM syndrome, an autosomal dominant disorder: clinical, hematological, and molecular studies. Am. J. Med. Genet. 2000;91:368–376. [PubMed] [Google Scholar]
- 24.Wengner AM, et al. The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation. Blood. 2008;111:42–49. doi: 10.1182/blood-2007-07-099648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arancibia SA, et al. Toll-like receptors are key participants in innate immune responses. Biol. Res. 2007;40:97–112. doi: 10.4067/s0716-97602007000200001. [DOI] [PubMed] [Google Scholar]
- 26.Zeytun A, et al. Induction of cytokines and chemokines by Toll-like receptor signaling: strategies for control of inflammation. Crit Rev. Immunol. 2010;30:53–67. doi: 10.1615/critrevimmunol.v30.i1.40. [DOI] [PubMed] [Google Scholar]
- 27.McDonald B, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 28.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Ley K, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 30.Phillipson M, et al. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J. Exp. Med. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson Z, et al. Interaction of chemokines and glycosaminoglycans: a new twist in the regulation of chemokine function with opportunities for therapeutic intervention. Cytokine Growth Factor Rev. 2005;16:625–636. doi: 10.1016/j.cytogfr.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Lou O, et al. CD99 is a key mediator of the transendothelial migration of neutrophils. J. Immunol. 2007;178:1136–1143. doi: 10.4049/jimmunol.178.2.1136. [DOI] [PubMed] [Google Scholar]
- 33.Wegmann F, et al. ESAM supports neutrophil extravasation, activation of Rho, and VEGF-induced vascular permeability. J. Exp. Med. 2006;203:1671–1677. doi: 10.1084/jem.20060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reutershan J, et al. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J. Clin. Invest. 2006;116:695–702. doi: 10.1172/JCI27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dangerfield J, et al. PECAM-1 (CD31) homophilic interaction up-regulates alpha6beta1 on transmigrated neutrophils in vivo and plays a functional role in the ability of alpha6 integrins to mediate leukocyte migration through the perivascular basement membrane. J. Exp. Med. 2002;196:1201–1211. doi: 10.1084/jem.20020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stefanidakis M, et al. Intracellular and cell surface localization of a complex between alphaMbeta2 integrin and promatrix metalloproteinase-9 progelatinase in neutrophils. J. Immunol. 2004;172:7060–7068. doi: 10.4049/jimmunol.172.11.7060. [DOI] [PubMed] [Google Scholar]
- 37.Wang S, et al. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J. Exp. Med. 2006;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young RE, et al. Role of neutrophil elastase in LTB4-induced neutrophil transmigration in vivo assessed with a specific inhibitor and neutrophil elastase deficient mice. Br. J. Pharmacol. 2007;151:628–637. doi: 10.1038/sj.bjp.0707267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coldren CD, et al. Functional and genomic changes induced by alveolar transmigration in human neutrophils. Am. J. Physiol Lung Cell Mol. Physiol. 2006;291:L1267–L1276. doi: 10.1152/ajplung.00097.2006. [DOI] [PubMed] [Google Scholar]
- 40.Nourshargh S, Marelli-Berg FM. Transmigration through venular walls: a key regulator of leukocyte phenotype and function. Trends Immunol. 2005;26:157–165. doi: 10.1016/j.it.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Hu M, et al. Transmigration across a lung epithelial monolayer delays apoptosis of polymorphonuclear leukocytes. Surgery. 2004;135:87–98. doi: 10.1016/s0039-6060(03)00347-7. [DOI] [PubMed] [Google Scholar]
- 42.Chou RC, et al. Lipid-Cytokine-Chemokine Cascade Drives Neutrophil Recruitment in a Murine Model of Inflammatory Arthritis. Immunity. 2010;33:266–278. doi: 10.1016/j.immuni.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen M, et al. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J. Exp. Med. 2006;203:837–842. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim ND, et al. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J. Exp. Med. 2006;203:829–835. doi: 10.1084/jem.20052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bernhagen J, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 2007;13:587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 46.Weber C, et al. Structural determinants of MIF functions in CXCR2-mediated inflammatory and atherogenic leukocyte recruitment. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16278–16283. doi: 10.1073/pnas.0804017105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santos LL, et al. Macrophage migration inhibitory factor regulates neutrophil chemotactic responses in inflammatory arthritis. Arthritis Rheum. 2010 doi: 10.1002/art.30203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saiwai H, et al. The LTB4-BLT1 axis mediates neutrophil infiltration and secondary injury in experimental spinal cord injury. Am. J. Pathol. 2010;176:2352–2366. doi: 10.2353/ajpath.2010.090839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cadene M, et al. Influence of low molecular mass heparin on the kinetics of neutrophil elastase inhibition by mucus proteinase inhibitor. J. Biol. Chem. 1995;270:13204–13209. doi: 10.1074/jbc.270.22.13204. [DOI] [PubMed] [Google Scholar]
- 50.Webb LM, et al. Binding to heparan sulfate or heparin enhances neutrophil responses to interleukin 8. Proc. Natl. Acad. Sci. U. S. A. 1993;90:7158–7162. doi: 10.1073/pnas.90.15.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quinton LJ, et al. Selective transport of cytokine-induced neutrophil chemoattractant from the lung to the blood facilitates pulmonary neutrophil recruitment. Am. J. Physiol Lung Cell Mol. Physiol. 2004;286:L465–L472. doi: 10.1152/ajplung.00153.2003. [DOI] [PubMed] [Google Scholar]
- 52.Zhang P, et al. The granulopoietic cytokine response and enhancement of granulopoiesis in mice during endotoxemia. Shock. 2005;23:344–352. doi: 10.1097/01.shk.0000158960.93832.de. [DOI] [PubMed] [Google Scholar]
- 53.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 54.Grespan R, et al. CXCR2-specific chemokines mediate leukotriene B4-dependent recruitment of neutrophils to inflamed joints in mice with antigen-induced arthritis. Arthritis Rheum. 2008;58:2030–2040. doi: 10.1002/art.23597. [DOI] [PubMed] [Google Scholar]
- 55.Lemos HP, et al. Prostaglandin mediates IL-23/IL-17-induced neutrophil migration in inflammation by inhibiting IL-12 and IFNgamma production. Proc. Natl. Acad. Sci. U. S. A. 2009;106:5954–5959. doi: 10.1073/pnas.0812782106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pelletier M, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115:335–343. doi: 10.1182/blood-2009-04-216085. [DOI] [PubMed] [Google Scholar]
- 57.Foxman EF, et al. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J. Cell Biol. 1997;139:1349–1360. doi: 10.1083/jcb.139.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foxman EF, et al. Integrating conflicting chemotactic signals. The role of memory in leukocyte navigation. J. Cell Biol. 1999;147:577–588. doi: 10.1083/jcb.147.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heit B, et al. PTEN functions to ‘prioritize’ chemotactic cues and prevent ‘distraction’ in migrating neutrophils. Nat. Immunol. 2008;9:743–752. doi: 10.1038/ni.1623. [DOI] [PubMed] [Google Scholar]
- 60.Khan AI, et al. Lipopolysaccharide: a p38 MAPK-dependent disrupter of neutrophil chemotaxis. Microcirculation. 2005;12:421–432. doi: 10.1080/10739680590960368. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y, et al. IL-17A and TNF-{alpha} Exert Synergistic Effects on Expression of CXCL5 by Alveolar Type II Cells In Vivo and In Vitro. J. Immunol. 2011 doi: 10.4049/jimmunol.1002016. [DOI] [PubMed] [Google Scholar]
- 62.Lee PY, et al. IL-1alpha modulates neutrophil recruitment in chronic inflammation induced by hydrocarbon oil. J. Immunol. 2011;186:1747–1754. doi: 10.4049/jimmunol.1001328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scapini P, et al. The neutrophil as a cellular source of chemokines. Immunol. Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 64.Lindemann SW, et al. Neutrophils alter the inflammatory milieu by signal-dependent translation of constitutive messenger RNAs. Proc. Natl. Acad. Sci. U. S. A. 2004;101:7076–7081. doi: 10.1073/pnas.0401901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guma M, et al. Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis Rheum. 2009;60:3642–3650. doi: 10.1002/art.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joosten LA, et al. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum. 2009;60:3651–3662. doi: 10.1002/art.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kasama T, et al. Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Curr. Drug Targets. Inflamm. Allergy. 2005;4:273–279. doi: 10.2174/1568010054022114. [DOI] [PubMed] [Google Scholar]
- 68.Cassatella MA. Neutrophil-derived proteins: selling cytokines by the pound. Adv. Immunol. 1999;73:369–509. doi: 10.1016/s0065-2776(08)60791-9. [DOI] [PubMed] [Google Scholar]
- 69.Joosten LA, et al. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum. 2009;60:3651–3662. doi: 10.1002/art.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van LP, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J. Leukoc. Biol. 2007;82:1375–1381. doi: 10.1189/jlb.0607338. [DOI] [PubMed] [Google Scholar]
- 71.Weathington NM, et al. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat. Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 72.Gaggar A, et al. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J. Immunol. 2008;180:5662–5669. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Snelgrove RJ, et al. A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science. 2010;330:90–94. doi: 10.1126/science.1190594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soehnlein O, et al. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114:4613–4623. doi: 10.1182/blood-2009-06-221630. [DOI] [PubMed] [Google Scholar]
- 75.Ariel A, et al. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat. Immunol. 2006;7:1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176–183. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 77.Zlotnik A, et al. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biology. 2006;7:243. doi: 10.1186/gb-2006-7-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]